Abstract
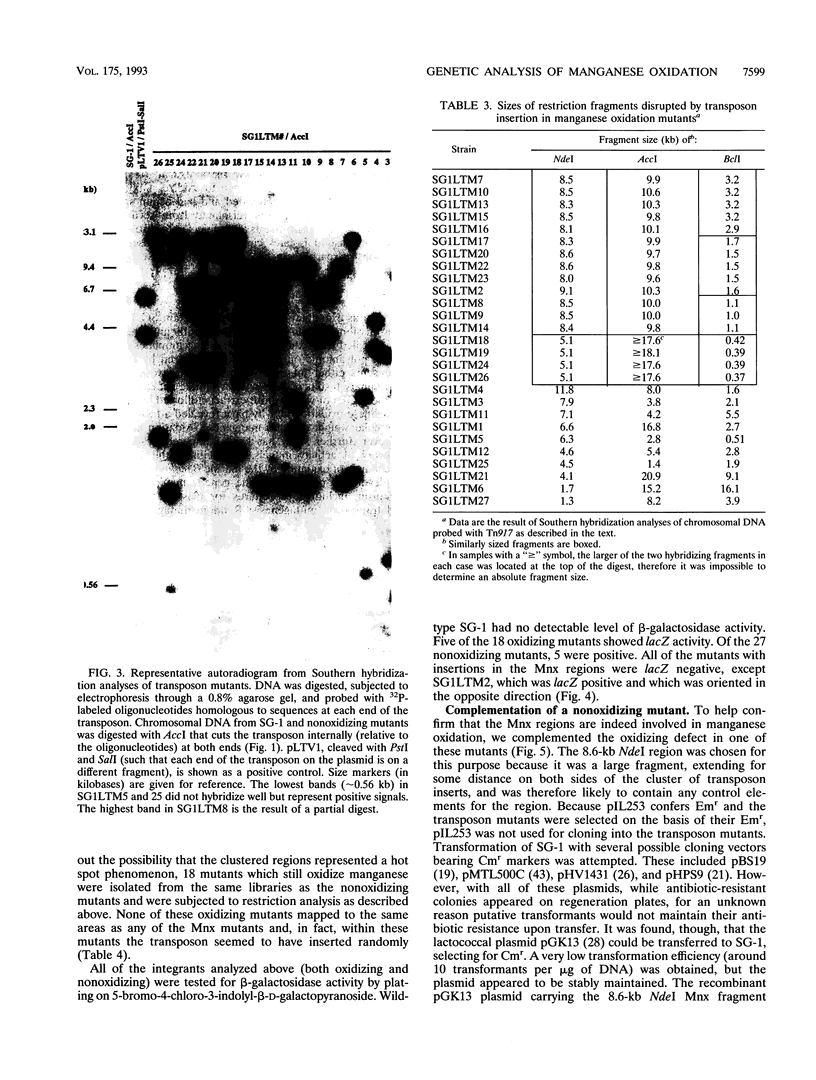
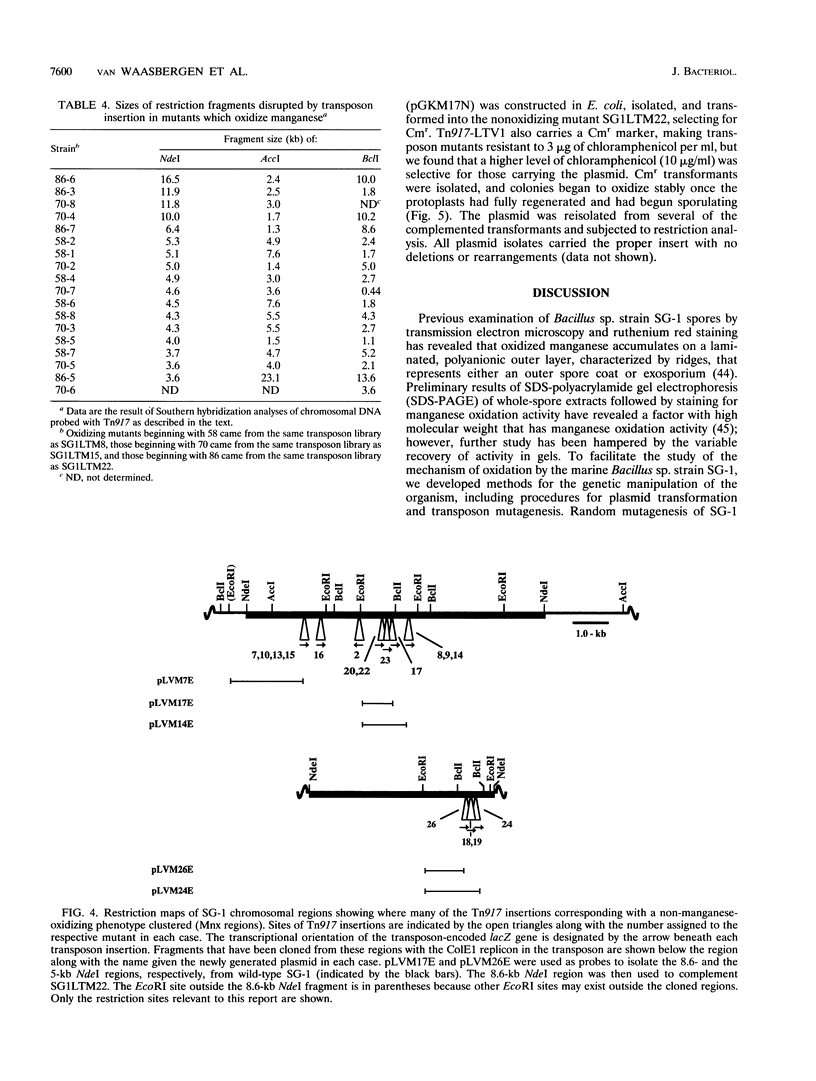
Mature spores of the marine Bacillus sp. strain SG-1 bind and oxidize manganese(II), thereby becoming encrusted with a manganese(IV) oxide. Both the function and mechanism of this oxidation are unknown, although evidence suggests that spore coat proteins are involved. To further study this phenomenon, methods of genetic analysis were developed for SG-1. By a modified protoplast transformation procedure, SG-1 was transformed (approximately 100 transformants per micrograms of DNA) with several different plasmids of gram-positive origin. Transposon Tn917, delivered on the temperature-sensitive plasmid pLTV1, was used to generate mutants of SG-1. Conditions were established that allowed 98% plasmid loss and insertions to be recovered at a frequency of 10(-3). Each mutant was found to be the result of a single insertion event. Restriction analysis of 27 mutants that do not oxidize manganese but still sporulate localized 17 of the insertions within two regions of the chromosome (termed Mnx regions), and a physical map of these regions was generated. Analysis of 18 transposon integrants in which manganese oxidation was unaffected revealed random transposon integration, with none of their insertions mapping within the Mnx regions. The Mnx regions were cloned from wild-type SG-1, and the largest region, carried on the lactococcal plasmid pGK13, was used to complement in trans one of the nonoxidizing mutants. These results demonstrate that the Mnx regions encode factors that are required for the oxidation of manganese, and this represents the first report identifying genes involved in bacterial manganese oxidation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki H., Slepecky R. A. Inducement of a heat-shock requirement for germination and production of increased heat resistance in Bacillus fastidiosus spores by manganous ions. J Bacteriol. 1973 Apr;114(1):137–143. doi: 10.1128/jb.114.1.137-143.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin B. Novel pharmaceutical compounds from marine bacteria. J Appl Bacteriol. 1989 Nov;67(5):461–470. doi: 10.1111/j.1365-2672.1989.tb02517.x. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brosius J. Superpolylinkers in cloning and expression vectors. DNA. 1989 Dec;8(10):759–777. doi: 10.1089/dna.1989.8.759. [DOI] [PubMed] [Google Scholar]
- CHARNEY J., FISHER W. P., HEGARTY C. P. Managanese as an essential element for sporulation in the genus Bacillus. J Bacteriol. 1951 Aug;62(2):145–148. doi: 10.1128/jb.62.2.145-148.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli A., Portnoy A., Youngman P. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J Bacteriol. 1990 Jul;172(7):3738–3744. doi: 10.1128/jb.172.7.3738-3744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Donovan W., Zheng L. B., Sandman K., Losick R. Genes encoding spore coat polypeptides from Bacillus subtilis. J Mol Biol. 1987 Jul 5;196(1):1–10. doi: 10.1016/0022-2836(87)90506-7. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E., Fisher S., Der C. L., Silver S. Manganese transport in Bacillus subtilis W23 during growth and sporulation. J Bacteriol. 1973 Mar;113(3):1363–1372. doi: 10.1128/jb.113.3.1363-1372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiorse W. C. Biology of iron- and manganese-depositing bacteria. Annu Rev Microbiol. 1984;38:515–550. doi: 10.1146/annurev.mi.38.100184.002503. [DOI] [PubMed] [Google Scholar]
- Haima P., van Sinderen D., Bron S., Venema G. An improved beta-galactosidase alpha-complementation system for molecular cloning in Bacillus subtilis. Gene. 1990 Sep 1;93(1):41–47. doi: 10.1016/0378-1119(90)90133-c. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A. Genetic studies with bacterial protoplasts. Annu Rev Microbiol. 1981;35:237–272. doi: 10.1146/annurev.mi.35.100181.001321. [DOI] [PubMed] [Google Scholar]
- Jannière L., Bruand C., Ehrlich S. D. Structurally stable Bacillus subtilis cloning vectors. Gene. 1990 Mar 1;87(1):53–61. doi: 10.1016/0378-1119(90)90495-d. [DOI] [PubMed] [Google Scholar]
- Kihm D. J., Hutton M. T., Hanlin J. H., Johnson E. A. Influence of transition metals added during sporulation on heat resistance of Clostridium botulinum 113B spores. Appl Environ Microbiol. 1990 Mar;56(3):681–685. doi: 10.1128/aem.56.3.681-685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok J., van der Vossen J. M., Venema G. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol. 1984 Oct;48(4):726–731. doi: 10.1128/aem.48.4.726-731.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa T., Yamazaki M., Yoshimi M., Ogawa S., Yamada A., Watabe K., Torii M. Surface hydrophobicity of spores of Bacillus spp. J Gen Microbiol. 1989 Oct;135(10):2717–2722. doi: 10.1099/00221287-135-10-2717. [DOI] [PubMed] [Google Scholar]
- Perkins J. B., Youngman P. J. A physical and functional analysis of Tn917, a Streptococcus transposon in the Tn3 family that functions in Bacillus. Plasmid. 1984 Sep;12(2):119–138. doi: 10.1016/0147-619x(84)90058-1. [DOI] [PubMed] [Google Scholar]
- Rosson R. A., Nealson K. H. Manganese binding and oxidation by spores of a marine bacillus. J Bacteriol. 1982 Aug;151(2):1027–1034. doi: 10.1128/jb.151.2.1027-1034.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEFFER P., IONESCO H. [Contribution to the genetic study of bacterial sporogenesis]. C R Hebd Seances Acad Sci. 1960 Dec 19;251:3125–3127. [PubMed] [Google Scholar]
- Simon D., Chopin A. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie. 1988 Apr;70(4):559–566. doi: 10.1016/0300-9084(88)90093-4. [DOI] [PubMed] [Google Scholar]
- Swinfield T. J., Oultram J. D., Thompson D. E., Brehm J. K., Minton N. P. Physical characterisation of the replication region of the Streptococcus faecalis plasmid pAM beta 1. Gene. 1990 Mar 1;87(1):79–90. [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Clewell D. B. Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1980 Mar;141(3):1366–1374. doi: 10.1128/jb.141.3.1366-1374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiencek K. M., Klapes N. A., Foegeding P. M. Hydrophobicity of Bacillus and Clostridium spores. Appl Environ Microbiol. 1990 Sep;56(9):2600–2605. doi: 10.1128/aem.56.9.2600-2605.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrind J. P., Boogerd F. C., de Vrind-de Jong E. W. Manganese reduction by a marine Bacillus species. J Bacteriol. 1986 Jul;167(1):30–34. doi: 10.1128/jb.167.1.30-34.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrind J. P., de Vrind-de Jong E. W., de Voogt J. W., Westbroek P., Boogerd F. C., Rosson R. A. Manganese oxidation by spores and spore coats of a marine bacillus species. Appl Environ Microbiol. 1986 Nov;52(5):1096–1100. doi: 10.1128/aem.52.5.1096-1100.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen W. L. Proceedings: Netherlands Society for Microbiology meeting at Utrecht on 2 May 1973. Biological xoidation of manganese in soils. Antonie Van Leeuwenhoek. 1973 Nov;39(4):657–662. doi: 10.1007/BF02578915. [DOI] [PubMed] [Google Scholar]