Abstract
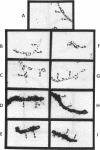
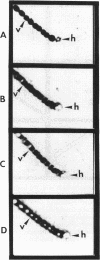
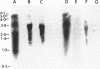
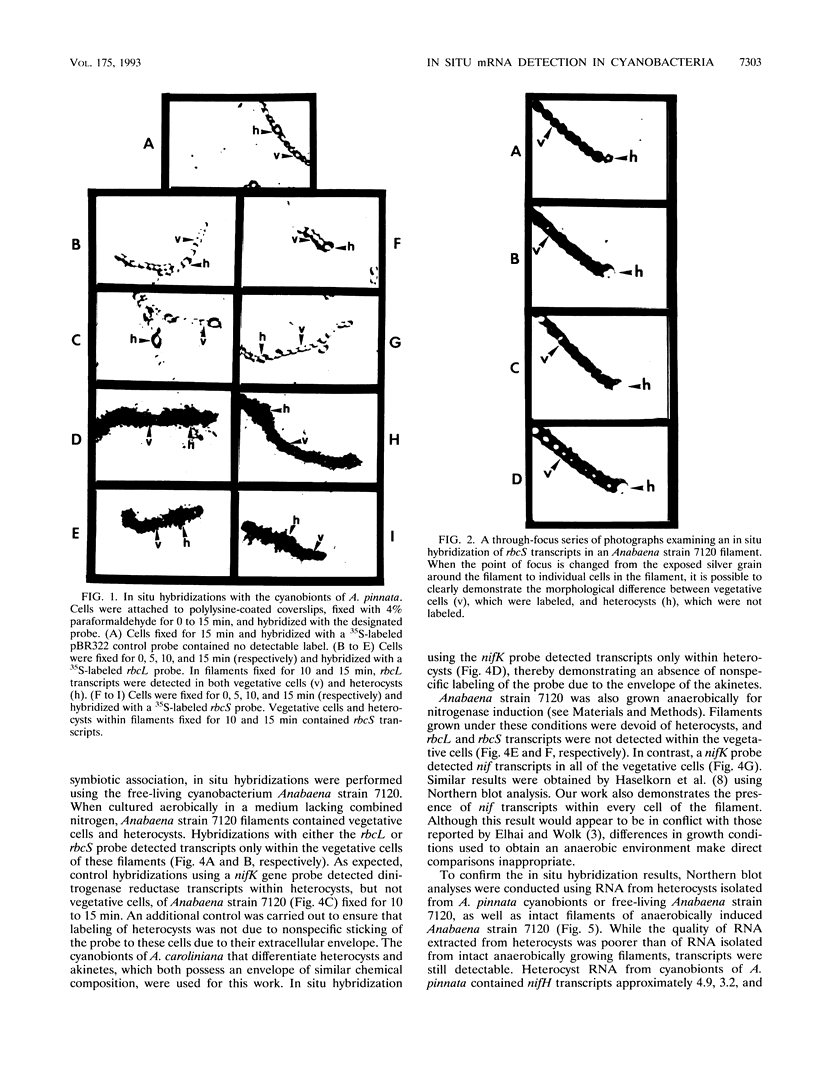
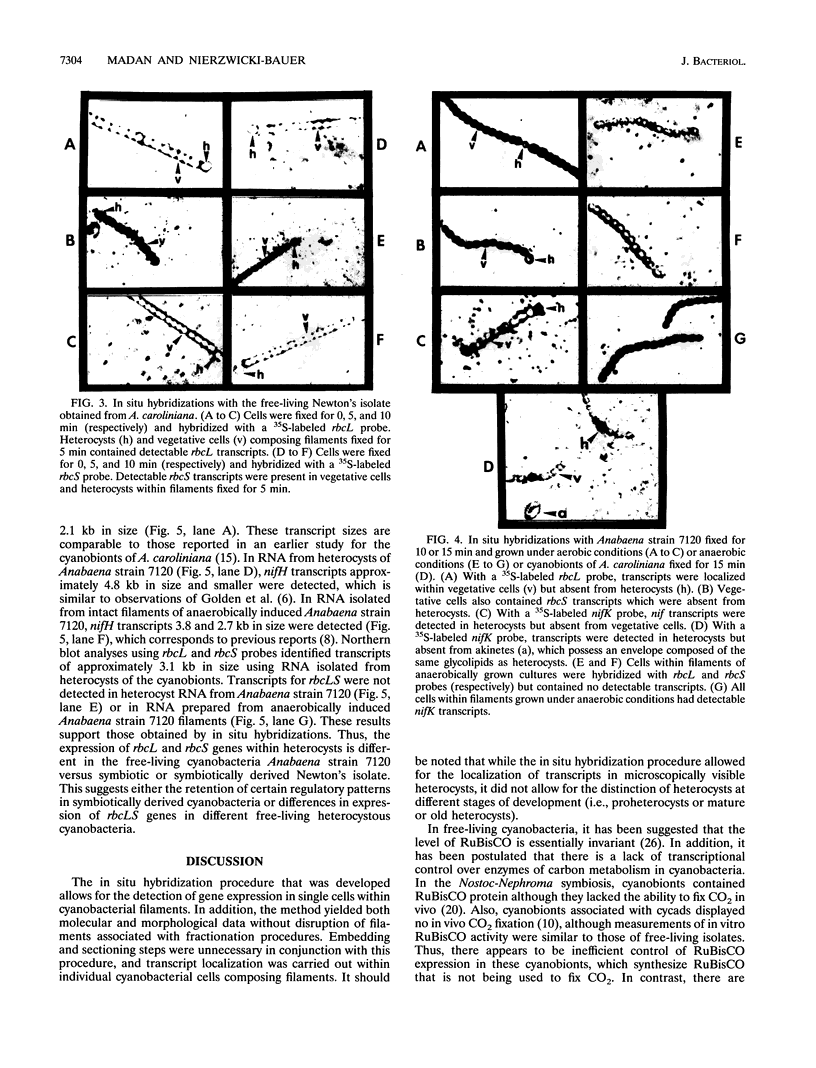
Heterocysts of free-living cyanobacteria lack ribulose-1,5-bisphosphate carboxylase activity. Nevertheless, using in situ hybridizations, we demonstrate that transcripts for the rbcL and rbcS genes are present in both heterocysts and vegetative cells of Anabaena spp. in association with, or isolated from, the Azolla-Anabaena symbiosis. In contrast, rbcLS transcripts were detected only in vegetative cells of the free-living cyanobacterium Anabaena strain 7120. Under anaerobic growth conditions that inhibited heterocyst differentiation, transcripts for nitrogenase were present in all cells composing Anabaena strain 7120 filaments, whereas rbcL and rbcS transcripts were not detected. Thus, transcriptional regulation of genes related to photosynthesis and nitrogen fixation is under environmental, as well as developmental, control in Anabaena spp. In addition, these results suggest either the possible retention of regulatory patterns in symbiotically derived cyanobacterial isolates or differences in expression of rbcLS genes in different free-living cyanobacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elhai J., Wolk C. P. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 1990 Oct;9(10):3379–3388. doi: 10.1002/j.1460-2075.1990.tb07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming H., Haselkorn R. The program of protein synthesis during heterocyst differentiation in nitrogen-fixing blue-green algae. Cell. 1974 Oct;3(2):169–170. doi: 10.1016/0092-8674(74)90121-4. [DOI] [PubMed] [Google Scholar]
- Giovannoni S. J., DeLong E. F., Olsen G. J., Pace N. R. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J Bacteriol. 1988 Feb;170(2):720–726. doi: 10.1128/jb.170.2.720-726.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Jensen B. B., Cox R. P., Burris R. H. Isolation of cyanobacterial heterocysts with high and sustained dinitrogen-fixation capacity supported by endogenous reductants. Arch Microbiol. 1986 Aug;145(3):241–247. doi: 10.1007/BF00443652. [DOI] [PubMed] [Google Scholar]
- Mevarech M., Rice D., Haselkorn R. Nucleotide sequence of a cyanobacterial nifH gene coding for nitrogenase reductase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6476–6480. doi: 10.1073/pnas.77.11.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Haselkorn R. Differences in mRNA levels in Anabaena living freely or in symbiotic association with Azolla. EMBO J. 1986 Jan;5(1):29–35. doi: 10.1002/j.1460-2075.1986.tb04173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. A., Mayne B. C. The Azolla, Anabaena azollae Relationship: II. Localization of Nitrogenase Activity as Assayed by Acetylene Reduction. Plant Physiol. 1974 Jun;53(6):820–824. doi: 10.1104/pp.53.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. A. The Azolla-Anabaena azzolae symbiosis. Basic Life Sci. 1977;9:231–258. doi: 10.1007/978-1-4684-0880-5_16. [DOI] [PubMed] [Google Scholar]
- Pierce J., Carlson T. J., Williams J. G. A cyanobacterial mutant requiring the expression of ribulose bisphosphate carboxylase from a photosynthetic anaerobe. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5753–5757. doi: 10.1073/pnas.86.15.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. B., Mayne B. C., Toia R. E., Peters G. A. Azolla-Anabaena Relationship: VIII. Photosynthetic Characterization of the Association and Individual Partners. Plant Physiol. 1979 Nov;64(5):791–795. doi: 10.1104/pp.64.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Steinberg N. A., Meeks J. C. Photosynthetic CO2 fixation and ribulose bisphosphate carboxylase/oxygenase activity of Nostoc sp. strain UCD 7801 in symbiotic association with Anthoceros punctatus. J Bacteriol. 1989 Nov;171(11):6227–6233. doi: 10.1128/jb.171.11.6227-6233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkenbach F., Wolk C. P. Activities of enzymes of the oxidative and the reductive pentose phosphate pathways in heterocysts of a blue-green alga. Plant Physiol. 1973 Nov;52(5):480–483. doi: 10.1104/pp.52.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P., Thomas J., Shaffer P. W., Austin S. M., Galonsky A. Pathway of nitrogen metabolism after fixation of 13N-labeled nitrogen gas by the cyanobacterium, Anabaena cylindrica. J Biol Chem. 1976 Aug 25;251(16):5027–5034. [PubMed] [Google Scholar]