Abstract
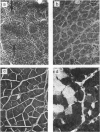
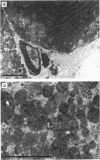
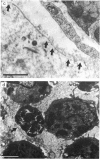
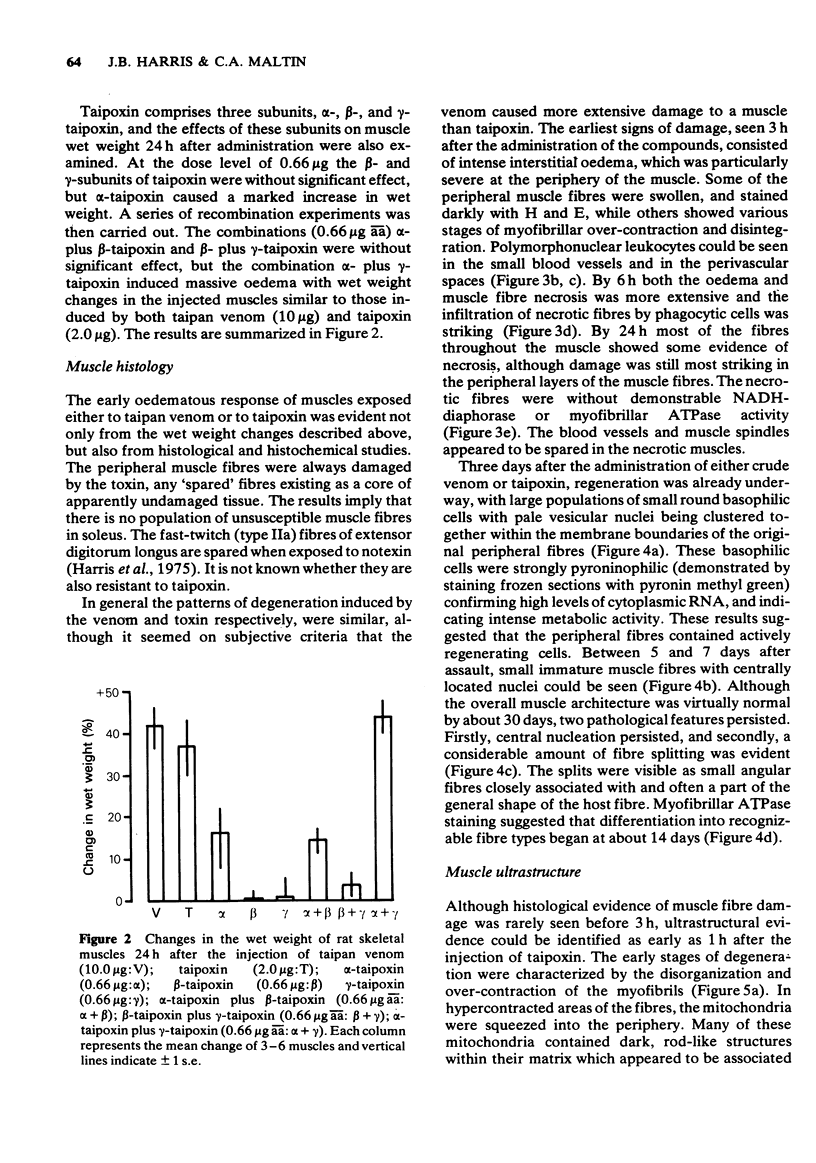
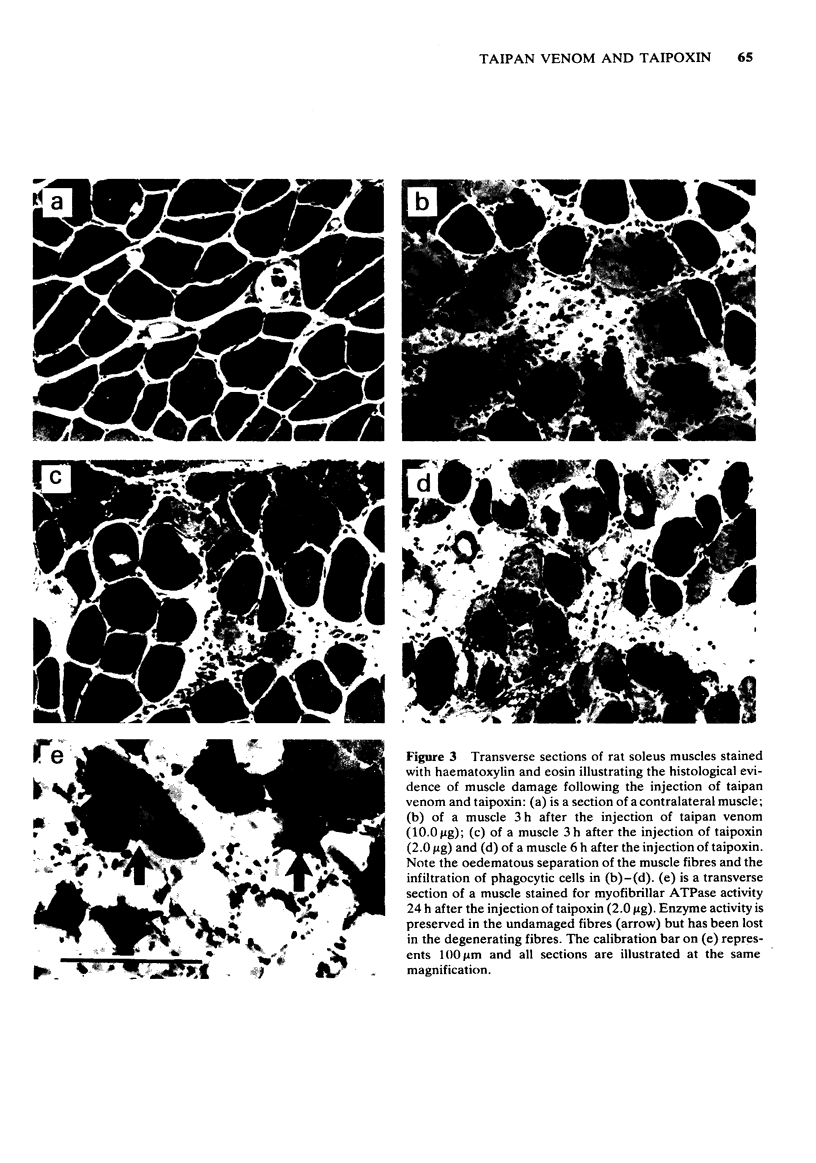
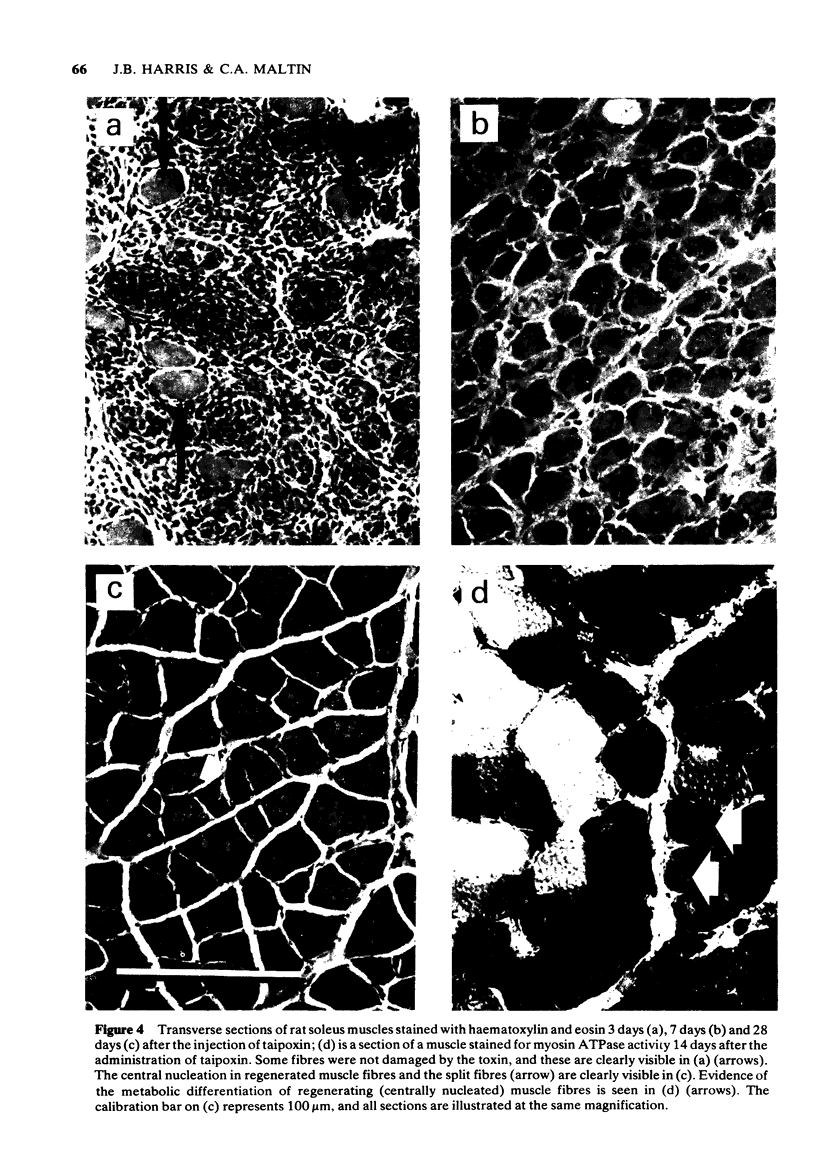
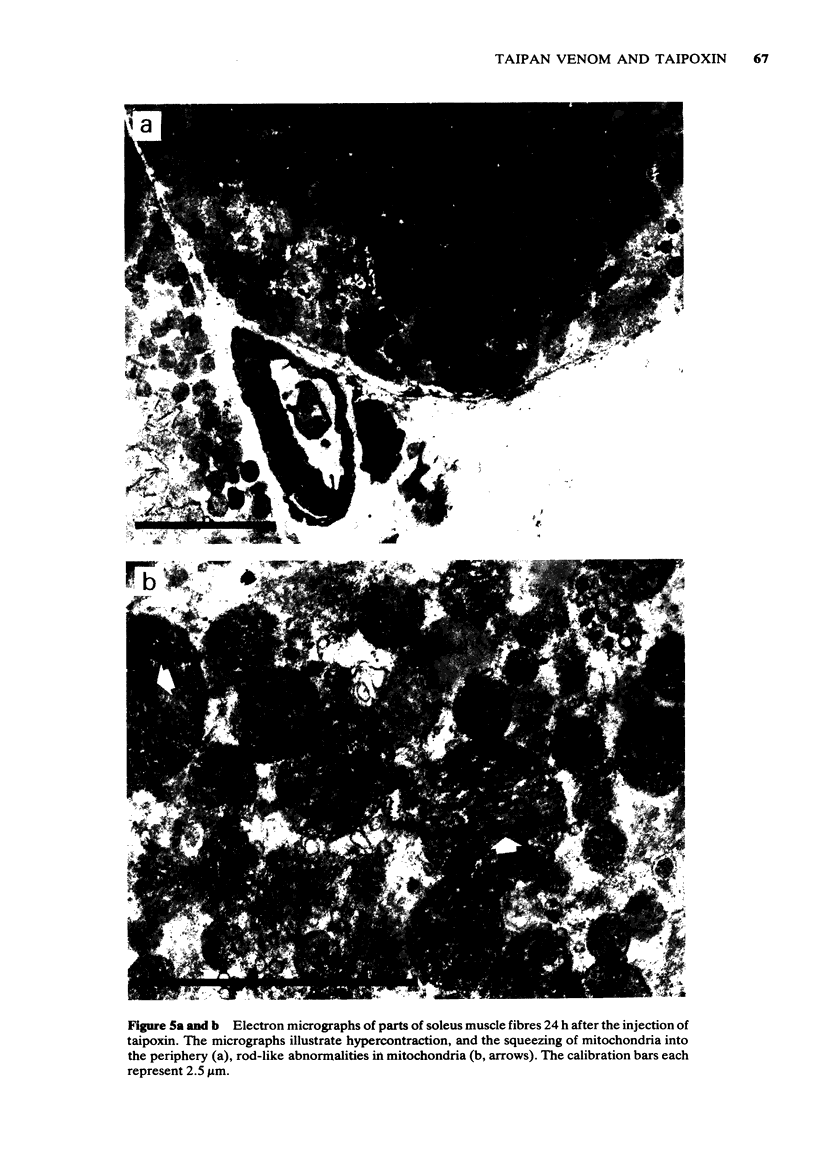
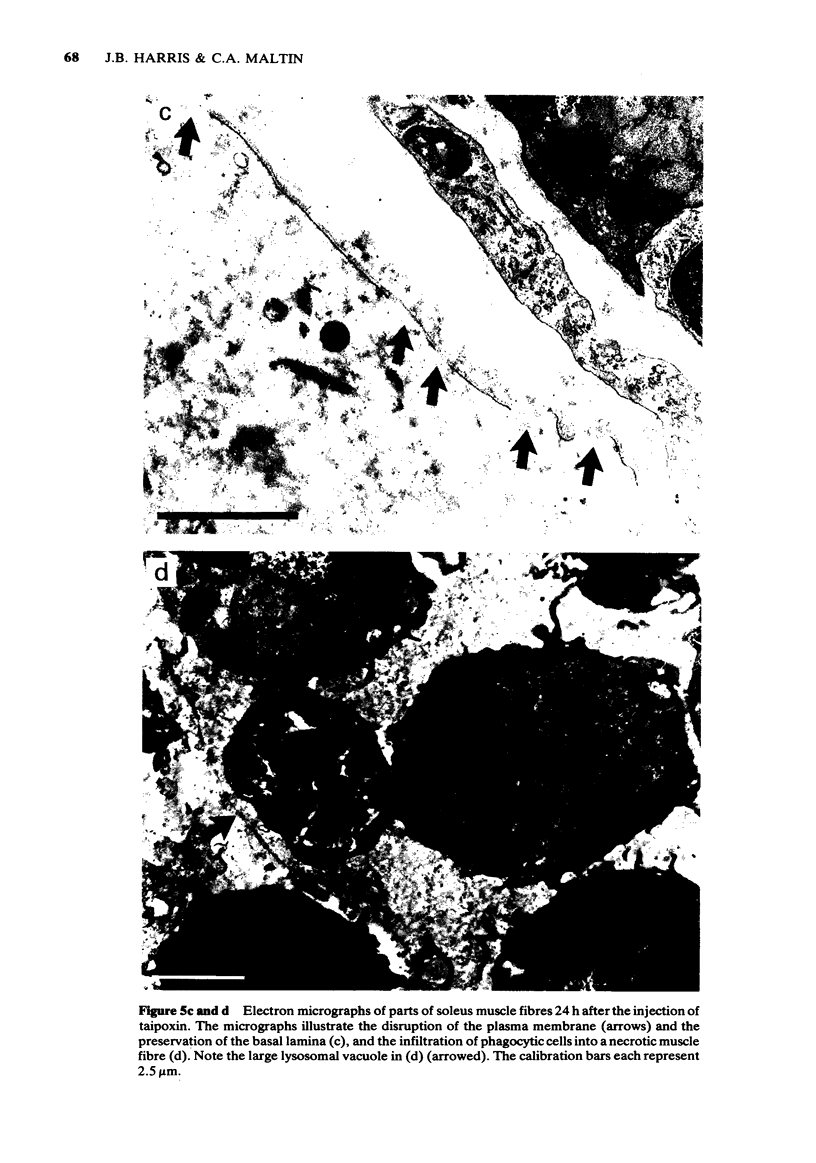
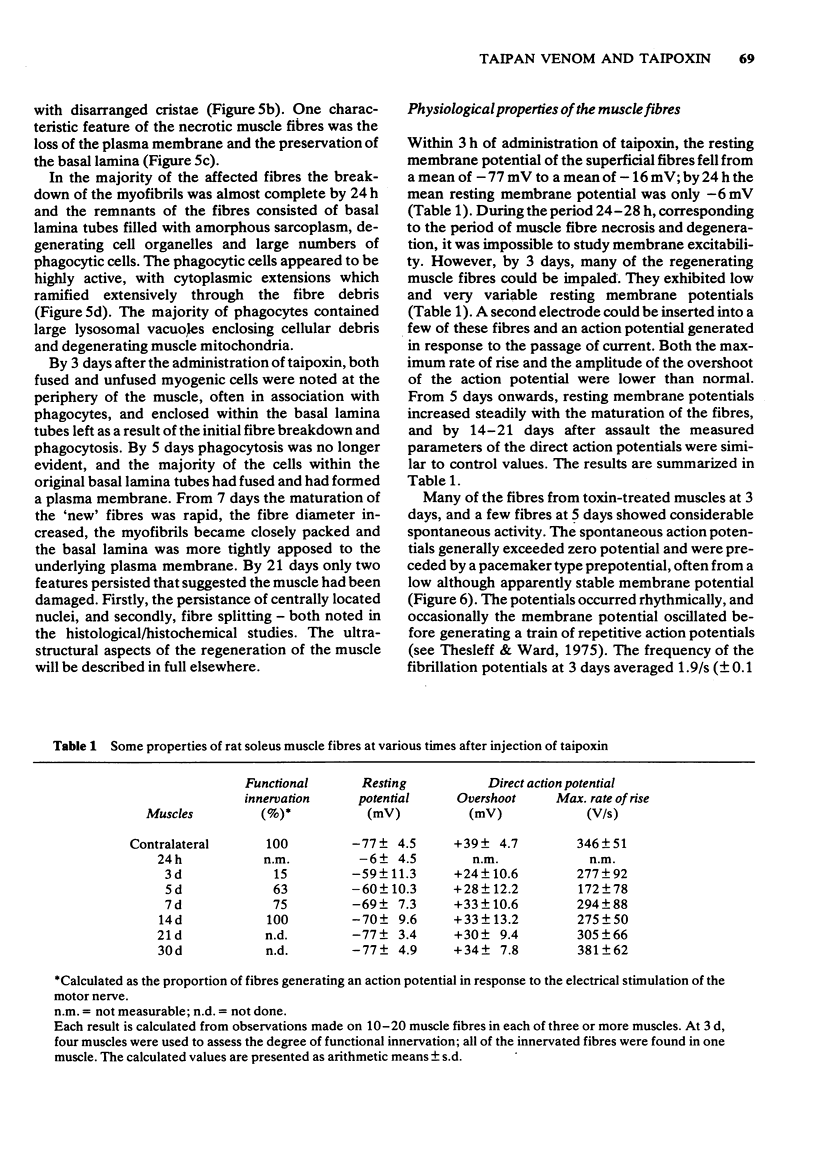
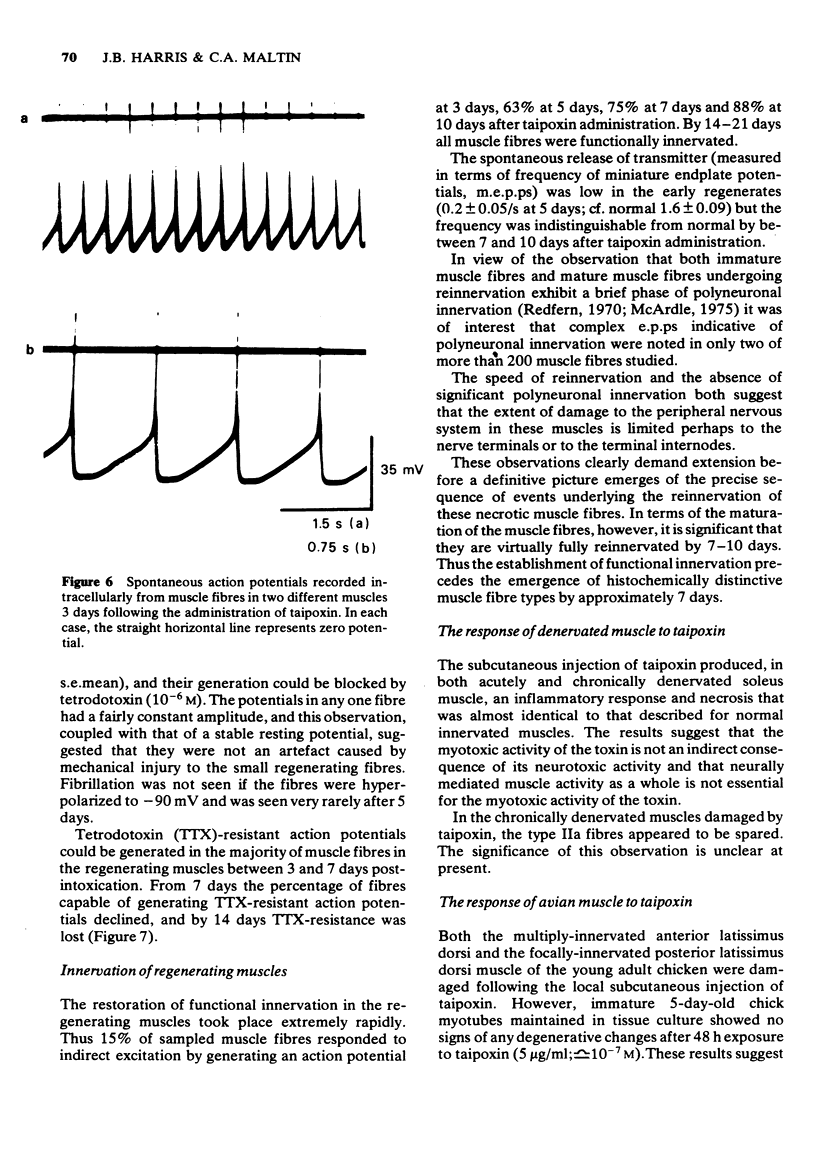
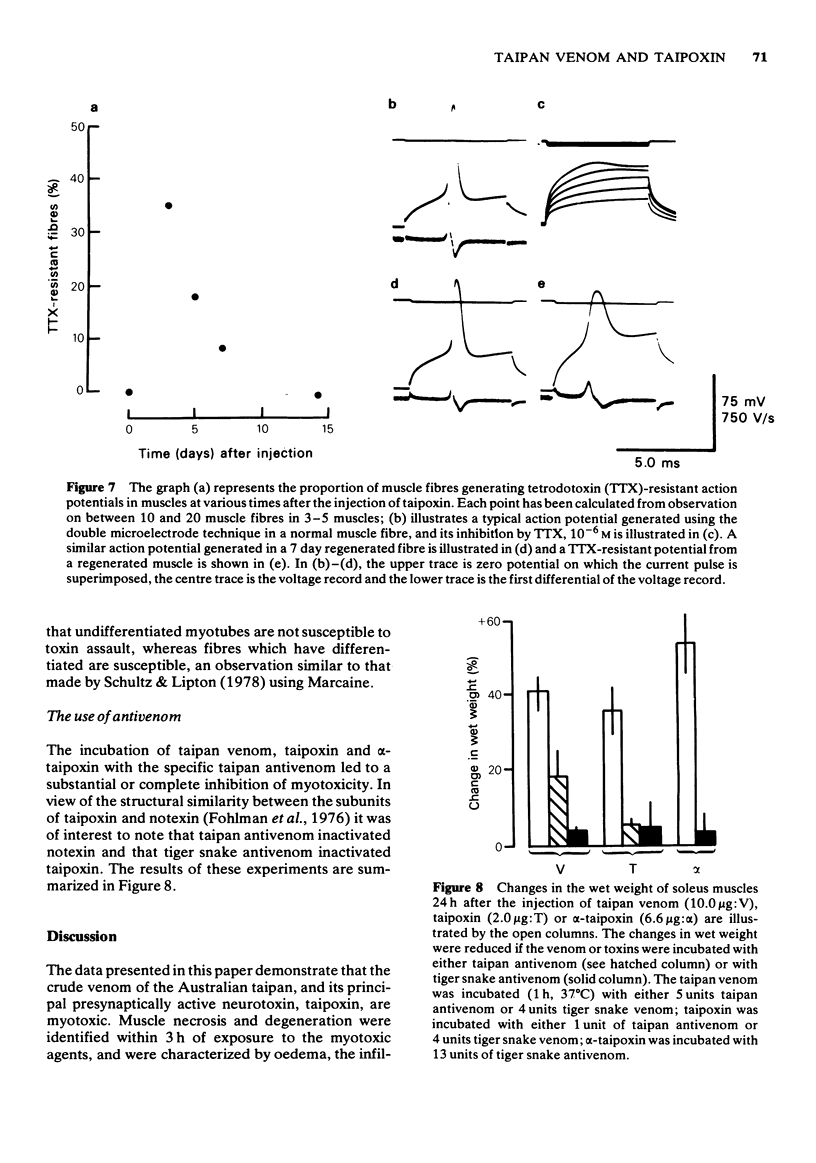
1 The crude venom of the Australian taipan. Oxyuranus scutellatus and its principal neurotoxin, taipoxin, were injected into the anterolateral aspect of one hind limb of the rat. 2 The effects of the venom and toxin on the morphology and physiology on the underlying soleus muscles were examined. 3 Both the crude venom and the toxin caused necrosis and degeneration of the muscle. Damage to the peripheral muscle fibres could be seen at the light microscopic level as early as 3 h after injection of the toxic compounds. 4 The necrotic response was accompanied by an infiltration of phagocytic cells and an extensive oedema. The wet weight of the damaged muscles was almost doubled by 6 h. 5 In individual muscle fibres, necrosis was associated with the disruption of the plasma membrane and the disorganization of the myofibrils. The basal lamina of the muscle fibres was left intact. 6 Denervated mammalian muscles and innervated avian muscles were also destroyed by tiapoxin, but immature avian muscle growing in tissue culture was resistant. 7 Of the 3 subunits of taipoxin, only the basic alpha-taipoxin was itself myotoxic. However, its potency was enhanced by the presence of the acid gamma subunit. The role of the neutral beta-subunit is unclear. 8 The period of necrosis and degeneration lasted for approximately 48 h, after which the muscle fibres began to regenerate. Regeneration took place within the surviving basal lamina, with the formation of myotubes by three days, and small, immature muscle fibres by five days. Regeneration was virtually complete by 21 days.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan W., Gascoigne K., Ludlow J., Smith J. W. A modular system of instruments for simple experiments on skeletal muscle using intracellular microelectrode techniques [proceedings]. Br J Pharmacol. 1977 Sep;61(1):155P–155P. [PMC free article] [PubMed] [Google Scholar]
- Campbell C. H. The taipan (Oxyuranus scutellatus) and the effect of its bite. Med J Aust. 1967 Apr 15;1(15):735–739. doi: 10.5694/j.1326-5377.1967.tb21596.x. [DOI] [PubMed] [Google Scholar]
- Cullen M. J., Mastaglia F. L. Morphological changes in dystrophic muscle. Br Med Bull. 1980 May;36(2):145–122. doi: 10.1093/oxfordjournals.bmb.a071630. [DOI] [PubMed] [Google Scholar]
- Dowdall M. J., Fohlman J. P., Eaker D. Inhibition of high-affinity choline transport in peripheral cholinergic endings by presynaptic snake venom neurotoxins. Nature. 1977 Oct 20;269(5630):700–702. doi: 10.1038/269700a0. [DOI] [PubMed] [Google Scholar]
- Dowdall M. J., Fohlman J. P., Watts A. Presynaptic action of snake venom neurotoxins on cholinergic systems. Adv Cytopharmacol. 1979;3:63–76. [PubMed] [Google Scholar]
- Fohlman J., Eaker D., Dowdall M. J., Lüllmann-Rauch R., Sjödin T., Leander S. Chemical modification of taipoxin and the consequences for phospholipase activity, pathophysiology, and inhibition of high-affinity choline uptake. Eur J Biochem. 1979 Mar;94(2):531–540. doi: 10.1111/j.1432-1033.1979.tb12922.x. [DOI] [PubMed] [Google Scholar]
- Fohlman J., Eaker D., Karlsoon E., Thesleff S. Taipoxin, an extremely potent presynaptic neurotoxin from the venom of the australian snake taipan (Oxyuranus s. scutellatus). Isolation, characterization, quaternary structure and pharmacological properties. Eur J Biochem. 1976 Sep 15;68(2):457–469. doi: 10.1111/j.1432-1033.1976.tb10833.x. [DOI] [PubMed] [Google Scholar]
- Hall-Craggs E. C. Rapid degeneration and regeneration of a whole skeletal muscle following treatment with bupivacaine (Marcain). Exp Neurol. 1974 May;43(2):349–358. doi: 10.1016/0014-4886(74)90176-9. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Johnson M. A. Further observations on the pathological responses of rat skeletal muscle to toxins isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin Exp Pharmacol Physiol. 1978 Nov-Dec;5(6):587–600. doi: 10.1111/j.1440-1681.1978.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Johnson M. A., Macdonell C. Taipoxin, a presynaptically active neurotoxin, destroys mammalian skeletal muscle [proceedings]. Br J Pharmacol. 1977 Sep;61(1):133P–133P. [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Karlsson E., Thesleff S. Effects of an isolated toxin from Australian tiger snake (Notechis scutatus scutatus) venom at the mammalian neuromuscular junction. Br J Pharmacol. 1973 Jan;47(1):141–146. doi: 10.1111/j.1476-5381.1973.tb08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., MacDonell C. A. Phospholipase A2 activity of notexin and its role in muscle damage. Toxicon. 1981;19(3):419–430. doi: 10.1016/0041-0101(81)90046-5. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Freiman D. G. An improved method of fixation for formalin-sensitive enzymes with special reference to myosin adenosine triphosphatase. J Histochem Cytochem. 1966 Aug;14(8):577–581. doi: 10.1177/14.8.577. [DOI] [PubMed] [Google Scholar]
- Hood V. L., Johnson J. R. Acute renal failure with myoglobinuria after tiger snake bite. Med J Aust. 1975 Oct 18;2(16):638–641. [PubMed] [Google Scholar]
- Howard B. D., Wu W. C. Evidence that beta-bungarotoxin acts at the exterior of nerve terminals. Brain Res. 1976 Feb 13;103(1):190–192. doi: 10.1016/0006-8993(76)90704-6. [DOI] [PubMed] [Google Scholar]
- Kamenskaya M. A., Thesleff S. The neuromuscular blocking action of an isolated toxin from the elapid (Oxyuranus scutellactus). Acta Physiol Scand. 1974 Apr;90(4):716–724. doi: 10.1111/j.1748-1716.1974.tb05639.x. [DOI] [PubMed] [Google Scholar]
- Libelius R. Evidence for endocytotic uptake of cobra neurotoxin in mouse skeletal muscle. J Neural Transm. 1975;37(1):61–71. doi: 10.1007/BF01249766. [DOI] [PubMed] [Google Scholar]
- McArdle J. J. Complex end-plate potentials at the regenerating neuromuscular junction of the rat. Exp Neurol. 1975 Dec;49(3):629–638. doi: 10.1016/0014-4886(75)90048-5. [DOI] [PubMed] [Google Scholar]
- Ng R. H., Howard B. D. Mitochondria and sarcoplasmic reticulum as model targets for neurotoxic and myotoxic phospholipases A2. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1346–1350. doi: 10.1073/pnas.77.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluskal M. G., Harris J. B., Pennington R. J., Eaker D. Some biochemical responses of rat skeletal muscle to a single subcutaneous injection of a toxin (notexin) isolated from the venom of the Australian tiger snake Notechis scutatus scutatus. Clin Exp Pharmacol Physiol. 1978 Mar-Apr;5(2):131–141. doi: 10.1111/j.1440-1681.1978.tb00663.x. [DOI] [PubMed] [Google Scholar]
- Publicover S. J., Duncan C. J., Smith J. L. Ultrastructural changes in muscle mitochondria in situ, including the apparent development of internal septa, associated with the uptake and release of calcium. Cell Tissue Res. 1977 Dec 19;185(3):373–385. doi: 10.1007/BF00220297. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. I. Quantitative aspects. Acta Physiol Scand. 1971 Apr;81(4):557–564. doi: 10.1111/j.1748-1716.1971.tb04932.x. [DOI] [PubMed] [Google Scholar]
- STEIN J. M., PADYKULA H. A. Histochemical classification of individual skeletal muscle fibers of the rat. Am J Anat. 1962 Mar;110:103–123. doi: 10.1002/aja.1001100203. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H. The morphology of regeneration of skeletal muscles in the rat. Tissue Cell. 1976;8(4):673–692. doi: 10.1016/0040-8166(76)90039-2. [DOI] [PubMed] [Google Scholar]
- Schultz E., Lipton B. H. The effect of Marcaine on muscle and non-muscle cells in vitro. Anat Rec. 1978 Jul;191(3):351–369. doi: 10.1002/ar.1091910308. [DOI] [PubMed] [Google Scholar]
- Sutherland S. K., Coulter A. R. Three instructive cases of tiger snake (Notechis scutatus) envenomation--and how a radioimmunoassay proved the diagnosis. Med J Aust. 1977 Aug 6;2(6):177–180. [PubMed] [Google Scholar]
- TREVAN D. J., SHARROCK A. A methyl green-pyronin-orange G stain for formalin-fixed tissues. J Pathol Bacteriol. 1951 Apr;63(2):326–329. doi: 10.1002/path.1700630215. [DOI] [PubMed] [Google Scholar]
- Thesleff S., Ward M. R. Studies on the mechanism of fibrillation potentials in denervated muscle. J Physiol. 1975 Jan;244(2):313–323. doi: 10.1113/jphysiol.1975.sp010800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinca J. C. Report of recovery from taipan bite. Med J Aust. 1969 Mar 8;1(10):514–516. doi: 10.5694/j.1326-5377.1969.tb92255.x. [DOI] [PubMed] [Google Scholar]
- Vracko R., Benditt E. P. Basal lamina: the scaffold for orderly cell replacement. Observations on regeneration of injured skeletal muscle fibers and capillaries. J Cell Biol. 1972 Nov;55(2):406–419. doi: 10.1083/jcb.55.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrogemann K., Pena S. D. Mitochondrial calcium overload: A general mechanism for cell-necrosis in muscle diseases. Lancet. 1976 Mar 27;1(7961):672–674. doi: 10.1016/s0140-6736(76)92781-1. [DOI] [PubMed] [Google Scholar]