Abstract
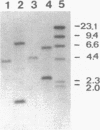
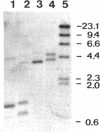
The nucleotide sequence of a chromosomal DNA fragment of Lactococcus lactis subsp. lactis SSL135, previously implicated in peptide utilization, has been determined. The genes oppDFBCA, encoding the oligopeptide transport system (Opp), and that encoding the endopeptidase PepO were located on this 8.9-kb DNA fragment. The oppDFBCA and pepO genes are probably organized in an operon. Analysis of the deduced amino acid sequences of the genes indicated that the oligopeptide transport system consists of two ATP-binding proteins OppD and OppF, two integral membrane proteins OppB and OppC, and a substrate-binding protein OppA. On the basis of the homology of OppF and OppD of L. lactis with other ABC (ATP-binding cassette) transporter proteins, the L. lactis Opp system can be classified as a member of this group. Two integration mutants, one defective in OppA and the other defective in PepO, were constructed. Growth of these mutants in a chemically defined medium with oligopeptides showed that the transport system, but not the endopeptidase, is essential for the utilization of peptides longer than three residues. Uptake of the pentapeptide Leu-enkephalin in glycolyzing lactococcal cells was followed by rapid hydrolysis of the peptide intracellularly. Importantly, extracellular hydrolysis of Leu-enkephalin is not observed. The OppA-deficient mutant was unable to transport Leu-enkephalin. Growth experiments with pasteurized milk revealed that transport of oligopeptides forms an essential part of the proteolytic system in lactococci.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alloing G., Trombe M. C., Claverys J. P. The ami locus of the gram-positive bacterium Streptococcus pneumoniae is similar to binding protein-dependent transport operons of gram-negative bacteria. Mol Microbiol. 1990 Apr;4(4):633–644. doi: 10.1111/j.1365-2958.1990.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Bacterial periplasmic permeases as model systems for the superfamily of traffic ATPases, including the multidrug resistance protein and the cystic fibrosis transmembrane conductance regulator. Int Rev Cytol. 1992;137:1–35. doi: 10.1016/s0074-7696(08)62672-8. [DOI] [PubMed] [Google Scholar]
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapot-Chartier M. P., Nardi M., Chopin M. C., Chopin A., Gripon J. C. Cloning and sequencing of pepC, a cysteine aminopeptidase gene from Lactococcus lactis subsp. cremoris AM2. Appl Environ Microbiol. 1993 Jan;59(1):330–333. doi: 10.1128/aem.59.1.330-333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Hellingwerf K. J., Konings W. N. Mechanism of energy coupling to entry and exit of neutral and branched chain amino acids in membrane vesicles of Streptococcus cremoris. J Biol Chem. 1987 Sep 15;262(26):12438–12443. [PubMed] [Google Scholar]
- Feirtag J. M., Petzel J. P., Pasalodos E., Baldwin K. A., McKay L. L. Thermosensitive plasmid replication, temperature-sensitive host growth, and chromosomal plasmid integration conferred by Lactococcus lactis subsp. cremoris lactose plasmids in Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1991 Feb;57(2):539–548. doi: 10.1128/aem.57.2.539-548.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983 Apr;154(1):1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Higgins C. F. Uptake of cell wall peptides by Salmonella typhimurium and Escherichia coli. J Bacteriol. 1987 Aug;169(8):3861–3865. doi: 10.1128/jb.169.8.3861-3865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Salmond G. P., Gill D. R., Downie J. A., Evans I. J., Holland I. B., Gray L., Buckel S. D., Bell A. W. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986 Oct 2;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- Hiles I. D., Gallagher M. P., Jamieson D. J., Higgins C. F. Molecular characterization of the oligopeptide permease of Salmonella typhimurium. J Mol Biol. 1987 May 5;195(1):125–142. doi: 10.1016/0022-2836(87)90332-9. [DOI] [PubMed] [Google Scholar]
- Holo H., Nes I. F. High-Frequency Transformation, by Electroporation, of Lactococcus lactis subsp. cremoris Grown with Glycine in Osmotically Stabilized Media. Appl Environ Microbiol. 1989 Dec;55(12):3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horng J. S., Polzin K. M., McKay L. L. Replication and temperature-sensitive maintenance functions of lactose plasmid pSK11L from Lactococcus lactis subsp. cremoris. J Bacteriol. 1991 Dec;173(23):7573–7581. doi: 10.1128/jb.173.23.7573-7581.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz J., van Sinderen D., Kok J., Konings W. N. Cell Wall-Associated Proteases of Streptococcus cremoris Wg2. Appl Environ Microbiol. 1987 Apr;53(4):853–859. doi: 10.1128/aem.53.4.853-859.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde S. C., Emsley P., Hartshorn M. J., Mimmack M. M., Gileadi U., Pearce S. R., Gallagher M. P., Gill D. R., Hubbard R. E., Higgins C. F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990 Jul 26;346(6282):362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Kok J. Genetics of the proteolytic system of lactic acid bacteria. FEMS Microbiol Rev. 1990 Sep;7(1-2):15–42. doi: 10.1111/j.1574-6968.1990.tb04877.x. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Poolman B., Driessen A. J. Bioenergetics and solute transport in lactococci. Crit Rev Microbiol. 1989;16(6):419–476. doi: 10.3109/10408418909104474. [DOI] [PubMed] [Google Scholar]
- Kunji E. R., Smid E. J., Plapp R., Poolman B., Konings W. N. Di-tripeptides and oligopeptides are taken up via distinct transport mechanisms in Lactococcus lactis. J Bacteriol. 1993 Apr;175(7):2052–2059. doi: 10.1128/jb.175.7.2052-2059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Law B. A., Kolstad J. Proteolytic systems in lactic acid bacteria. Antonie Van Leeuwenhoek. 1983 Sep;49(3):225–245. doi: 10.1007/BF00399500. [DOI] [PubMed] [Google Scholar]
- Law B. A. Peptide utilization by group N streptococci. J Gen Microbiol. 1978 Mar;105(1):113–118. doi: 10.1099/00221287-105-1-113. [DOI] [PubMed] [Google Scholar]
- Leenhouts K. J., Kok J., Venema G. Stability of Integrated Plasmids in the Chromosome of Lactococcus lactis. Appl Environ Microbiol. 1990 Sep;56(9):2726–2735. doi: 10.1128/aem.56.9.2726-2735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig W., Seewaldt E., Kilpper-Bälz R., Schleifer K. H., Magrum L., Woese C. R., Fox G. E., Stackebrandt E. The phylogenetic position of Streptococcus and Enterococcus. J Gen Microbiol. 1985 Mar;131(3):543–551. doi: 10.1099/00221287-131-3-543. [DOI] [PubMed] [Google Scholar]
- Mathiopoulos C., Mueller J. P., Slack F. J., Murphy C. G., Patankar S., Bukusoglu G., Sonenshein A. L. A Bacillus subtilis dipeptide transport system expressed early during sporulation. Mol Microbiol. 1991 Aug;5(8):1903–1913. doi: 10.1111/j.1365-2958.1991.tb00814.x. [DOI] [PubMed] [Google Scholar]
- Mayo B., Kok J., Bockelmann W., Haandrikman A., Leenhouts K. J., Venema G. Effect of X-Prolyl Dipeptidyl Aminopeptidase Deficiency on Lactococcus lactis. Appl Environ Microbiol. 1993 Jul;59(7):2049–2055. doi: 10.1128/aem.59.7.2049-2055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo B., Kok J., Venema K., Bockelmann W., Teuber M., Reinke H., Venema G. Molecular cloning and sequence analysis of the X-prolyl dipeptidyl aminopeptidase gene from Lactococcus lactis subsp. cremoris. Appl Environ Microbiol. 1991 Jan;57(1):38–44. doi: 10.1128/aem.57.1.38-44.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi M., Chopin M. C., Chopin A., Cals M. M., Gripon J. C. Cloning and DNA sequence analysis of an X-prolyl dipeptidyl aminopeptidase gene from Lactococcus lactis subsp. lactis NCDO 763. Appl Environ Microbiol. 1991 Jan;57(1):45–50. doi: 10.1128/aem.57.1.45-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M., Higgins C. F., Pearce S. R., Gallagher M. P., Hoch J. A. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol Microbiol. 1991 Jan;5(1):173–185. doi: 10.1111/j.1365-2958.1991.tb01838.x. [DOI] [PubMed] [Google Scholar]
- Petzel J. P., McKay L. L. Molecular characterization of the integration of the lactose plasmid from Lactococcus lactis subsp. cremoris SK11 into the chromosome of L. lactis subsp. lactis. Appl Environ Microbiol. 1992 Jan;58(1):125–131. doi: 10.1128/aem.58.1.125-131.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Konings W. N. Relation of growth of Streptococcus lactis and Streptococcus cremoris to amino acid transport. J Bacteriol. 1988 Feb;170(2):700–707. doi: 10.1128/jb.170.2.700-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Veldkamp H., Konings W. N. Bioenergetic consequences of lactose starvation for continuously cultured Streptococcus cremoris. J Bacteriol. 1987 Apr;169(4):1460–1468. doi: 10.1128/jb.169.4.1460-1468.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner D. Z., LeDeaux J. R., Ireton K., Grossman A. D. The spo0K locus of Bacillus subtilis is homologous to the oligopeptide permease locus and is required for sporulation and competence. J Bacteriol. 1991 Feb;173(4):1388–1398. doi: 10.1128/jb.173.4.1388-1398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid E. J., Driessen A. J., Konings W. N. Mechanism and energetics of dipeptide transport in membrane vesicles of Lactococcus lactis. J Bacteriol. 1989 Jan;171(1):292–298. doi: 10.1128/jb.171.1.292-298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid E. J., Plapp R., Konings W. N. Peptide uptake is essential for growth of Lactococcus lactis on the milk protein casein. J Bacteriol. 1989 Nov;171(11):6135–6140. doi: 10.1128/jb.171.11.6135-6140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøman P. Sequence of a gene (lap) encoding a 95.3-kDa aminopeptidase from Lactococcus lactis ssp. cremoris Wg2. Gene. 1992 Apr 1;113(1):107–112. doi: 10.1016/0378-1119(92)90676-g. [DOI] [PubMed] [Google Scholar]
- Tan P. S., Chapot-Chartier M. P., Pos K. M., Rousseau M., Boquien C. Y., Gripon J. C., Konings W. N. Localization of peptidases in lactococci. Appl Environ Microbiol. 1992 Jan;58(1):285–290. doi: 10.1128/aem.58.1.285-290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P. S., van Alen-Boerrigter I. J., Poolman B., Siezen R. J., de Vos W. M., Konings W. N. Characterization of the Lactococcus lactis pepN gene encoding an aminopeptidase homologous to mammalian aminopeptidase N. FEBS Lett. 1992 Jul 13;306(1):9–16. doi: 10.1016/0014-5793(92)80827-4. [DOI] [PubMed] [Google Scholar]
- Tapuhi Y., Schmidt D. E., Lindner W., Karger B. L. Dansylation of amino acids for high-performance liquid chromatography analysis. Anal Biochem. 1981 Jul 15;115(1):123–129. doi: 10.1016/0003-2697(81)90534-0. [DOI] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tynkkynen S., von Wright A. Characterization of a cloned chromosomal fragment affecting the proteinase activity of Streptococcus lactis ssp. lactis. Biochimie. 1988 Apr;70(4):531–534. doi: 10.1016/0300-9084(88)90089-2. [DOI] [PubMed] [Google Scholar]
- Tynkkynen S., von Wright A., Syväoja E. L. Peptide Utilization Encoded by Lactococcus lactis subsp. lactis SSL135 Chromosomal DNA. Appl Environ Microbiol. 1989 Oct;55(10):2690–2695. doi: 10.1128/aem.55.10.2690-2695.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmeier V. T., Porterfield S. P., Hendrich C. E. Quantitation of Dns-amino acids from body tissues and fluids using high-performance liquid chromatography. J Chromatogr. 1982 Sep 10;231(2):410–417. doi: 10.1016/s0378-4347(00)81865-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Alen-Boerrigter I. J., Baankreis R., de Vos W. M. Characterization and overexpression of the Lactococcus lactis pepN gene and localization of its product, aminopeptidase N. Appl Environ Microbiol. 1991 Sep;57(9):2555–2561. doi: 10.1128/aem.57.9.2555-2561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vossen J. M., van der Lelie D., Venema G. Isolation and characterization of Streptococcus cremoris Wg2-specific promoters. Appl Environ Microbiol. 1987 Oct;53(10):2452–2457. doi: 10.1128/aem.53.10.2452-2457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. The structure of signal peptides from bacterial lipoproteins. Protein Eng. 1989 May;2(7):531–534. doi: 10.1093/protein/2.7.531. [DOI] [PubMed] [Google Scholar]
- von Wright A., Tynkkynen S., Suominen M. Cloning of a Streptococcus lactis subsp. lactis Chromosomal Fragment Associated with the Ability To Grow in Milk. Appl Environ Microbiol. 1987 Jul;53(7):1584–1588. doi: 10.1128/aem.53.7.1584-1588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]