Abstract
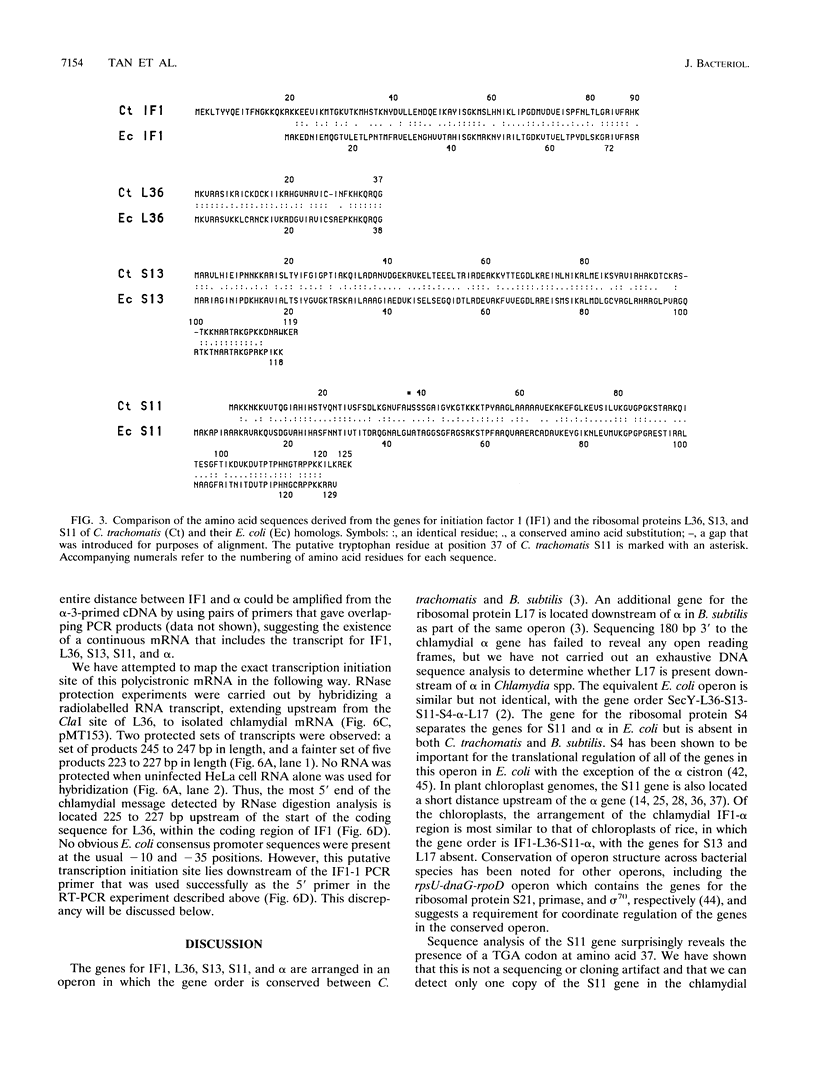
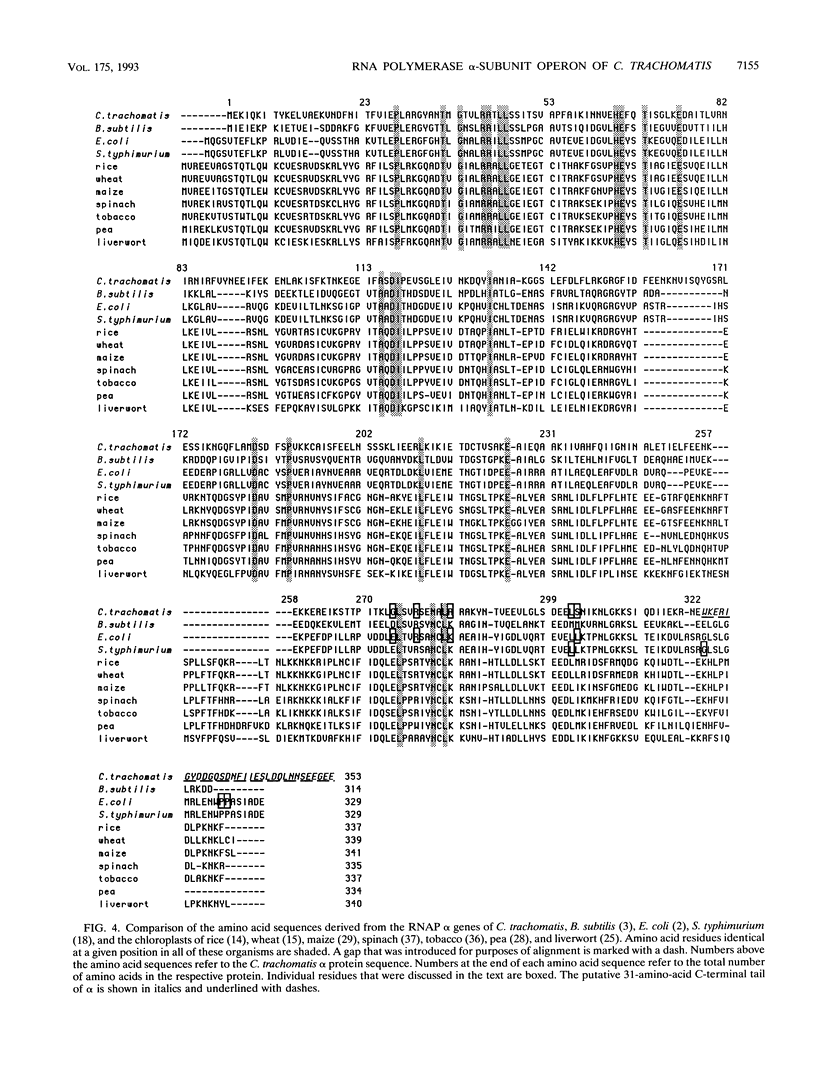
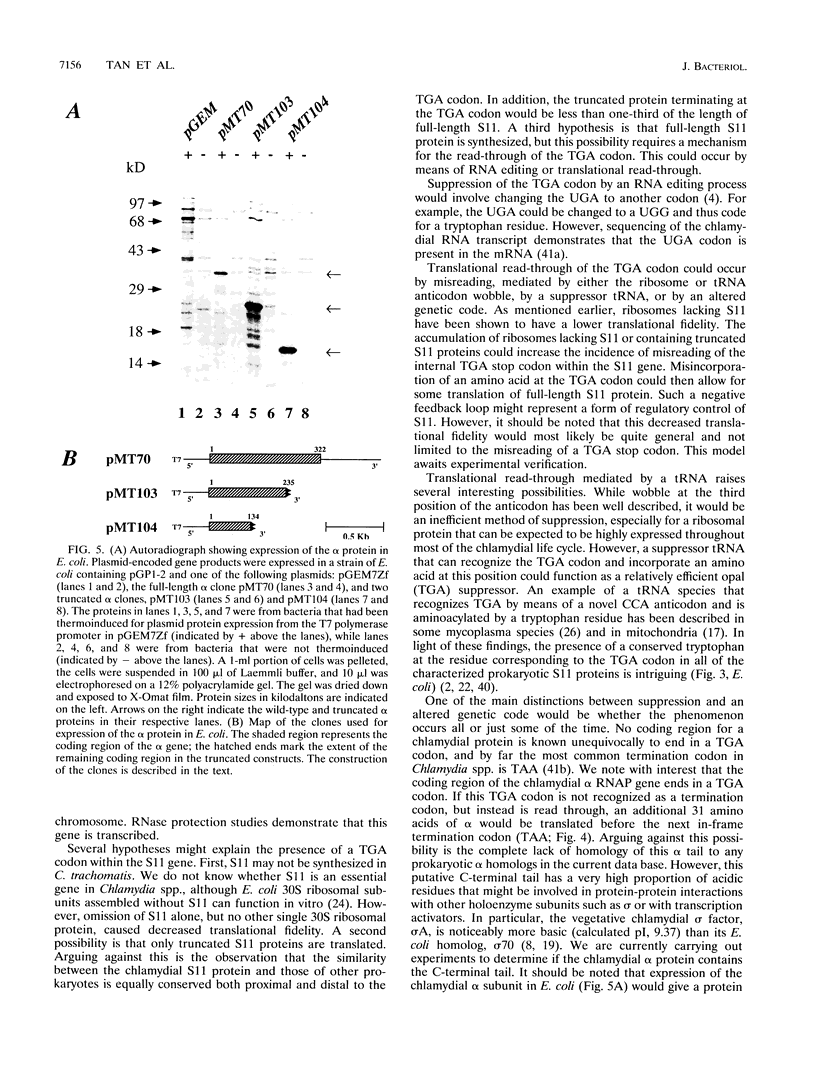
We have cloned the chlamydial operon that encodes the initiation factor IF1, the ribosomal proteins L36, S13, and S11, and the alpha subunit of RNA polymerase. The genes for S11 and alpha are closely linked in Escherichia coli, Bacillus subtilis, and plant chloroplast genomes, and this arrangement is conserved in Chlamydia spp. The S11 ribosomal protein gene potentially encodes a protein of 125 amino acids with 41 to 42% identity over its entire length to its E. coli and B. subtilis homologs; the gene encoding the alpha subunit specifies a protein of 322 amino acids with 25 to 30% identity over its entire length to its E. coli and B. subtilis homologs. In a T7-based expression system in E. coli, the chlamydial alpha gene directed the synthesis of a 36-kDa protein. Mapping of the chlamydial mRNA transcript by RNase protection studies and by a combination of reverse transcription and the polymerase chain reaction demonstrates that IF1, L36, S13, S11, and alpha are transcribed as a polycistronic transcript.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. E., Stephens R. S. Identification by sequence analysis of two-site posttranslational processing of the cysteine-rich outer membrane protein 2 of Chlamydia trachomatis serovar L2. J Bacteriol. 1989 Jan;171(1):285–291. doi: 10.1128/jb.171.1.285-291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell D., Davis G., Gosink M., Post L., Nomura M., Kestler H., Zengel J. M., Lindahl L. Nucleotide sequence of the alpha ribosomal protein operon of Escherichia coli. Nucleic Acids Res. 1985 Jun 11;13(11):3891–3903. doi: 10.1093/nar/13.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan S. A., Suh J. W., Thomas S. M., Price C. W. Gene encoding the alpha core subunit of Bacillus subtilis RNA polymerase is cotranscribed with the genes for initiation factor 1 and ribosomal proteins B, S13, S11, and L17. J Bacteriol. 1989 May;171(5):2553–2562. doi: 10.1128/jb.171.5.2553-2562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R. Messenger RNA editing and the genetic code. Experientia. 1990 Dec 1;46(11-12):1142–1148. doi: 10.1007/BF01936924. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Dean D., Davis G. R., Bedwell D. M., Nomura M. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 1983 May 11;11(9):2599–2616. doi: 10.1093/nar/11.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebright R. H. Transcription activation at Class I CAP-dependent promoters. Mol Microbiol. 1993 May;8(5):797–802. doi: 10.1111/j.1365-2958.1993.tb01626.x. [DOI] [PubMed] [Google Scholar]
- Engel J. N., Ganem D. A polymerase chain reaction-based approach to cloning sigma factors from eubacteria and its application to the isolation of a sigma-70 homolog from Chlamydia trachomatis. J Bacteriol. 1990 May;172(5):2447–2455. doi: 10.1128/jb.172.5.2447-2455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. N., Ganem D. Chlamydial rRNA operons: gene organization and identification of putative tandem promoters. J Bacteriol. 1987 Dec;169(12):5678–5685. doi: 10.1128/jb.169.12.5678-5685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. N., Pollack J., Malik F., Ganem D. Cloning and characterization of RNA polymerase core subunits of Chlamydia trachomatis by using the polymerase chain reaction. J Bacteriol. 1990 Oct;172(10):5732–5741. doi: 10.1128/jb.172.10.5732-5741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. N., Pollack J., Perara E., Ganem D. Heat shock response of murine Chlamydia trachomatis. J Bacteriol. 1990 Dec;172(12):6959–6972. doi: 10.1128/jb.172.12.6959-6972.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahr M. J., Sriprakash K. S., Hatch T. P. Convergent and overlapping transcripts of the Chlamydia trachomatis 7.5-kb plasmid. Plasmid. 1992 Nov;28(3):247–257. doi: 10.1016/0147-619x(92)90056-g. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Hird S. M., Dyer T. A., Gray J. C. Nucleotide sequence of the rpoA gene in wheat chloroplast DNA. Nucleic Acids Res. 1989 Aug 11;17(15):6394–6394. doi: 10.1093/nar/17.15.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihama A. Role of the RNA polymerase alpha subunit in transcription activation. Mol Microbiol. 1992 Nov;6(22):3283–3288. doi: 10.1111/j.1365-2958.1992.tb02196.x. [DOI] [PubMed] [Google Scholar]
- Jukes T. H., Osawa S. The genetic code in mitochondria and chloroplasts. Experientia. 1990 Dec 1;46(11-12):1117–1126. doi: 10.1007/BF01936921. [DOI] [PubMed] [Google Scholar]
- Kimura M., Kimura J., Hatakeyama T. Amino acid sequences of ribosomal proteins S11 from Bacillus stearothermophilus and S19 from Halobacterium marismortui. Comparison of the ribosomal protein S11 family. FEBS Lett. 1988 Nov 21;240(1-2):15–20. doi: 10.1016/0014-5793(88)80332-6. [DOI] [PubMed] [Google Scholar]
- Koehler J. E., Burgess R. R., Thompson N. E., Stephens R. S. Chlamydia trachomatis RNA polymerase major sigma subunit. Sequence and structural comparison of conserved and unique regions with Escherichia coli sigma 70 and Bacillus subtilis sigma 43. J Biol Chem. 1990 Aug 5;265(22):13206–13214. [PubMed] [Google Scholar]
- Lambden P. R., Everson J. S., Ward M. E., Clarke I. N. Sulfur-rich proteins of Chlamydia trachomatis: developmentally regulated transcription of polycistronic mRNA from tandem promoters. Gene. 1990 Mar 1;87(1):105–112. doi: 10.1016/0378-1119(90)90500-q. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Sor F., Archer R. H., Nomura M., Zengel J. M. Transcriptional organization of the S10, spc and alpha operons of Escherichia coli. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):337–342. doi: 10.1016/0167-4781(90)90191-4. [DOI] [PubMed] [Google Scholar]
- Lombardo M. J., Bagga D., Miller C. G. Mutations in rpoA affect expression of anaerobically regulated genes in Salmonella typhimurium. J Bacteriol. 1991 Dec;173(23):7511–7518. doi: 10.1128/jb.173.23.7511-7518.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markmann-Mulisch U., Subramanian A. R. Nucleotide sequence of maize chloroplast rpS11 with conserved amino acid sequence between eukaryotes, bacteria and plastids. Biochem Int. 1988 Oct;17(4):655–664. [PubMed] [Google Scholar]
- Moulder J. W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991 Mar;55(1):143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Mizushima S., Ozaki M., Traub P., Lowry C. V. Structure and function of ribosomes and their molecular components. Cold Spring Harb Symp Quant Biol. 1969;34:49–61. doi: 10.1101/sqb.1969.034.01.009. [DOI] [PubMed] [Google Scholar]
- Osawa S., Muto A., Ohama T., Andachi Y., Tanaka R., Yamao F. Prokaryotic genetic code. Experientia. 1990 Dec 1;46(11-12):1097–1106. doi: 10.1007/BF01936919. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton S., Gray J. C. The plastid rpoA gene encoding a protein homologous to the bacterial RNA polymerase alpha subunit is expressed in pea chloroplasts. Mol Gen Genet. 1989 May;217(1):77–84. doi: 10.1007/BF00330945. [DOI] [PubMed] [Google Scholar]
- Ruf M., Kössel H. Structure and expression of the gene coding for the alpha-subunit of DNA-dependent RNA polymerase from the chloroplast genome of Zea mays. Nucleic Acids Res. 1988 Jul 11;16(13):5741–5754. doi: 10.1093/nar/16.13.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo F. D., Silhavy T. J. Alpha: the Cinderella subunit of RNA polymerase. J Biol Chem. 1992 Jul 25;267(21):14515–14518. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardinia L. M., Engel J. N., Ganem D. Chlamydial gene encoding a 70-kilodalton antigen in Escherichia coli: analysis of expression signals and identification of the gene product. J Bacteriol. 1989 Jan;171(1):335–341. doi: 10.1128/jb.171.1.335-341.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Schachter J. The intracellular life of Chlamydia. Curr Top Microbiol Immunol. 1988;138:109–139. [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijben-Müller G., Hallick R. B., Alt J., Westhoff P., Herrmann R. G. Spinach plastid genes coding for initiation factor IF-1, ribosomal protein S11 and RNA polymerase alpha-subunit. Nucleic Acids Res. 1986 Jan 24;14(2):1029–1044. doi: 10.1093/nar/14.2.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauch J. M., Russo F. D., Silhavy T. J. Suppressor mutations in rpoA suggest that OmpR controls transcription by direct interaction with the alpha subunit of RNA polymerase. J Bacteriol. 1991 Dec;173(23):7501–7510. doi: 10.1128/jb.173.23.7501-7510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Wagar E. A., Edman U. Developmental regulation of tandem promoters for the major outer membrane protein gene of Chlamydia trachomatis. J Bacteriol. 1988 Feb;170(2):744–750. doi: 10.1128/jb.170.2.744-750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J. W., Boylan S. A., Price C. W. Gene for the alpha subunit of Bacillus subtilis RNA polymerase maps in the ribosomal protein gene cluster. J Bacteriol. 1986 Oct;168(1):65–71. doi: 10.1128/jb.168.1.65-71.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. S., Bedwell D. M., Nomura M. Regulation of alpha operon gene expression in Escherichia coli. A novel form of translational coupling. J Mol Biol. 1987 Jul 20;196(2):333–345. doi: 10.1016/0022-2836(87)90694-2. [DOI] [PubMed] [Google Scholar]
- Thomas M. S., Glass R. E. Escherichia coli rpoA mutation which impairs transcription of positively regulated systems. Mol Microbiol. 1991 Nov;5(11):2719–2725. doi: 10.1111/j.1365-2958.1991.tb01980.x. [DOI] [PubMed] [Google Scholar]
- Versalovic J., Koeuth T., Britton R., Geszvain K., Lupski J. R. Conservation and evolution of the rpsU-dnaG-rpoD macromolecular synthesis operon in bacteria. Mol Microbiol. 1993 Apr;8(2):343–355. doi: 10.1111/j.1365-2958.1993.tb01578.x. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Arfsten A. E., Nomura M. In vitro expression of Escherichia coli ribosomal protein genes: autogenous inhibition of translation. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1837–1841. doi: 10.1073/pnas.77.4.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]