Abstract
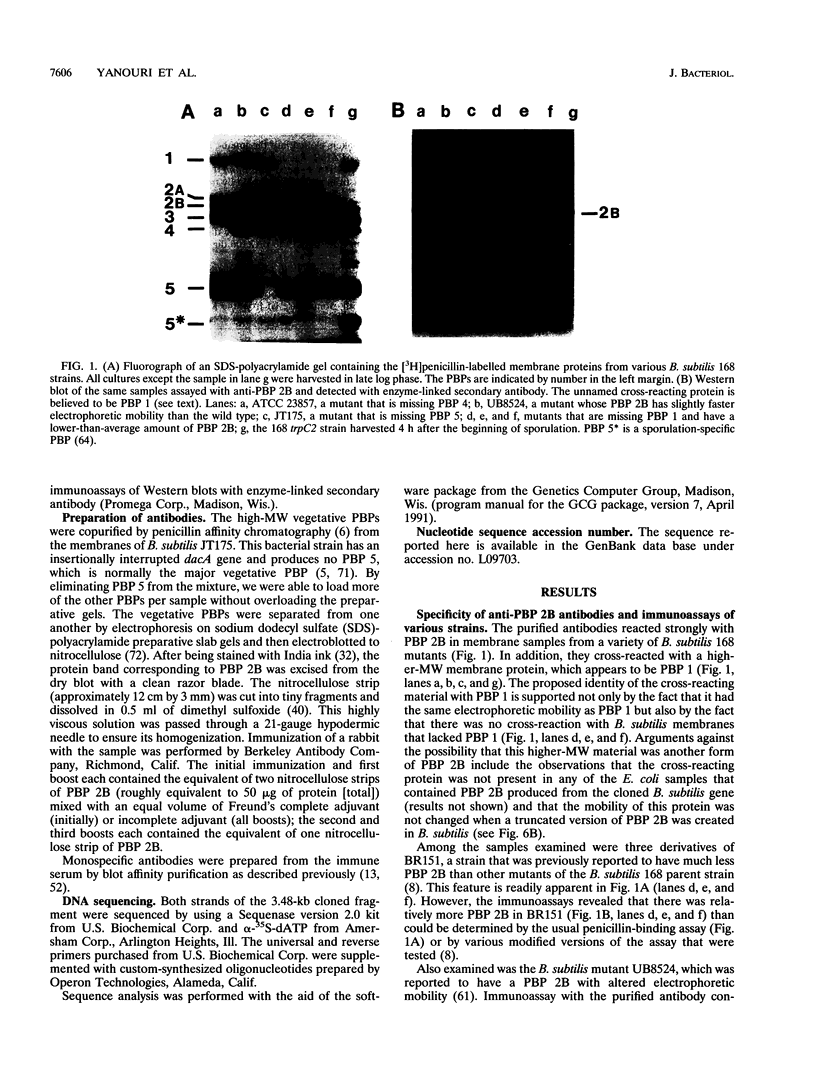
The pbpB gene, which encodes penicillin-binding protein (PBP) 2B of Bacillus subtilis, has been cloned, sequenced, mapped, and mutagenized. The sequence of PBP 2B places it among the class B high-molecular-weight PBPs. It appears to contain three functional domains: an N-terminal domain homologous to the corresponding domain of other class B PBPs, a penicillin-binding domain, and a lengthy carboxy extension. The PBP has a noncleaved signal sequence at its N terminus that presumably serves as its anchor in the cell membrane. Previous studies led to the hypothesis that PBP 2B is required for both vegetative cell division and sporulation septation. Its sequence, map site, and mutant phenotype support this hypothesis. PBP 2B is homologous to PBP 3, the cell division protein encoded by pbpB of Escherichia coli. Moreover, both pbpB genes are located in the same relative position within a cluster of cell division and cell wall genes on their respective chromosomes. However, immediately adjacent to the B. subtilis pbpB gene is spoVD, which appears to be a sporulation-specific homolog of pbpB. Inactivation of SpoVD blocked synthesis of the cortical peptidoglycan in the spore, whereas carboxy truncation of PBP 2B caused cells to grow as filaments. Thus, it appears that a gene duplication has occurred in B. subtilis and that one PBP has evolved to serve a common role in septation during both vegetative growth and sporulation, whereas the other PBP serves a specialized role in sporulation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beall B., Lowe M., Lutkenhaus J. Cloning and characterization of Bacillus subtilis homologs of Escherichia coli cell division genes ftsZ and ftsA. J Bacteriol. 1988 Oct;170(10):4855–4864. doi: 10.1128/jb.170.10.4855-4864.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Spratt B. G., Donachie W. D. Interaction between membrane proteins PBP3 and rodA is required for normal cell shape and division in Escherichia coli. J Bacteriol. 1986 Sep;167(3):1004–1008. doi: 10.1128/jb.167.3.1004-1008.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Takasuga A., Edwards D. H., Dewar S. J., Spratt B. G., Adachi H., Ohta T., Matsuzawa H., Donachie W. D. The balance between different peptidoglycan precursors determines whether Escherichia coli cells will elongate or divide. J Bacteriol. 1990 Dec;172(12):6697–6703. doi: 10.1128/jb.172.12.6697-6703.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Covalent affinity chromatography of penicillin-binding components from bacterial membranes. Methods Enzymol. 1974;34:401–405. doi: 10.1016/s0076-6879(74)34046-3. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Five penicillin-binding components occur in Bacillus subtilis membranes. J Biol Chem. 1972 Dec 25;247(24):8107–8113. [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Inactivation of D-alanine carboxypeptidase by penicillins and cephalosporins is not lethal in Bacillus subtilis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2814–2817. doi: 10.1073/pnas.68.11.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E. Absence of penicillin-binding protein 4 from an apparently normal strain of Bacillus subtilis. J Bacteriol. 1987 Nov;169(11):5301–5303. doi: 10.1128/jb.169.11.5301-5303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Gustafson A. Mapping of the gene for a major penicillin-binding protein to a genetically conserved region of the Bacillus subtilis chromosome and conservation of the protein among related species of Bacillus. J Bacteriol. 1991 Mar;173(5):1807–1809. doi: 10.1128/jb.173.5.1807-1809.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Gustafson A. Mutagenesis and mapping of the gene for a sporulation-specific penicillin-binding protein in Bacillus subtilis. J Bacteriol. 1992 Aug;174(16):5430–5435. doi: 10.1128/jb.174.16.5430-5435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Ling M. L. Isolation and sequence analysis of dacB, which encodes a sporulation-specific penicillin-binding protein in Bacillus subtilis. J Bacteriol. 1992 Mar;174(6):1717–1725. doi: 10.1128/jb.174.6.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Neyman S. L. Correlation of penicillin-binding protein composition with different functions of two membranes in Bacillus subtilis forespores. J Bacteriol. 1986 Feb;165(2):498–503. doi: 10.1128/jb.165.2.498-503.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K., Lutkenhaus J. The proper ratio of FtsZ to FtsA is required for cell division to occur in Escherichia coli. J Bacteriol. 1992 Oct;174(19):6145–6151. doi: 10.1128/jb.174.19.6145-6151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R. A., Errington J. DNA sequence of the murE-murD region of Bacillus subtilis 168. J Gen Microbiol. 1993 Feb;139(2):361–370. doi: 10.1099/00221287-139-2-361. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson C. G., Jephcott A. E., Gough K. R., Spratt B. G. Penicillin-binding protein 2 genes of non-beta-lactamase-producing, penicillin-resistant strains of Neisseria gonorrhoeae. Mol Microbiol. 1989 Jan;3(1):35–41. doi: 10.1111/j.1365-2958.1989.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993 Mar;57(1):1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J., Mandelstam J. Use of a lacZ gene fusion to determine the dependence pattern of sporulation operon spoIIA in spo mutants of Bacillus subtilis. J Gen Microbiol. 1986 Nov;132(11):2967–2976. doi: 10.1099/00221287-132-11-2967. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Serine beta-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- Guzman L. M., Barondess J. J., Beckwith J. FtsL, an essential cytoplasmic membrane protein involved in cell division in Escherichia coli. J Bacteriol. 1992 Dec;174(23):7716–7728. [PMC free article] [PubMed] [Google Scholar]
- Gómez M. J., Fluoret B., van Heijenoort J., Ayala J. A. Nucleotide sequence of the regulatory region of the gene pbpB of Escherichia coli. Nucleic Acids Res. 1990 May 11;18(9):2813–2813. doi: 10.1093/nar/18.9.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M., Hederstedt L. Cloning and characterization of the Bacillus subtilis hemEHY gene cluster, which encodes protoheme IX biosynthetic enzymes. J Bacteriol. 1992 Dec;174(24):8081–8093. doi: 10.1128/jb.174.24.8081-8093.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H., Nishimura Y., Kato J., Suzuki H., Nagasawa H., Suzuki A., Hirota Y. Genetic analyses of processing involving C-terminal cleavage in penicillin-binding protein 3 of Escherichia coli. J Bacteriol. 1989 Nov;171(11):5882–5889. doi: 10.1128/jb.171.11.5882-5889.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H., Yamamoto Y., Higashitani A., Suzuki H., Nishimura Y. Cloning, mapping, and characterization of the Escherichia coli prc gene, which is involved in C-terminal processing of penicillin-binding protein 3. J Bacteriol. 1991 Aug;173(15):4799–4813. doi: 10.1128/jb.173.15.4799-4813.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques A. O., de Lencastre H., Piggot P. J. A Bacillus subtilis morphogene cluster that includes spoVE is homologous to the mra region of Escherichia coli. Biochimie. 1992 Jul-Aug;74(7-8):735–748. doi: 10.1016/0300-9084(92)90146-6. [DOI] [PubMed] [Google Scholar]
- Ikeda M., Sato T., Wachi M., Jung H. K., Ishino F., Kobayashi Y., Matsuhashi M. Structural similarity among Escherichia coli FtsW and RodA proteins and Bacillus subtilis SpoVE protein, which function in cell division, cell elongation, and spore formation, respectively. J Bacteriol. 1989 Nov;171(11):6375–6378. doi: 10.1128/jb.171.11.6375-6378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino F., Park W., Tomioka S., Tamaki S., Takase I., Kunugita K., Matsuzawa H., Asoh S., Ohta T., Spratt B. G. Peptidoglycan synthetic activities in membranes of Escherichia coli caused by overproduction of penicillin-binding protein 2 and rodA protein. J Biol Chem. 1986 May 25;261(15):7024–7031. [PubMed] [Google Scholar]
- Joris B., Ghuysen J. M., Dive G., Renard A., Dideberg O., Charlier P., Frère J. M., Kelly J. A., Boyington J. C., Moews P. C. The active-site-serine penicillin-recognizing enzymes as members of the Streptomyces R61 DD-peptidase family. Biochem J. 1988 Mar 1;250(2):313–324. doi: 10.1042/bj2500313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Suzuki H., Hirota Y. Overlapping of the coding regions for alpha and gamma components of penicillin-binding protein 1 b in Escherichia coli. Mol Gen Genet. 1984;196(3):449–457. doi: 10.1007/BF00436192. [DOI] [PubMed] [Google Scholar]
- Kemp E. H., Sammons R. L., Moir A., Sun D., Setlow P. Analysis of transcriptional control of the gerD spore germination gene of Bacillus subtilis 168. J Bacteriol. 1991 Aug;173(15):4646–4652. doi: 10.1128/jb.173.15.4646-4652.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppe G., Yu W., Strominger J. L. Penicillin-binding proteins in Bacillus subtilis mutants. Antimicrob Agents Chemother. 1982 Jun;21(6):979–983. doi: 10.1128/aac.21.6.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K. A. Proteins transferred to nitrocellulose for use as immunogens. Anal Biochem. 1985 Jun;147(2):285–288. doi: 10.1016/0003-2697(85)90273-8. [DOI] [PubMed] [Google Scholar]
- Kuroda A., Rashid M. H., Sekiguchi J. Molecular cloning and sequencing of the upstream region of the major Bacillus subtilis autolysin gene: a modifier protein exhibiting sequence homology to the major autolysin and the spoIID product. J Gen Microbiol. 1992 Jun;138(6):1067–1076. doi: 10.1099/00221287-138-6-1067. [DOI] [PubMed] [Google Scholar]
- Laible G., Hakenbeck R., Sicard M. A., Joris B., Ghuysen J. M. Nucleotide sequences of the pbpX genes encoding the penicillin-binding proteins 2x from Streptococcus pneumoniae R6 and a cefotaxime-resistant mutant, C506. Mol Microbiol. 1989 Oct;3(10):1337–1348. doi: 10.1111/j.1365-2958.1989.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Michaud C., Parquet C., Flouret B., Blanot D., van Heijenoort J. Revised interpretation of the sequence containing the murE gene encoding the UDP-N-acetylmuramyl-tripeptide synthetase of Escherichia coli. Biochem J. 1990 Jul 1;269(1):277–278. doi: 10.1042/bj2690277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa H., Sakagami Y., Suzuki A., Suzuki H., Hara H., Hirota Y. Determination of the cleavage site involved in C-terminal processing of penicillin-binding protein 3 of Escherichia coli. J Bacteriol. 1989 Nov;171(11):5890–5893. doi: 10.1128/jb.171.11.5890-5893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Maruyama I. N., Soma M., Kato J., Suzuki H., Horota Y. On the process of cellular division in Escherichia coli: nucleotide sequence of the gene for penicillin-binding protein 3. Mol Gen Genet. 1983;191(1):1–9. doi: 10.1007/BF00330881. [DOI] [PubMed] [Google Scholar]
- Neyman S. L., Buchanan C. E. Restoration of vegetative penicillin-binding proteins during germination and outgrowth of Bacillus subtilis spores: relationship of individual proteins to specific cell cycle events. J Bacteriol. 1985 Jan;161(1):164–168. doi: 10.1128/jb.161.1.164-168.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas R. A., Strominger J. L., Suzuki H., Hirota Y. Identification of the active site in penicillin-binding protein 3 of Escherichia coli. J Bacteriol. 1985 Oct;164(1):456–460. doi: 10.1128/jb.164.1.456-460.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J. B. Analysis of cytoskeletal structures using blot-purified monospecific antibodies. Methods Enzymol. 1986;134:467–472. doi: 10.1016/0076-6879(86)34112-0. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras G., Raze D., el Kharroubi A., Hastir D., Englebert S., Coyette J., Ghuysen J. M. Cloning and sequencing of the low-affinity penicillin-binding protein 3r-encoding gene of Enterococcus hirae S185: modular design and structural organization of the protein. J Bacteriol. 1993 May;175(10):2844–2852. doi: 10.1128/jb.175.10.2844-2852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popham D. L., Setlow P. Cloning, nucleotide sequence, and regulation of the Bacillus subtilis pbpE operon, which codes for penicillin-binding protein 4* and an apparent amino acid racemase. J Bacteriol. 1993 May;175(10):2917–2925. doi: 10.1128/jb.175.10.2917-2925.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popham D. L., Setlow P. Cloning, nucleotide sequence, and regulation of the Bacillus subtilis pbpF gene, which codes for a putative class A high-molecular-weight penicillin-binding protein. J Bacteriol. 1993 Aug;175(15):4870–4876. doi: 10.1128/jb.175.15.4870-4876.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats R., Gomez M., Pla J., Blasco B., Ayala J. A. A new beta-lactam-binding protein derived from penicillin-binding protein 3 of Escherichia coli. J Bacteriol. 1989 Sep;171(9):5194–5198. doi: 10.1128/jb.171.9.5194-5198.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. W., Doi R. H. Genetic mapping of rpoD implicates the major sigma factor of Bacillus subtilis RNA polymerase in sporulation initiation. Mol Gen Genet. 1985;201(1):88–95. doi: 10.1007/BF00397991. [DOI] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohayeb M., Chopra I. Mutations affecting penicillin-binding proteins 2a, 2b and 3 in Bacillus subtilis alter cell shape and peptidoglycan metabolism. J Gen Microbiol. 1987 Jul;133(7):1733–1742. doi: 10.1099/00221287-133-7-1733. [DOI] [PubMed] [Google Scholar]
- Silber K. R., Keiler K. C., Sauer R. T. Tsp: a tail-specific protease that selectively degrades proteins with nonpolar C termini. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):295–299. doi: 10.1073/pnas.89.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Elledge S., Sweetser D., Young R. A., Davis R. W. Lambda gt 11: gene isolation with antibody probes and other applications. Methods Enzymol. 1987;154:107–128. doi: 10.1016/0076-6879(87)54073-3. [DOI] [PubMed] [Google Scholar]
- Sowell M. O., Buchanan C. E. Changes in penicillin-binding proteins during sporulation of Bacillus subtilis. J Bacteriol. 1983 Mar;153(3):1331–1337. doi: 10.1128/jb.153.3.1331-1337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol. 1977 Jul;131(1):293–305. doi: 10.1128/jb.131.1.293-305.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel R., Reiss B., Schaller H. Translationally coupled initiation of protein synthesis in Bacillus subtilis. Nucleic Acids Res. 1985 Feb 11;13(3):893–909. doi: 10.1093/nar/13.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J. W., Boylan S. A., Price C. W. Gene for the alpha subunit of Bacillus subtilis RNA polymerase maps in the ribosomal protein gene cluster. J Bacteriol. 1986 Oct;168(1):65–71. doi: 10.1128/jb.168.1.65-71.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Bone E. J., Ellar D. J. The sporulation-specific penicillin-binding protein 5a from Bacillus subtilis is a DD-carboxypeptidase in vitro. Biochem J. 1985 Sep 15;230(3):825–828. doi: 10.1042/bj2300825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Roberts A. N., Johnstone K., Piggot P. J., Winter G., Ellar D. J. Reduced heat resistance of mutant spores after cloning and mutagenesis of the Bacillus subtilis gene encoding penicillin-binding protein 5. J Bacteriol. 1986 Jul;167(1):257–264. doi: 10.1128/jb.167.1.257-264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki M., Wachi M., Jung H. K., Ishino F., Matsuhashi M. Escherichia coli mraR gene involved in cell growth and division. J Bacteriol. 1992 Dec;174(23):7841–7843. doi: 10.1128/jb.174.23.7841-7843.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. J., Schuch R., Piggot P. J. Characterization of a Bacillus subtilis sporulation operon that includes genes for an RNA polymerase sigma factor and for a putative DD-carboxypeptidase. J Bacteriol. 1992 Aug;174(15):4885–4892. doi: 10.1128/jb.174.15.4885-4892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E., Smith C., Reilly B. E. Chromosomal location of genes regulating resistance to bacteriophage in Bacillus subtilis. J Bacteriol. 1969 Jun;98(3):1087–1097. doi: 10.1128/jb.98.3.1087-1097.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. Y., Jones D. M., Sáez Nieto J. A., Pérez Trallero E., Spratt B. G. Genetic diversity of penicillin-binding protein 2 genes of penicillin-resistant strains of Neisseria meningitidis revealed by fingerprinting of amplified DNA. Antimicrob Agents Chemother. 1990 Aug;34(8):1523–1528. doi: 10.1128/aac.34.8.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. Y., Spratt B. G. Nucleotide sequence of the penicillin-binding protein 2 gene of Neisseria meningitidis. Nucleic Acids Res. 1989 Jul 11;17(13):5383–5383. doi: 10.1093/nar/17.13.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heijenoort Y., Gómez M., Derrien M., Ayala J., van Heijenoort J. Membrane intermediates in the peptidoglycan metabolism of Escherichia coli: possible roles of PBP 1b and PBP 3. J Bacteriol. 1992 Jun;174(11):3549–3557. doi: 10.1128/jb.174.11.3549-3557.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988 Jul 1;174(4):671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]