Abstract
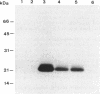
The C3 exoenzyme gene is located on a bacteriophage in Clostridium botulinum types C and D (M. R. Popoff, D. Hauser, P. Boquet, M. W. Eklund, and D. M. Gill, Infect. Immun. 59:3673-3679, 1991). A derivative CN phage from phage C of C. botulinum Stockholm (C-St) (K. Oguma, H. Iida, and K. Inoue, Jpn. J. Microbiol. 19:167-172, 1975), isolated as neurotoxin negative, also does not produce exoenzyme C3. The botulinal neurotoxin C1 gene is present on the CN phage but contains a stop mutation in the DNA region encoding the N-terminal part of the heavy chain (codon 553). The putative truncated botulinal neurotoxin C1 protein was not recovered in a C. botulinum strain harboring the CN phage. We found that the C3 gene is localized on a 21.5-kbp DNA fragment flanked by the core motif 5'-AAGGAG-3' in DNAs of phage C of C. botulinum 468 (C-468), C-St phage, and phage D of C. botulinum 1873 (D-1873). The 21.5-kbp DNA fragment is deleted in CN phage DNA, and the motif 5'-AAGGAG-3' is present only in one copy at the deletion junction, but the deletion in the CN phage could be nonspecific, since this phage was obtained by nitrosoguanidine treatment. These findings could indicate that the C3 gene is localized on a 21.5-kbp mobile element. C. botulinum type C strain 003-9 produces a C3 exoenzyme (Y. Nemoto, T. Namba, S. Kozaki, and S. Narumiya, J. Biol. Chem. 266:19312-19319, 1991), and Staphylococcus aureus E1 produces a related C3 enzyme which is named epidernmal cell differentiation inhibitor (S. Inoue, M. Sugai, Y. Murooka, S. Y. Paik, Y. M. Hong, H. Oghai, and H. Suginaka, Biochem. Biophys. Res. Comm. 174:459-464, 1991) and which shares 80.6 and 56.6% similarity, respectively with the C3 enzymes from C-468 or C-St and D-1873 phages athe amino acid level. The features of the putative 21.5-kbp transposon were not found in C. botulinum 003-9 and S. aureus E1, as determined by analysis of the C3 and epidermal cell differentiation inhibitor gene-flanking DNA regions. These data suggest a common ancestral origin and divergent evolution of the C3 genes in these three groups of bacterial strains and dissemination of a 21.5-kbp element carrying the C3 gene C-468, C-St, and D-1873 phages.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham L. J., Rood J. I. Identification of Tn4451 and Tn4452, chloramphenicol resistance transposons from Clostridium perfringens. J Bacteriol. 1987 Apr;169(4):1579–1584. doi: 10.1128/jb.169.4.1579-1584.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktories K., Bärmann M., Ohishi I., Tsuyama S., Jakobs K. H., Habermann E. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986 Jul 24;322(6077):390–392. doi: 10.1038/322390a0. [DOI] [PubMed] [Google Scholar]
- Belyi YuF, Tartakovskii I. S., Vertiev YuV, Prosorovskii S. V. Partial purification and characterization of ADP-ribosyltransferase produced by Legionella pneumophila. Biomed Sci. 1991;2(2):169–174. [PubMed] [Google Scholar]
- Binz T., Kurazono H., Popoff M. R., Eklund M. W., Sakaguchi G., Kozaki S., Krieglstein K., Henschen A., Gill D. M., Niemann H. Nucleotide sequence of the gene encoding Clostridium botulinum neurotoxin type D. Nucleic Acids Res. 1990 Sep 25;18(18):5556–5556. doi: 10.1093/nar/18.18.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P., Boquet P., Madaule P., Popoff M. R., Rubin E. J., Gill D. M. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989 Apr;8(4):1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan A. J., Nemoto Y., Narumiya S., Kozaki S., Haley B. E. NAD+ binding site of Clostridium botulinum C3 ADP-ribosyltransferase. Identification of peptide in the adenine ring binding domain using 2-azido NAD. J Biol Chem. 1992 Jul 25;267(21):14866–14870. [PubMed] [Google Scholar]
- Daube G., Simon P., Kaeckenbeeck A. IS1151, an IS-like element of Clostridium perfringens. Nucleic Acids Res. 1993 Jan 25;21(2):352–352. doi: 10.1093/nar/21.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenighini M., Montecucco C., Ripka W. C., Rappuoli R. Computer modelling of the NAD binding site of ADP-ribosylating toxins: active-site structure and mechanism of NAD binding. Mol Microbiol. 1991 Jan;5(1):23–31. doi: 10.1111/j.1365-2958.1991.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T. Interconversion of type C and D strains of Clostridium botulinum by specific bacteriophages. Appl Microbiol. 1974 Jan;27(1):251–258. doi: 10.1128/am.27.1.251-258.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T., Reed S. M., Smith C. A. Bacteriophage and the toxigenicity of Clostridium botulinum type C. Science. 1971 Apr 30;172(3982):480–482. doi: 10.1126/science.172.3982.480. [DOI] [PubMed] [Google Scholar]
- Fujii N., Oguma K., Yokosawa N., Kimura K., Tsuzuki K. Characterization of bacteriophage nucleic acids obtained from Clostridium botulinum types C and D. Appl Environ Microbiol. 1988 Jan;54(1):69–73. doi: 10.1128/aem.54.1.69-73.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser D., Eklund M. W., Kurazono H., Binz T., Niemann H., Gill D. M., Boquet P., Popoff M. R. Nucleotide sequence of Clostridium botulinum C1 neurotoxin. Nucleic Acids Res. 1990 Aug 25;18(16):4924–4924. doi: 10.1093/nar/18.16.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hächler H., Kayser F. H., Berger-Bächi B. Homology of a transferable tetracycline resistance determinant of Clostridium difficile with Streptococcus (Enterococcus) faecalis transposon Tn916. Antimicrob Agents Chemother. 1987 Jul;31(7):1033–1038. doi: 10.1128/aac.31.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Sugai M., Murooka Y., Paik S. Y., Hong Y. M., Ohgai H., Suginaka H. Molecular cloning and sequencing of the epidermal cell differentiation inhibitor gene from Staphylococcus aureus. Biochem Biophys Res Commun. 1991 Jan 31;174(2):459–464. doi: 10.1016/0006-291x(91)91438-i. [DOI] [PubMed] [Google Scholar]
- Just I., Mohr C., Schallehn G., Menard L., Didsbury J. R., Vandekerckhove J., van Damme J., Aktories K. Purification and characterization of an ADP-ribosyltransferase produced by Clostridium limosum. J Biol Chem. 1992 May 25;267(15):10274–10280. [PubMed] [Google Scholar]
- Just I., Schallehn G., Aktories K. ADP-ribosylation of small GTP-binding proteins by Bacillus cereus. Biochem Biophys Res Commun. 1992 Mar 31;183(3):931–936. doi: 10.1016/s0006-291x(05)80279-7. [DOI] [PubMed] [Google Scholar]
- Khorana H. G. Rhodopsin, photoreceptor of the rod cell. An emerging pattern for structure and function. J Biol Chem. 1992 Jan 5;267(1):1–4. [PubMed] [Google Scholar]
- Kimura K., Fujii N., Tsuzuki K., Murakami T., Indoh T., Yokosawa N., Takeshi K., Syuto B., Oguma K. The complete nucleotide sequence of the gene coding for botulinum type C1 toxin in the C-ST phage genome. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1304–1311. doi: 10.1016/0006-291x(90)90828-b. [DOI] [PubMed] [Google Scholar]
- Kozaki S., Kamata Y., Nagai T., Ogasawara J., Sakaguchi G. The use of monoclonal antibodies to analyze the structure of Clostridium botulinum type E derivative toxin. Infect Immun. 1986 Jun;52(3):786–791. doi: 10.1128/iai.52.3.786-791.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriishi K., Syuto B., Yokosawa N., Oguma K., Saito M. Purification and characterization of ADP-ribosyltransferases (exoenzyme C3) of Clostridium botulinum type C and D strains. J Bacteriol. 1991 Oct;173(19):6025–6029. doi: 10.1128/jb.173.19.6025-6029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullany P., Wilks M., Lamb I., Clayton C., Wren B., Tabaqchali S. Genetic analysis of a tetracycline resistance element from Clostridium difficile and its conjugal transfer to and from Bacillus subtilis. J Gen Microbiol. 1990 Jul;136(7):1343–1349. doi: 10.1099/00221287-136-7-1343. [DOI] [PubMed] [Google Scholar]
- Nemoto Y., Namba T., Kozaki S., Narumiya S. Clostridium botulinum C3 ADP-ribosyltransferase gene. Cloning, sequencing, and expression of a functional protein in Escherichia coli. J Biol Chem. 1991 Oct 15;266(29):19312–19319. [PubMed] [Google Scholar]
- Oguma K., Iida H., Inoue K. Observations on nonconverting phage, c-n71, obtained from a nontoxigenic strain of Clostridium botulinum type C. Jpn J Microbiol. 1975 Jun;19(3):167–172. doi: 10.1111/j.1348-0421.1975.tb00864.x. [DOI] [PubMed] [Google Scholar]
- Popoff M. R., Hauser D., Boquet P., Eklund M. W., Gill D. M. Characterization of the C3 gene of Clostridium botulinum types C and D and its expression in Escherichia coli. Infect Immun. 1991 Oct;59(10):3673–3679. doi: 10.1128/iai.59.10.3673-3679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff M., Boquet P., Gill D. M., Eklund M. W. DNA sequence of exoenzyme C3, an ADP-ribosyltransferase encoded by Clostridium botulinum C and D phages. Nucleic Acids Res. 1990 Mar 11;18(5):1291–1291. doi: 10.1093/nar/18.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E. J., Gill D. M., Boquet P., Popoff M. R. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. Mol Cell Biol. 1988 Jan;8(1):418–426. doi: 10.1128/mcb.8.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R., Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992 Oct 29;359(6398):832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]