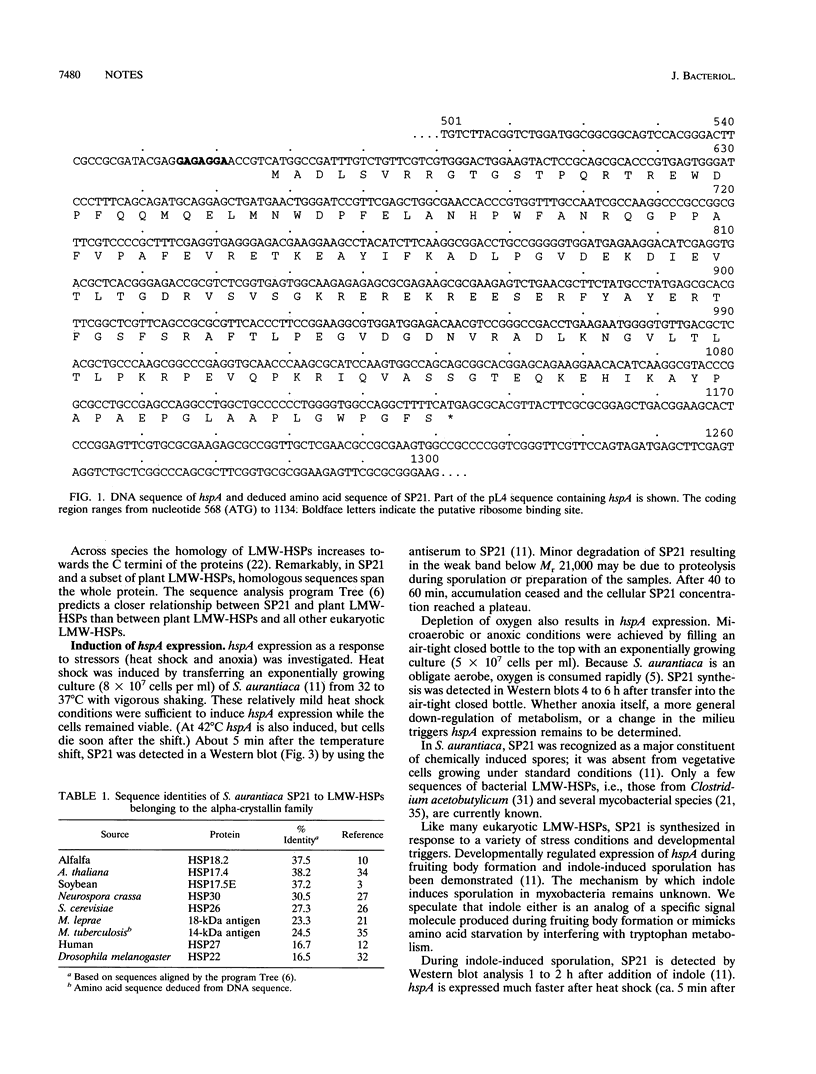
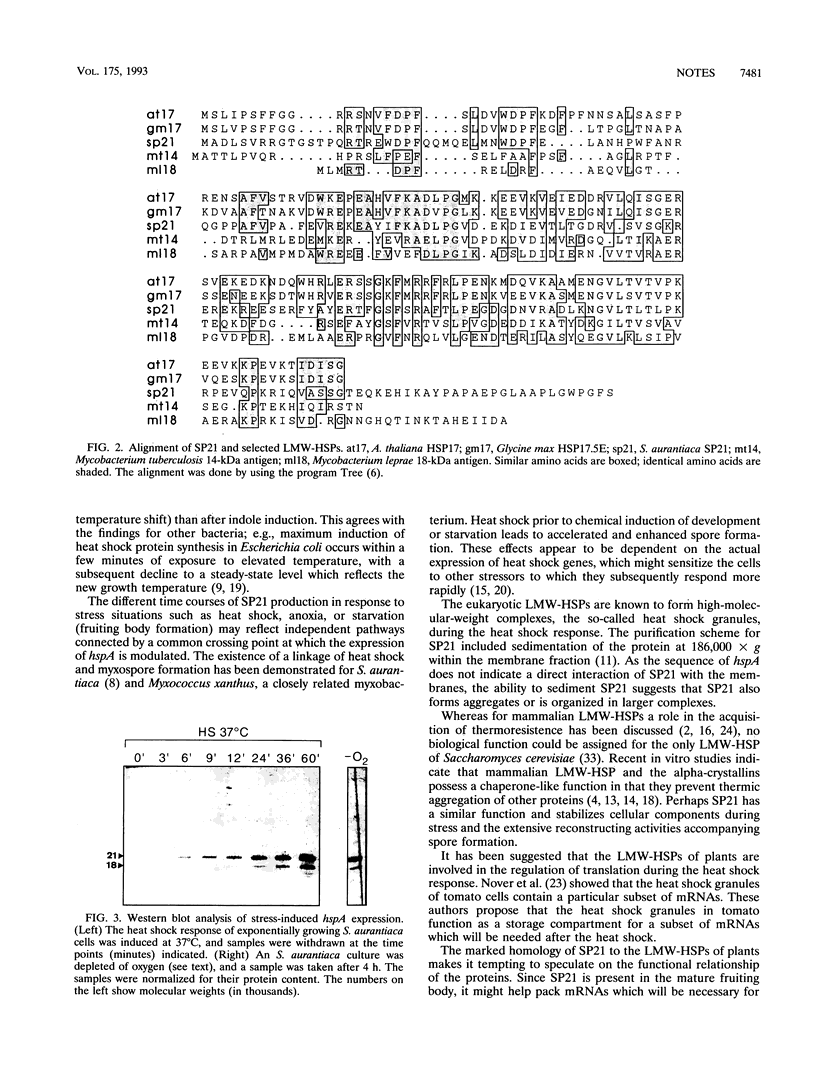
Abstract
In the fruiting body-forming myxobacterium Stigmatella aurantiaca a 21,000-M(r) protein, SP21, is synthesized during fruiting, heat shock, and stress induced by oxygen limitation. The corresponding gene was isolated from a gene expression library in lambda gt11 with an antiserum to the purified protein. The DNA sequence of the gene reveals that SP21 is a member of the alpha-crystallin family of low-molecular-weight heat shock proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arrigo A. P., Michel M. R. Decreased heat- and tumor necrosis factor-mediated hsp28 phosphorylation in thermotolerant HeLa cells. FEBS Lett. 1991 Apr 22;282(1):152–156. doi: 10.1016/0014-5793(91)80466-g. [DOI] [PubMed] [Google Scholar]
- Czarnecka E., Gurley W. B., Nagao R. T., Mosquera L. A., Key J. L. DNA sequence and transcript mapping of a soybean gene encoding a small heat shock protein. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3726–3730. doi: 10.1073/pnas.82.11.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M., NIEDERPRUEM D. J. ELECTRON TRANSPORT SYSTEM IN VEGETATIVE CELLS AND MICROCYSTS OF MYXOCOCCUS XANTHUS. J Bacteriol. 1964 Feb;87:316–322. doi: 10.1128/jb.87.2.316-322.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Györgyey J., Gartner A., Németh K., Magyar Z., Hirt H., Heberle-Bors E., Dudits D. Alfalfa heat shock genes are differentially expressed during somatic embryogenesis. Plant Mol Biol. 1991 Jun;16(6):999–1007. doi: 10.1007/BF00016072. [DOI] [PubMed] [Google Scholar]
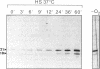
- Heidelbach M., Skladny H., Schairer H. U. Purification and characterization of SP21, a development-specific protein of the myxobacterium Stigmatella aurantiaca. J Bacteriol. 1993 Feb;175(3):905–908. doi: 10.1128/jb.175.3.905-908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Potter R., Stein G., Stein J., Weber L. A. Sequence and organization of genes encoding the human 27 kDa heat shock protein. Nucleic Acids Res. 1986 May 27;14(10):4127–4145. doi: 10.1093/nar/14.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob U., Gaestel M., Engel K., Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993 Jan 25;268(3):1517–1520. [PubMed] [Google Scholar]
- Killeen K. P., Nelson D. R. Acceleration of starvation- and glycerol-induced myxospore formation by prior heat shock in Myxococcus xanthus. J Bacteriol. 1988 Nov;170(11):5200–5207. doi: 10.1128/jb.170.11.5200-5207.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J., Chrétien P., Laszlo A., Lambert H. Phosphorylation of HSP27 during development and decay of thermotolerance in Chinese hamster cells. J Cell Physiol. 1991 Apr;147(1):93–101. doi: 10.1002/jcp.1041470113. [DOI] [PubMed] [Google Scholar]
- Merck K. B., Groenen P. J., Voorter C. E., de Haard-Hoekman W. A., Horwitz J., Bloemendal H., de Jong W. W. Structural and functional similarities of bovine alpha-crystallin and mouse small heat-shock protein. A family of chaperones. J Biol Chem. 1993 Jan 15;268(2):1046–1052. [PubMed] [Google Scholar]
- Nelson D. R., Killeen K. P. Heat shock proteins of vegetative and fruiting Myxococcus xanthus cells. J Bacteriol. 1986 Dec;168(3):1100–1106. doi: 10.1128/jb.168.3.1100-1106.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerland A. H., Mustafa A. S., Sweetser D., Godal T., Young R. A. A protein antigen of Mycobacterium leprae is related to a family of small heat shock proteins. J Bacteriol. 1988 Dec;170(12):5919–5921. doi: 10.1128/jb.170.12.5919-5921.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L., Scharf K. D., Neumann D. Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol. 1989 Mar;9(3):1298–1308. doi: 10.1128/mcb.9.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterreich S., Schunck H., Benndorf R., Bielka H. Cisplatin induces the small heat shock protein hsp25 and thermotolerance in Ehrlich ascites tumor cells. Biochem Biophys Res Commun. 1991 Oct 15;180(1):243–248. doi: 10.1016/s0006-291x(05)81283-5. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petko L., Lindquist S. Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell. 1986 Jun 20;45(6):885–894. doi: 10.1016/0092-8674(86)90563-5. [DOI] [PubMed] [Google Scholar]
- Plesofsky-Vig N., Brambl R. Gene sequence and analysis of hsp30, a small heat shock protein of Neurospora crassa which associates with mitochondria. J Biol Chem. 1990 Sep 15;265(26):15432–15440. [PubMed] [Google Scholar]
- Qualls G. T., Stephens K., White D. Light-stimulated morphogenesis in the fruiting myxobacterium Stigmatella aurantiaca. Science. 1978 Aug 4;201(4354):444–445. doi: 10.1126/science.96528. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer U., Dürre P. Sequence and molecular characterization of a DNA region encoding a small heat shock protein of Clostridium acetobutylicum. J Bacteriol. 1993 Jun;175(11):3394–3400. doi: 10.1128/jb.175.11.3394-3400.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate R., Ayme A., Voellmy R. Nucleotide sequence analysis of the Drosophila small heat shock gene cluster at locus 67B. J Mol Biol. 1983 Mar 25;165(1):35–57. doi: 10.1016/s0022-2836(83)80241-1. [DOI] [PubMed] [Google Scholar]
- Susek R. E., Lindquist S. L. hsp26 of Saccharomyces cerevisiae is related to the superfamily of small heat shock proteins but is without a demonstrable function. Mol Cell Biol. 1989 Nov;9(11):5265–5271. doi: 10.1128/mcb.9.11.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Komeda Y. Characterization of two genes encoding small heat-shock proteins in Arabidopsis thaliana. Mol Gen Genet. 1989 Nov;219(3):365–372. doi: 10.1007/BF00259608. [DOI] [PubMed] [Google Scholar]
- Verbon A., Hartskeerl R. A., Schuitema A., Kolk A. H., Young D. B., Lathigra R. The 14,000-molecular-weight antigen of Mycobacterium tuberculosis is related to the alpha-crystallin family of low-molecular-weight heat shock proteins. J Bacteriol. 1992 Feb;174(4):1352–1359. doi: 10.1128/jb.174.4.1352-1359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong W. W., Leunissen J. A., Voorter C. E. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993 Jan;10(1):103–126. doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]