Abstract
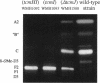
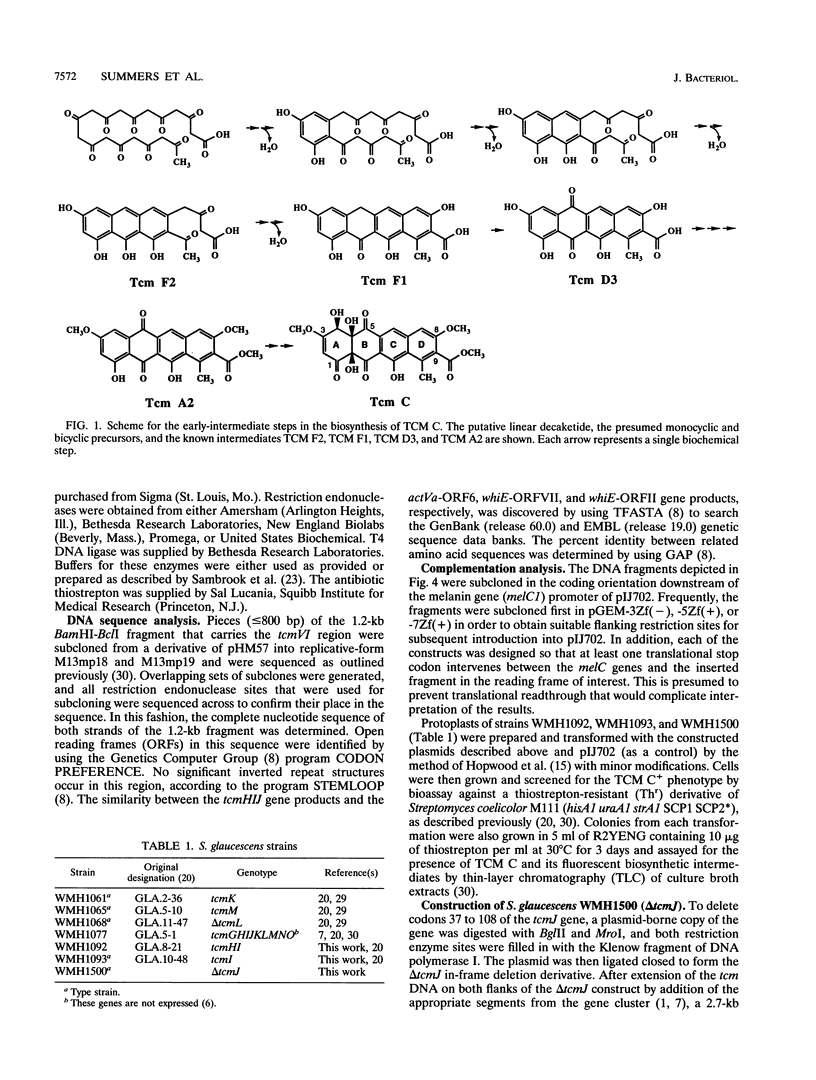
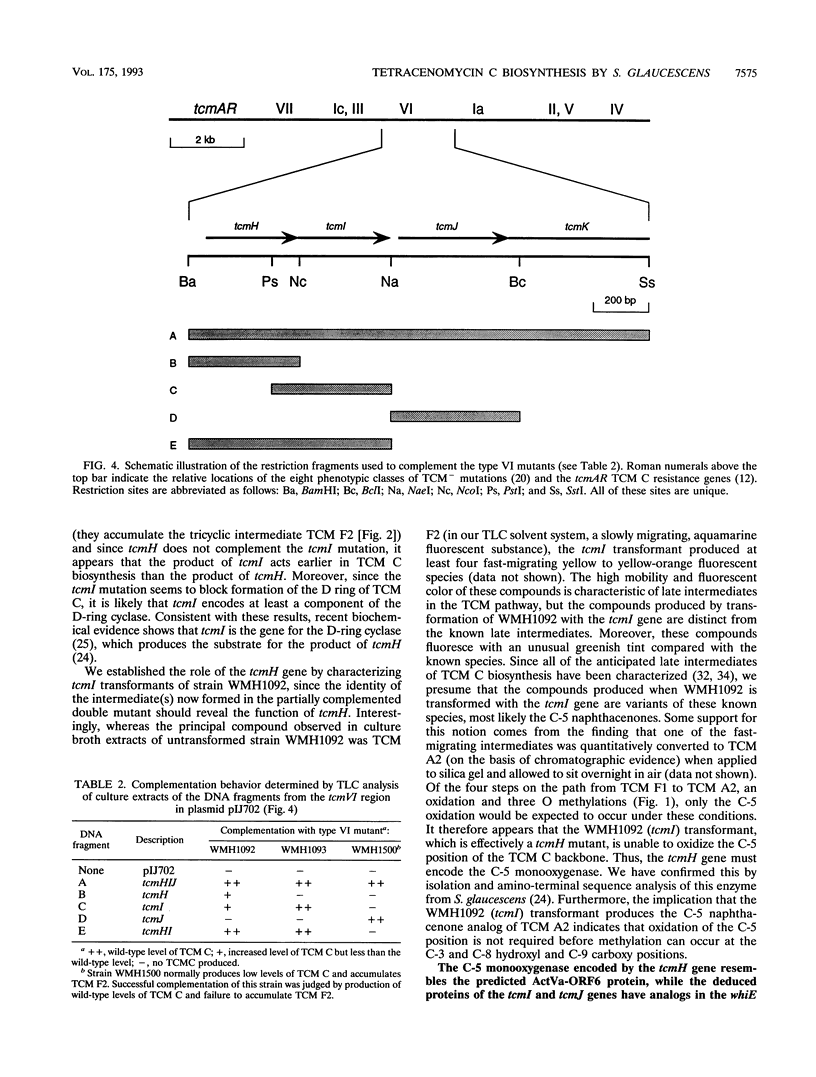
Certain mutations in the tcmVI region of the Streptomyces glaucescens chromosome affect formation of the D ring of the polyketide antibiotic tetracenomycin C (TCM C). This region lies immediately upstream from the TCM C polyketide synthase genes (tcmKLM), and the nucleotide sequence reveals the presence of three small genes, tcmH, tcmI, and tcmJ. On the basis of the phenotypes of mutants and the effects of these genes, when coupled on a plasmid with the tcmKLMN177 genes (tcmN177 is a 3'-truncated version of tcmN), on the production of TCM intermediates in a TCM- mutant, the tcmH gene encodes the C-5 monooxygenase that converts TCM F1 to TCM D3, the tcmI gene encodes the D-ring cyclase that converts TCM F2 to TCM F1 (mutations in this gene are responsible for the type VI phenotype), and the tcmJ gene most likely encodes the B-ring cyclase that acts in the biosynthesis of TCM F2. Furthermore, it appears that the N-terminal domain of the tcmN gene product (encoded by the tcmN177 gene) acts later in the biosynthesis of TCM F2 than the product of tcmJ, suggesting that the N-terminal domain of the TcmN protein is the C-ring cyclase.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibb M. J., Biró S., Motamedi H., Collins J. F., Hutchinson C. R. Analysis of the nucleotide sequence of the Streptomyces glaucescens tcmI genes provides key information about the enzymology of polyketide antibiotic biosynthesis. EMBO J. 1989 Sep;8(9):2727–2736. doi: 10.1002/j.1460-2075.1989.tb08414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Cohen S. N. Gene expression in Streptomyces: construction and application of promoter-probe plasmid vectors in Streptomyces lividans. Mol Gen Genet. 1982;187(2):265–277. doi: 10.1007/BF00331128. [DOI] [PubMed] [Google Scholar]
- Birch A. J. Biosynthesis of polyketides and related compounds. Science. 1967 Apr 14;156(3772):202–206. doi: 10.1126/science.156.3772.202. [DOI] [PubMed] [Google Scholar]
- Caballero J. L., Martinez E., Malpartida F., Hopwood D. A. Organisation and functions of the actVA region of the actinorhodin biosynthetic gene cluster of Streptomyces coelicolor. Mol Gen Genet. 1991 Dec;230(3):401–412. doi: 10.1007/BF00280297. [DOI] [PubMed] [Google Scholar]
- Davis N. K., Chater K. F. Spore colour in Streptomyces coelicolor A3(2) involves the developmentally regulated synthesis of a compound biosynthetically related to polyketide antibiotics. Mol Microbiol. 1990 Oct;4(10):1679–1691. doi: 10.1111/j.1365-2958.1990.tb00545.x. [DOI] [PubMed] [Google Scholar]
- Decker H., Hutchinson C. R. Transcriptional analysis of the Streptomyces glaucescens tetracenomycin C biosynthesis gene cluster. J Bacteriol. 1993 Jun;175(12):3887–3892. doi: 10.1128/jb.175.12.3887-3892.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker H., Motamedi H., Hutchinson C. R. Nucleotide sequences and heterologous expression of tcmG and tcmP, biosynthetic genes for tetracenomycin C in Streptomyces glaucescens. J Bacteriol. 1993 Jun;175(12):3876–3886. doi: 10.1128/jb.175.12.3876-3886.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Moreno M. A., Caballero J. L., Hopwood D. A., Malpartida F. The act cluster contains regulatory and antibiotic export genes, direct targets for translational control by the bldA tRNA gene of Streptomyces. Cell. 1991 Aug 23;66(4):769–780. doi: 10.1016/0092-8674(91)90120-n. [DOI] [PubMed] [Google Scholar]
- Fernández-Moreno M. A., Martínez E., Boto L., Hopwood D. A., Malpartida F. Nucleotide sequence and deduced functions of a set of cotranscribed genes of Streptomyces coelicolor A3(2) including the polyketide synthase for the antibiotic actinorhodin. J Biol Chem. 1992 Sep 25;267(27):19278–19290. [PubMed] [Google Scholar]
- Gramajo H. C., White J., Hutchinson C. R., Bibb M. J. Overproduction and localization of components of the polyketide synthase of Streptomyces glaucescens involved in the production of the antibiotic tetracenomycin C. J Bacteriol. 1991 Oct;173(20):6475–6483. doi: 10.1128/jb.173.20.6475-6483.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoile P. G., Hutchinson C. R. Sequence and transcriptional analysis of the Streptomyces glaucescens tcmAR tetracenomycin C resistance and repressor gene loci. J Bacteriol. 1992 Jun;174(11):3651–3658. doi: 10.1128/jb.174.11.3651-3658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam S. E., Malpartida F., Hopwood D. A. Nucleotide sequence, transcription and deduced function of a gene involved in polyketide antibiotic synthesis in Streptomyces coelicolor. Gene. 1988 Dec 30;74(2):305–320. doi: 10.1016/0378-1119(88)90165-5. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Sherman D. H. Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet. 1990;24:37–66. doi: 10.1146/annurev.ge.24.120190.000345. [DOI] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Katz L., Donadio S. Polyketide synthesis: prospects for hybrid antibiotics. Annu Rev Microbiol. 1993;47:875–912. doi: 10.1146/annurev.mi.47.100193.004303. [DOI] [PubMed] [Google Scholar]
- Motamedi H., Hutchinson C. R. Cloning and heterologous expression of a gene cluster for the biosynthesis of tetracenomycin C, the anthracycline antitumor antibiotic of Streptomyces glaucescens. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4445–4449. doi: 10.1073/pnas.84.13.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamedi H., Wendt-Pienkowski E., Hutchinson C. R. Isolation of tetracenomycin C-nonproducing Streptomyces glaucescens mutants. J Bacteriol. 1986 Aug;167(2):575–580. doi: 10.1128/jb.167.2.575-580.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd B. A., Hopwood D. A. Genetics of actinorhodin biosynthesis by Streptomyces coelicolor A3(2). J Gen Microbiol. 1979 Sep;114(1):35–43. doi: 10.1099/00221287-114-1-35. [DOI] [PubMed] [Google Scholar]
- Shen B., Hutchinson C. R. Tetracenomycin F1 monooxygenase: oxidation of a naphthacenone to a naphthacenequinone in the biosynthesis of tetracenomycin C in Streptomyces glaucescens. Biochemistry. 1993 Jul 6;32(26):6656–6663. doi: 10.1021/bi00077a019. [DOI] [PubMed] [Google Scholar]
- Shen B., Nakayama H., Hutchinson C. R. Isolation and structural elucidation of tetracenomycin F2 and tetracenomycin F1: early intermediates in the biosynthesis of tetracenomycin C in Streptomyces glaucescens. J Nat Prod. 1993 Aug;56(8):1288–1293. doi: 10.1021/np50098a013. [DOI] [PubMed] [Google Scholar]
- Summers R. G., Wendt-Pienkowski E., Motamedi H., Hutchinson C. R. Nucleotide sequence of the tcmII-tcmIV region of the tetracenomycin C biosynthetic gene cluster of Streptomyces glaucescens and evidence that the tcmN gene encodes a multifunctional cyclase-dehydratase-O-methyl transferase. J Bacteriol. 1992 Mar;174(6):1810–1820. doi: 10.1128/jb.174.6.1810-1820.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Janssen G. R., Kieser T., Bibb M. J., Buttner M. J., Bibb M. J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986 Jun;203(3):468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- Weber W., Zähner H., Siebers J., Schröder K., Zeeck A. Stoffwechselprodukte von Mikroorganismen. 175. Mitteilung. Tetracenomycin C. Arch Microbiol. 1979 May;121(2):111–116. doi: 10.1007/BF00689973. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yue S., Motamedi H., Wendt-Pienkowski E., Hutchinson C. R. Anthracycline metabolites of tetracenomycin C-nonproducing Streptomyces glaucescens mutants. J Bacteriol. 1986 Aug;167(2):581–586. doi: 10.1128/jb.167.2.581-586.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]