Abstract
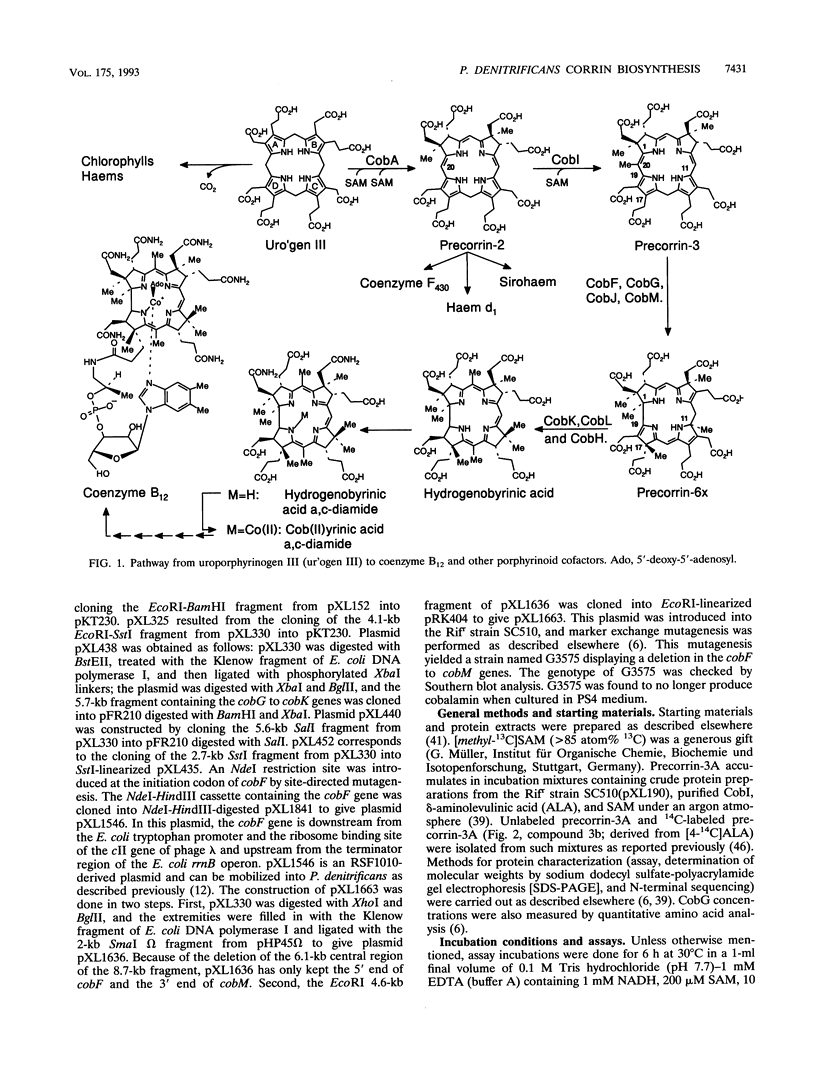
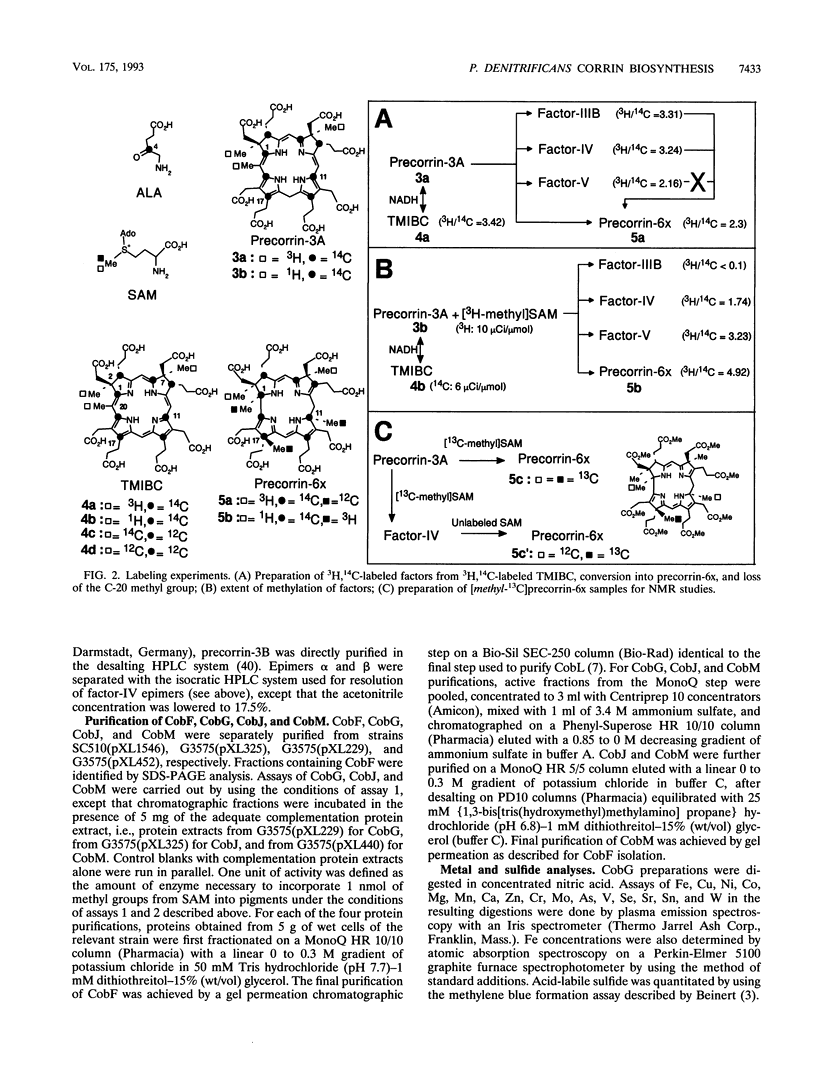
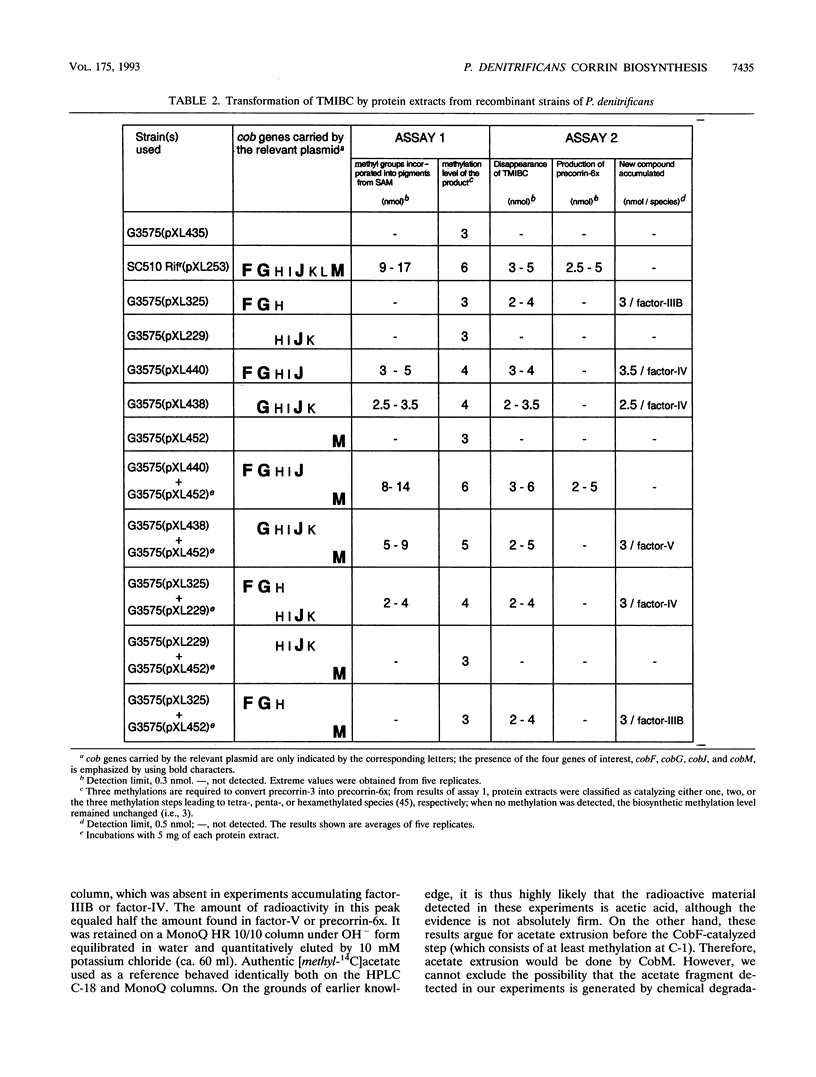
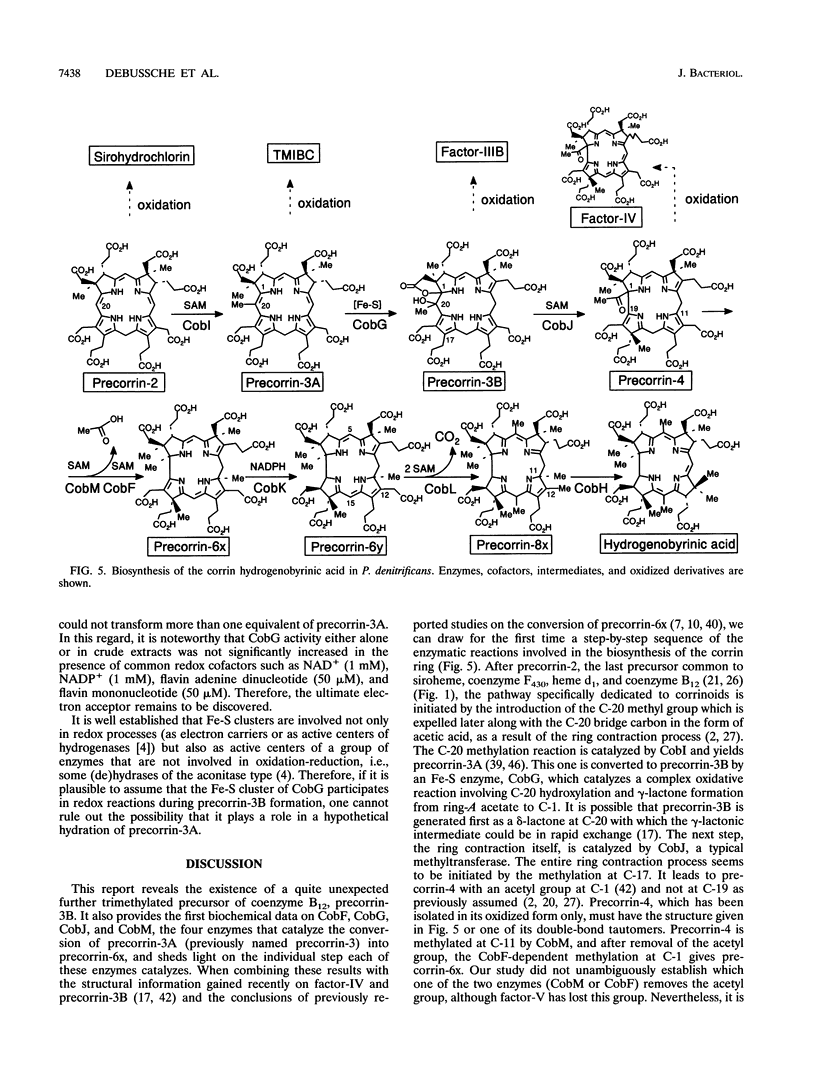
Studies with cell-free protein preparations from a series of recombinant strains of Pseudomonas denitrificans demonstrated that precorrin-3 is converted into a further trimethylated intermediate, named precorrin-3B, along the pathway to coenzyme B12. It was then shown that the part of the pathway from precorrin-3 (called precorrin-3A hereafter) to precorrin-6x involves three intermediates, precorrin-3B, precorrin-4, and precorrin-5. Precorrin-3B was isolated in its native (reduced) as well as its oxidized (factor-IIIB) states, and precorrin-4 was isolated in its oxidized form only (factor-IV). Both factors were in vitro precursors of precorrin-6x. The synthesis of precorrin-6x from precorrin-3A was shown to be catalyzed by four enzymes, CobG, CobJ, CobM, and CobF, intervening in this order. They were purified to homogeneity. CobG, which converts precorrin-3A to precorrin-3B, was found to be an iron-sulfur protein responsible for the oxidation known to occur between precorrin-3A and precorrin-6x, and CobJ, CobM, and CobF are the C-17, C-11, and C-1 methylases, respectively. The acetate fragment is extruded after precorrin-4 formation. This study combined with our recent structural studies on factor-IV (D. Thibaut, L. Debussche, D. Fréchet, F. Herman, M. Vuilhorgne, and F. Blanche, J. Chem. Soc. Chem. Commun. 1993:513-515, 1993) and precorrin-3B (L. Debussche, D. Thibaut, M. Danzer, F. Debu, D. Fréchet, F. Herman, F. Blanche, and M. Vuilhorgne, J. Chem. Soc. Chem. Commun. 1993:1100-1103, 1993) provides a first step-by-step picture of the sequence of the enzymatic reactions leading to the corrin ring in P. denitrificans.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Battersby A. R., Bushell M. J., Jones C., Lewis N. G., Pfenninger A. Biosynthesis of vitamin B12: identity of fragment extruded during ring contraction to the corrin macrocycle. Proc Natl Acad Sci U S A. 1981 Jan;78(1):13–15. doi: 10.1073/pnas.78.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinert H. Recent developments in the field of iron-sulfur proteins. FASEB J. 1990 May;4(8):2483–2491. doi: 10.1096/fasebj.4.8.2185975. [DOI] [PubMed] [Google Scholar]
- Beinert H. Semi-micro methods for analysis of labile sulfide and of labile sulfide plus sulfane sulfur in unusually stable iron-sulfur proteins. Anal Biochem. 1983 Jun;131(2):373–378. doi: 10.1016/0003-2697(83)90186-0. [DOI] [PubMed] [Google Scholar]
- Benner S. A., Ellington A. D., Tauer A. Modern metabolism as a palimpsest of the RNA world. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7054–7058. doi: 10.1073/pnas.86.18.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Debussche L., Thibaut D., Crouzet J., Cameron B. Purification and characterization of S-adenosyl-L-methionine: uroporphyrinogen III methyltransferase from Pseudomonas denitrificans. J Bacteriol. 1989 Aug;171(8):4222–4231. doi: 10.1128/jb.171.8.4222-4231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Famechon A., Thibaut D., Debussche L., Cameron B., Crouzet J. Biosynthesis of vitamin B12 in Pseudomonas denitrificans: the biosynthetic sequence from precorrin-6y to precorrin-8x is catalyzed by the cobL gene product. J Bacteriol. 1992 Feb;174(3):1050–1052. doi: 10.1128/jb.174.3.1050-1052.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Maton L., Debussche L., Thibaut D. Purification and characterization of Cob(II)yrinic acid a,c-diamide reductase from Pseudomonas denitrificans. J Bacteriol. 1992 Nov;174(22):7452–7454. doi: 10.1128/jb.174.22.7452-7454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Robin C., Couder M., Faucher D., Cauchois L., Cameron B., Crouzet J. Purification, characterization, and molecular cloning of S-adenosyl-L-methionine: uroporphyrinogen III methyltransferase from Methanobacterium ivanovii. J Bacteriol. 1991 Aug;173(15):4637–4645. doi: 10.1128/jb.173.15.4637-4645.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Thibaut D., Famechon A., Debussche L., Cameron B., Crouzet J. Precorrin-6x reductase from Pseudomonas denitrificans: purification and characterization of the enzyme and identification of the structural gene. J Bacteriol. 1992 Feb;174(3):1036–1042. doi: 10.1128/jb.174.3.1036-1042.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron B., Briggs K., Pridmore S., Brefort G., Crouzet J. Cloning and analysis of genes involved in coenzyme B12 biosynthesis in Pseudomonas denitrificans. J Bacteriol. 1989 Jan;171(1):547–557. doi: 10.1128/jb.171.1.547-557.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Crouzet J., Cameron B., Cauchois L., Rigault S., Rouyez M. C., Blanche F., Thibaut D., Debussche L. Genetic and sequence analysis of an 8.7-kilobase Pseudomonas denitrificans fragment carrying eight genes involved in transformation of precorrin-2 to cobyrinic acid. J Bacteriol. 1990 Oct;172(10):5980–5990. doi: 10.1128/jb.172.10.5980-5990.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet J., Levy-Schil S., Cameron B., Cauchois L., Rigault S., Rouyez M. C., Blanche F., Debussche L., Thibaut D. Nucleotide sequence and genetic analysis of a 13.1-kilobase-pair Pseudomonas denitrificans DNA fragment containing five cob genes and identification of structural genes encoding Cob(I)alamin adenosyltransferase, cobyric acid synthase, and bifunctional cobinamide kinase-cobinamide phosphate guanylyltransferase. J Bacteriol. 1991 Oct;173(19):6074–6087. doi: 10.1128/jb.173.19.6074-6087.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debussche L., Couder M., Thibaut D., Cameron B., Crouzet J., Blanche F. Assay, purification, and characterization of cobaltochelatase, a unique complex enzyme catalyzing cobalt insertion in hydrogenobyrinic acid a,c-diamide during coenzyme B12 biosynthesis in Pseudomonas denitrificans. J Bacteriol. 1992 Nov;174(22):7445–7451. doi: 10.1128/jb.174.22.7445-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg R., Kriemler H. P., Bergmann K. H., Müller G. Zur Cobyrinsäure-Biosynthese. Neuartige, methylierte Hydroporphyrine und deren Bedeutung bei der Cobyrinsäure-Bildung. Hoppe Seylers Z Physiol Chem. 1977 Mar;358(3):339–352. [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Leong S. A., Ditta G. S., Helinski D. R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982 Aug 10;257(15):8724–8730. [PubMed] [Google Scholar]
- Mombelli L., Nussbaumer C., Weber H., Müller G., Arigoni D. Biosynthesis of vitamin B12: nature of the volatile fragment generated during formation of the corrin ring system. Proc Natl Acad Sci U S A. 1981 Jan;78(1):11–12. doi: 10.1073/pnas.78.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski J., Wu J. Y., Rueger D. C., Miller B. E., Siegel L. M., Kredich N. M. Characterization of the cysJIH regions of Salmonella typhimurium and Escherichia coli B. DNA sequences of cysI and cysH and a model for the siroheme-Fe4S4 active center of sulfite reductase hemoprotein based on amino acid homology with spinach nitrite reductase. J Biol Chem. 1989 Sep 15;264(26):15726–15737. [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Roessner C. A., Warren M. J., Santander P. J., Atshaves B. P., Ozaki S., Stolowich N. J., Iida K., Scott A. I. Expression of 9 Salmonella typhimurium enzymes for cobinamide synthesis. Identification of the 11-methyl and 20-methyl transferases of corrin biosynthesis. FEBS Lett. 1992 Apr 13;301(1):73–78. doi: 10.1016/0014-5793(92)80213-z. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Lawrence J. G., Rubenfield M., Kieffer-Higgins S., Church G. M. Characterization of the cobalamin (vitamin B12) biosynthetic genes of Salmonella typhimurium. J Bacteriol. 1993 Jun;175(11):3303–3316. doi: 10.1128/jb.175.11.3303-3316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut D., Blanche F., Debussche L., Leeper F. J., Battersby A. R. Biosynthesis of vitamin B12: structure of precorrin-6x octamethyl ester. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8800–8804. doi: 10.1073/pnas.87.22.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut D., Couder M., Crouzet J., Debussche L., Cameron B., Blanche F. Assay and purification of S-adenosyl-L-methionine:precorrin-2 methyltransferase from Pseudomonas denitrificans. J Bacteriol. 1990 Nov;172(11):6245–6251. doi: 10.1128/jb.172.11.6245-6251.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut D., Couder M., Famechon A., Debussche L., Cameron B., Crouzet J., Blanche F. The final step in the biosynthesis of hydrogenobyrinic acid is catalyzed by the cobH gene product with precorrin-8x as the substrate. J Bacteriol. 1992 Feb;174(3):1043–1049. doi: 10.1128/jb.174.3.1043-1049.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut D., Debussche L., Blanche F. Biosynthesis of vitamin B12: isolation of precorrin-6x, a metal-free precursor of the corrin macrocycle retaining five S-adenosylmethionine-derived peripheral methyl groups. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8795–8799. doi: 10.1073/pnas.87.22.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M. J., Roessner C. A., Ozaki S., Stolowich N. J., Santander P. J., Scott A. I. Enzymatic synthesis and structure of precorrin-3, a trimethyldipyrrocorphin intermediate in vitamin B12 biosynthesis. Biochemistry. 1992 Jan 21;31(2):603–609. doi: 10.1021/bi00117a043. [DOI] [PubMed] [Google Scholar]


