Abstract
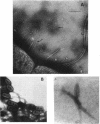
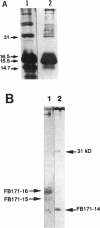
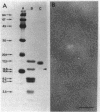
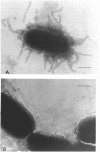
Enteropathogenic Escherichia coli (EPEC) express rope-like bundles of filaments, termed bundle-forming pili (BFP) (J. A. Girón, A. S. Y. Ho, and G. K. Schoolnik, Science 254:710-713, 1991). Expression of BFP is associated with localized adherence to HEp-2 cells and the presence of the EPEC adherence factor plasmid. In this study, we describe the identification of rod-like fimbriae and fibrillae expressed simultaneously on the bacterial surface of three prototype EPEC strains. Upon fimbrial extraction from EPEC B171 (O111:NM), three fimbrial subunits with masses of 16.5, 15.5, and 14.7 kDa were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Their N-terminal amino acid sequence showed homology with F9 and F7(2) fimbriae of uropathogenic E. coli and F1845 of diffuse-adhering E. coli, respectively. The mixture of fimbrial subunits (called FB171) exhibited mannose-resistant agglutination of human erythrocytes only, and this activity was not inhibited by alpha-D-Gal(1-4)-beta-Gal disaccharide or any other described receptor analogs for P, S, F, M, G, and Dr hemagglutinins of uropathogenic E. coli, which suggests a different receptor specificity. Hemagglutination was inhibited by extracellular matrix glycoproteins, i.e., collagen type IV, laminin, and fibronectin, and to a lesser extent by gangliosides, fetuin, and asialofetuin. Scanning electron microscopic studies performed on clusters of bacteria adhering to HEp-2 cells revealed the presence of structures resembling BFP and rod-like fimbriae linking bacteria to bacteria and bacteria to the eukaryotic cell membrane. We suggest a role of these surface appendages in the interaction of EPEC with eukaryotic cells as well as in the overall pathogenesis of intestinal disease caused by EPEC.
Full text
PDF


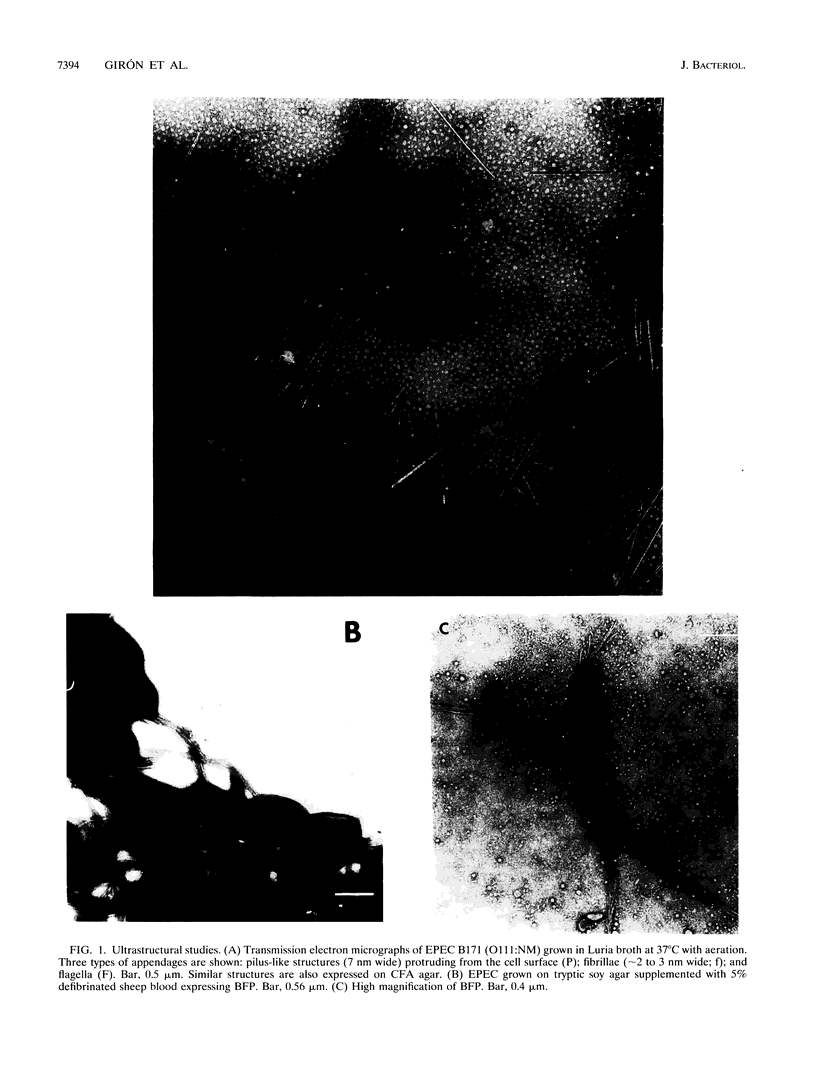
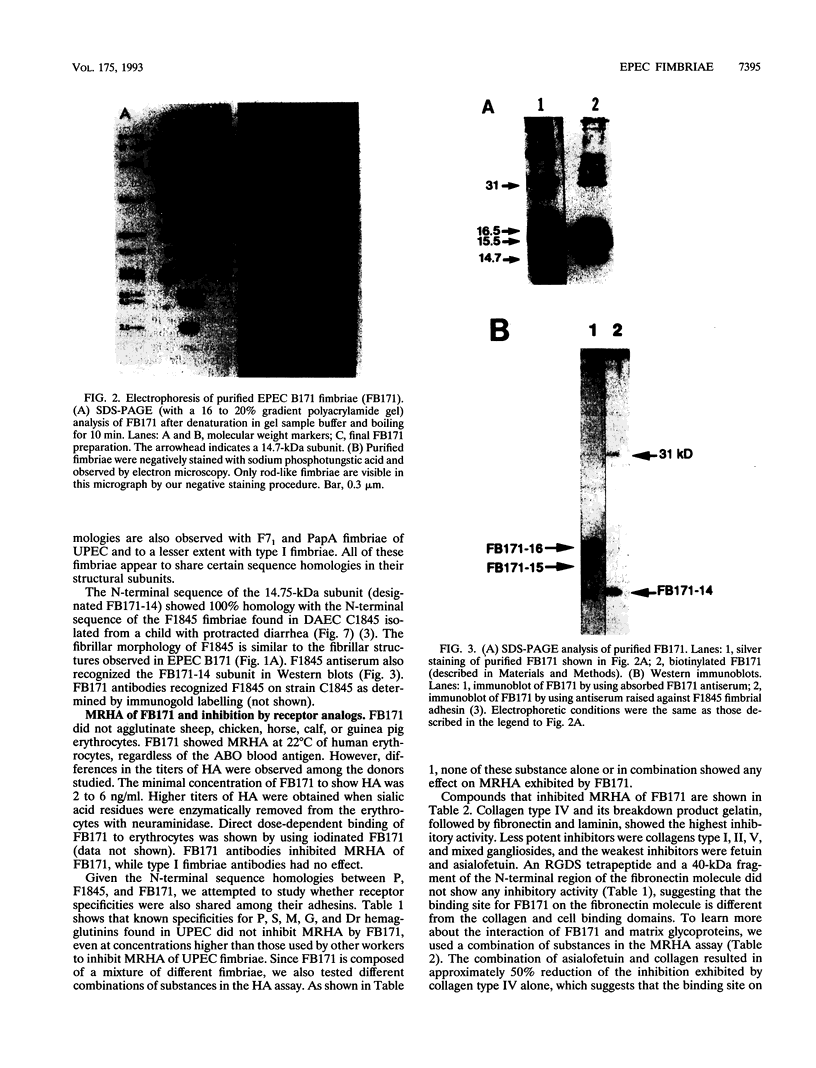

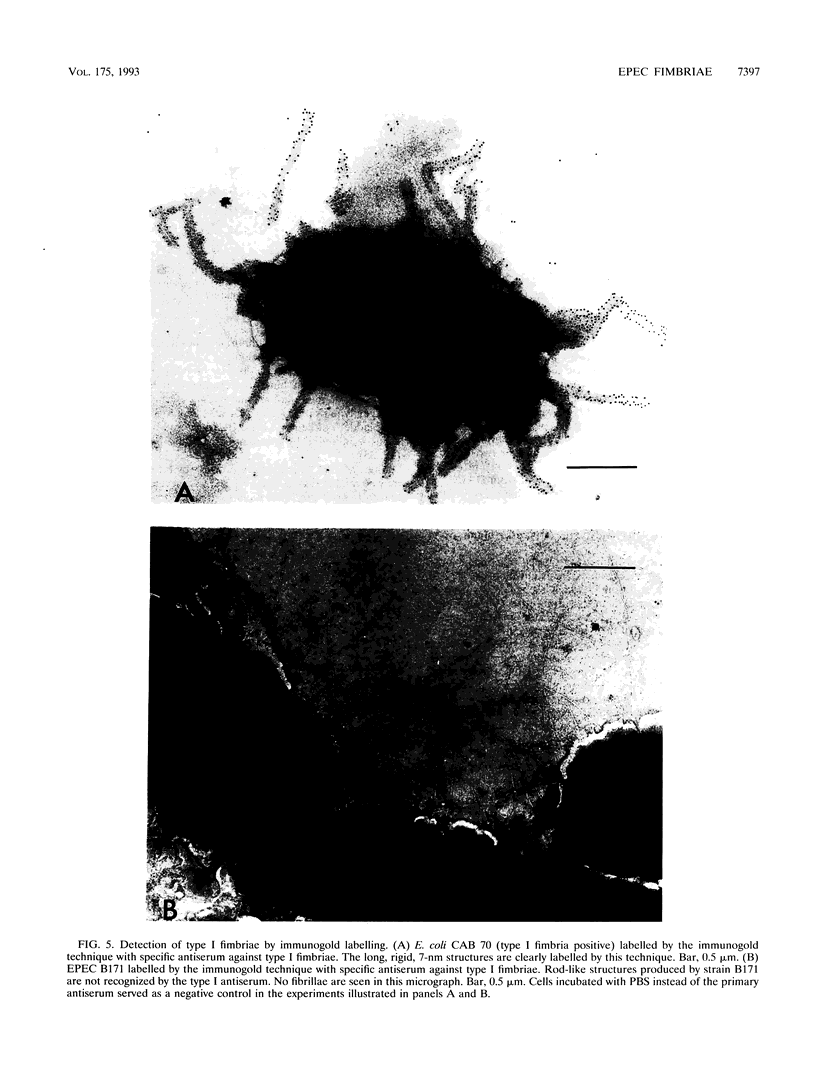




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade J. R., Da Veiga V. F., De Santa Rosa M. R., Suassuna I. An endocytic process in HEp-2 cells induced by enteropathogenic Escherichia coli. J Med Microbiol. 1989 Jan;28(1):49–57. doi: 10.1099/00222615-28-1-49. [DOI] [PubMed] [Google Scholar]
- Baldini M. M., Kaper J. B., Levine M. M., Candy D. C., Moon H. W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Bilge S. S., Clausen C. R., Lau W., Moseley S. L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989 Aug;171(8):4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chart H., Rowe B. The outer membrane protein of enteropathogenic Escherichia coli, described as the 'localised adherence factor', is OmpF and probably not involved in adhesion to HEp-2 cells. FEMS Microbiol Lett. 1989 Oct 15;52(3):291–295. doi: 10.1016/0378-1097(89)90213-9. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Donohue-Rolfe A., Keusch G. T. Epithelial cell invasion: an overlooked property of enteropathogenic Escherichia coli (EPEC) associated with the EPEC adherence factor. J Infect Dis. 1989 Sep;160(3):452–459. doi: 10.1093/infdis/160.3.452. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., DuPont H. L. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect Immun. 1979 Feb;23(2):336–346. doi: 10.1128/iai.23.2.336-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. L., Jerse A. E., Kaper J. B., Falkow S. Characterization of interactions of enteropathogenic Escherichia coli O127:H6 with mammalian cells in vitro. J Infect Dis. 1991 Oct;164(4):693–703. doi: 10.1093/infdis/164.4.693. [DOI] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Faris A., Wadström T., Hök M. Binding of Escherichia coli to fibronectin. A mechanism of tissue adherence. J Biol Chem. 1984 Dec 10;259(23):14899–14905. [PubMed] [Google Scholar]
- Girón J. A., Donnenberg M. S., Martin W. C., Jarvis K. G., Kaper J. B. Distribution of the bundle-forming pilus structural gene (bfpA) among enteropathogenic Escherichia coli. J Infect Dis. 1993 Oct;168(4):1037–1041. doi: 10.1093/infdis/168.4.1037. [DOI] [PubMed] [Google Scholar]
- Girón J. A., Ho A. S., Schoolnik G. K. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991 Nov 1;254(5032):710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- Jerse A. E., Yu J., Tall B. D., Kaper J. B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Heesemann J., Laufs R., Kroll H. P., Kaper J. B., Levine M. M. Serological response to type 1-like somatic fimbriae in diarrheal infection due to classical enteropathogenic Escherichia coli. Microb Pathog. 1987 Jun;2(6):425–434. doi: 10.1016/0882-4010(87)90049-0. [DOI] [PubMed] [Google Scholar]
- Kist M. L., Salit I. E., Hofmann T. Purification and characterization of the Dr hemagglutinins expressed by two uropathogenic Escherichia coli strains. Infect Immun. 1990 Mar;58(3):695–702. doi: 10.1128/iai.58.3.695-702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Martin G. R., Fishman P. H. Ganglioside inhibition of fibronectin-mediated cell adhesion to collagen. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3367–3371. doi: 10.1073/pnas.76.7.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., McNeish A. S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987 Jan;55(1):69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984 Aug;159(2):762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen V., Saxén H., Hultberg H., Svenson S. B. P-antigen-recognizing fimbriae from human uropathogenic Escherichia coli strains. Infect Immun. 1982 Jul;37(1):286–291. doi: 10.1128/iai.37.1.286-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987 Mar;155(3):377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Marklund B. I., Normark S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Marklund B. I., Strömberg N., Lindberg F., Karlsson K. A., Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988 Mar;2(2):255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- McKeown-Longo P. J. Fibronectin-cell surface interactions. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S322–S334. doi: 10.1093/clinids/9.supplement_4.s322. [DOI] [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Structure of common pili from Escherichia coli. J Bacteriol. 1979 Jun;138(3):969–975. doi: 10.1128/jb.138.3.969-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moch T., Hoschützky H., Hacker J., Kröncke K. D., Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(10):3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro J. P., Baldini M. M., Kaper J. B., Black R. E., Bravo N., Levine M. M. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J Infect Dis. 1985 Sep;152(3):560–565. doi: 10.1093/infdis/152.3.560. [DOI] [PubMed] [Google Scholar]
- Nowicki B., Labigne A., Moseley S., Hull R., Hull S., Moulds J. The Dr hemagglutinin, afimbrial adhesins AFA-I and AFA-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with Dr receptor recognition. Infect Immun. 1990 Jan;58(1):279–281. doi: 10.1128/iai.58.1.279-281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Tavendale A., Yakubu D. E. Some strains of Escherichia coli of putative enteroadherent-aggregative serotypes produce an unusual fibrillar haemagglutinin. FEMS Microbiol Lett. 1989 May;50(1-2):87–91. doi: 10.1016/0378-1097(89)90464-3. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Rhen M., Klemm P., Korhonen T. K. Identification of two new hemagglutinins of Escherichia coli, N-acetyl-D-glucosamine-specific fimbriae and a blood group M-specific agglutinin, by cloning the corresponding genes in Escherichia coli K-12. J Bacteriol. 1986 Dec;168(3):1234–1242. doi: 10.1128/jb.168.3.1234-1242.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. W., Junio L. N., Schoolnik G. K. HeLa cell invasion by a strain of enteropathogenic Escherichia coli that lacks the O-antigenic polysaccharide. Mol Microbiol. 1990 Oct;4(10):1661–1666. doi: 10.1111/j.1365-2958.1990.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Rothbaum R., McAdams A. J., Giannella R., Partin J. C. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982 Aug;83(2):441–454. [PubMed] [Google Scholar]
- Salit I. E., Vavougios J., Hofmann T. Isolation and characterization of Escherichia coli pili from diverse clinical sources. Infect Immun. 1983 Nov;42(2):755–762. doi: 10.1128/iai.42.2.755-762.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaletsky I. C., Milani S. R., Trabulsi L. R., Travassos L. R. Isolation and characterization of the localized adherence factor of enteropathogenic Escherichia coli. Infect Immun. 1988 Nov;56(11):2979–2983. doi: 10.1128/iai.56.11.2979-2983.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaletsky I. C., Silva M. L., Trabulsi L. R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984 Aug;45(2):534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Wadström T., Timpl R. Binding of the basement membrane protein laminin to Escherichia coli. FEBS Lett. 1982 Sep 6;146(1):55–58. doi: 10.1016/0014-5793(82)80704-7. [DOI] [PubMed] [Google Scholar]
- Svanborg Edén C., Gotschlich E. C., Korhonen T. K., Leffler H., Schoolnik G. Aspects on structure and function of pili on uropathogenic Escherichia coli. Prog Allergy. 1983;33:189–202. doi: 10.1159/000318330. [DOI] [PubMed] [Google Scholar]
- Swanson J., Bergström S., Barrera O., Robbins K., Corwin D. Pilus- gonococcal variants. Evidence for multiple forms of piliation control. J Exp Med. 1985 Aug 1;162(2):729–744. doi: 10.1084/jem.162.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen V., Korhonen T. K., Jokinen M., Gahmberg C. G., Ehnholm C. Blood group M specific haemagglutinin in pyelonephritogenic Escherichia coli. Lancet. 1982 May 22;1(8282):1192–1192. doi: 10.1016/s0140-6736(82)92264-4. [DOI] [PubMed] [Google Scholar]
- Wadström T., Adegbola R. A., Baloda S. B., Ljungh A., Sethi S. K., Yuk Y. R. Non-haemagglutinating fimbriae of enteropathogenic Escherichia coli (EPEC). Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Jul;261(4):417–424. doi: 10.1016/s0176-6724(86)80073-6. [DOI] [PubMed] [Google Scholar]
- Westerlund B., Kuusela P., Risteli J., Risteli L., Vartio T., Rauvala H., Virkola R., Korhonen T. K. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol Microbiol. 1989 Mar;3(3):329–337. doi: 10.1111/j.1365-2958.1989.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Westerlund B., Kuusela P., Vartio T., van Die I., Korhonen T. K. A novel lectin-independent interaction of P fimbriae of Escherichia coli with immobilized fibronectin. FEBS Lett. 1989 Jan 30;243(2):199–204. doi: 10.1016/0014-5793(89)80129-2. [DOI] [PubMed] [Google Scholar]
- Yakubu D. E., Old D. C., Tavendale A. Production of a mannose-resistant fibrillar haemagglutinin by strains of Escherichia coli of EPEC serotype O111:H12. FEMS Microbiol Lett. 1991 Jun 15;65(2):233–237. doi: 10.1016/0378-1097(91)90308-w. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W., Grotendorst G. R., Momoi T. Glycolipids: receptors for fibronectin? J Cell Physiol. 1981 Nov;109(2):343–351. doi: 10.1002/jcp.1041090218. [DOI] [PubMed] [Google Scholar]
- van Die I., Bergmans H. Nucleotide sequence of the gene encoding the F72 fimbrial subunit of a uropathogenic Escherichia coli strain. Gene. 1984 Dec;32(1-2):83–90. doi: 10.1016/0378-1119(84)90035-0. [DOI] [PubMed] [Google Scholar]