Abstract
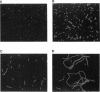
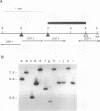
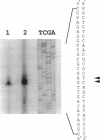
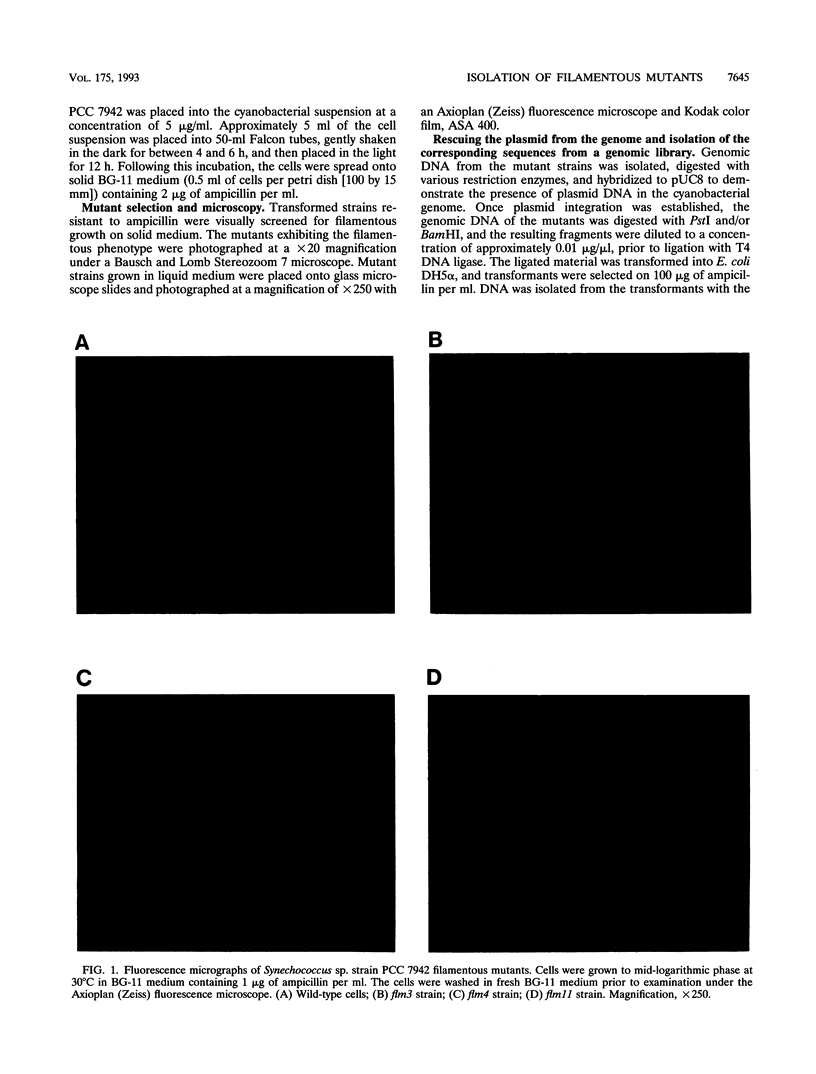
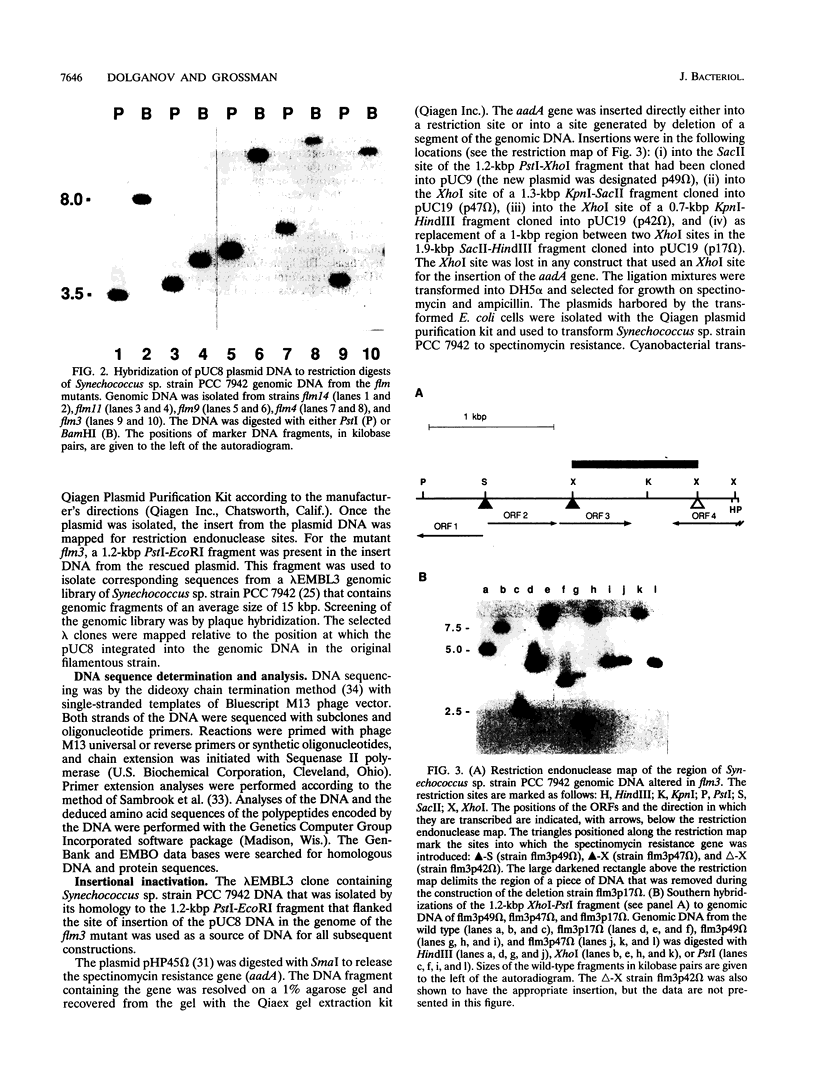
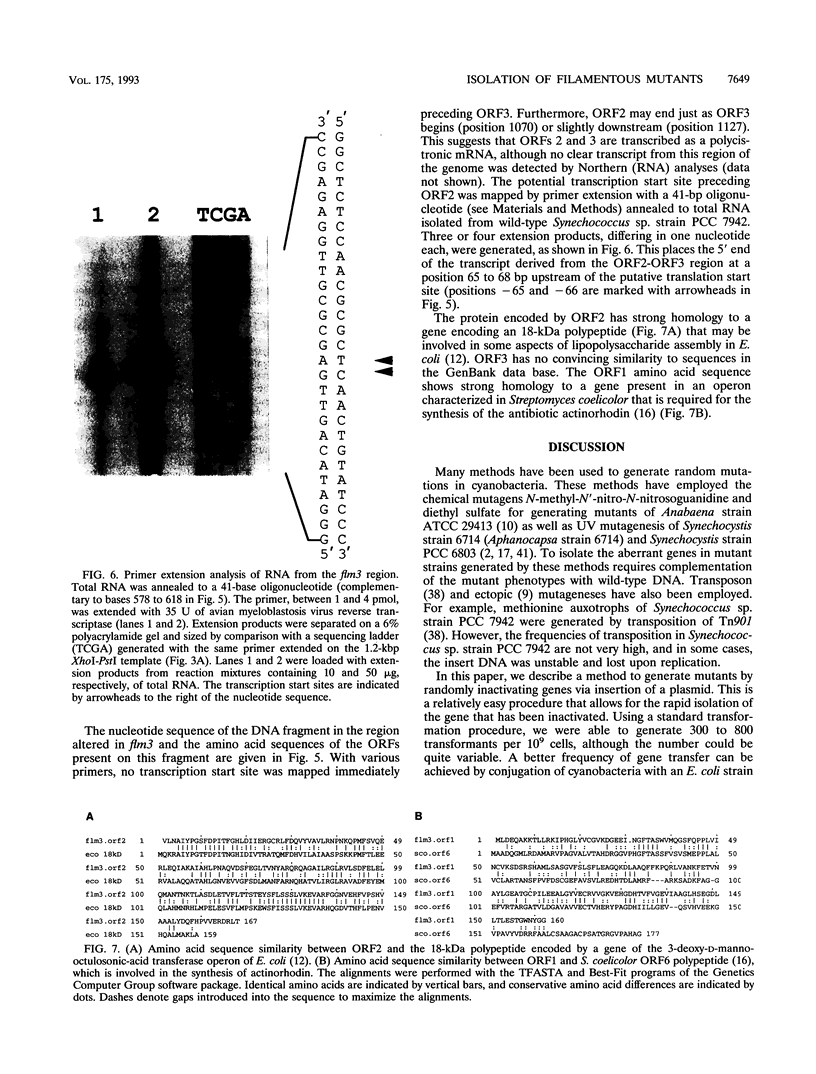
We have developed a simple procedure for generating mutants of the cyanobacterium Synechococcus sp. strain PCC 7942 in which the site of the lesion can be readily identified. This procedure involves transforming Synechococcus sp. strain PCC 7942 with a library of its own DNA that was fully digested with Sau3A and ligated into the plasmid vector pUC8. The homologous integration of the recombinant plasmid into the genome will often result in the disruption of a gene and the loss of gene function. We have used this method to generate many mutants of Synechococcus sp. strain PCC 7942 which grow as multicellular filaments rather than as unicells. Since the gene harboring the lesion was tagged with pUC8, it was easily isolated. In this paper, we discuss the usefulness of this procedure for the generation of mutants, and we characterize one mutant in which the lesion may be in an operon involved in the assembly of lipopolysaccharides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beall B., Lowe M., Lutkenhaus J. Cloning and characterization of Bacillus subtilis homologs of Escherichia coli cell division genes ftsZ and ftsA. J Bacteriol. 1988 Oct;170(10):4855–4864. doi: 10.1128/jb.170.10.4855-4864.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall B., Lutkenhaus J. FtsZ in Bacillus subtilis is required for vegetative septation and for asymmetric septation during sporulation. Genes Dev. 1991 Mar;5(3):447–455. doi: 10.1101/gad.5.3.447. [DOI] [PubMed] [Google Scholar]
- Bruns B. U., Briggs W. R., Grossman A. R. Molecular characterization of phycobilisome regulatory mutants of Fremyella diplosiphon. J Bacteriol. 1989 Feb;171(2):901–908. doi: 10.1128/jb.171.2.901-908.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buikema W. J., Haselkorn R. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 1991 Feb;5(2):321–330. doi: 10.1101/gad.5.2.321. [DOI] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Expression of the Escherichia coli lacZ gene on a plasmid vector in a cyanobacterium. Science. 1985 Nov 15;230(4727):805–807. doi: 10.1126/science.2997920. [DOI] [PubMed] [Google Scholar]
- Chiang G. G., Schaefer M. R., Grossman A. R. Complementation of a red-light-indifferent cyanobacterial mutant. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9415–9419. doi: 10.1073/pnas.89.20.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz T., Raetz C. R. A gene coding for 3-deoxy-D-manno-octulosonic-acid transferase in Escherichia coli. Identification, mapping, cloning, and sequencing. J Biol Chem. 1991 May 25;266(15):9687–9696. [PubMed] [Google Scholar]
- Cobley J. G., Miranda R. D. Mutations affecting chromatic adaptation in the cyanobacterium Fremyella diplosiphon. J Bacteriol. 1983 Mar;153(3):1486–1492. doi: 10.1128/jb.153.3.1486-1492.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus R. J., Barry B. A., Sithole I., Babcock G. T., McIntosh L. Directed mutagenesis indicates that the donor to P+680 in photosystem II is tyrosine-161 of the D1 polypeptide. Biochemistry. 1988 Dec 27;27(26):9071–9074. doi: 10.1021/bi00426a001. [DOI] [PubMed] [Google Scholar]
- Ernst A., Black T., Cai Y., Panoff J. M., Tiwari D. N., Wolk C. P. Synthesis of nitrogenase in mutants of the cyanobacterium Anabaena sp. strain PCC 7120 affected in heterocyst development or metabolism. J Bacteriol. 1992 Oct;174(19):6025–6032. doi: 10.1128/jb.174.19.6025-6032.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Moreno M. A., Martínez E., Boto L., Hopwood D. A., Malpartida F. Nucleotide sequence and deduced functions of a set of cotranscribed genes of Streptomyces coelicolor A3(2) including the polyketide synthase for the antibiotic actinorhodin. J Biol Chem. 1992 Sep 25;267(27):19278–19290. [PubMed] [Google Scholar]
- Flores E., Schmetterer G. Interaction of fructose with the glucose permease of the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol. 1986 May;166(2):693–696. doi: 10.1128/jb.166.2.693-696.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Haselkorn R. Mutation to herbicide resistance maps within the psbA gene of Anacystis nidulans R2. Science. 1985 Sep 13;229(4718):1104–1107. doi: 10.1126/science.3929379. [DOI] [PubMed] [Google Scholar]
- Golden S. S. Mutagenesis of cyanobacteria by classical and gene-transfer-based methods. Methods Enzymol. 1988;167:714–727. doi: 10.1016/0076-6879(88)67083-2. [DOI] [PubMed] [Google Scholar]
- Grossman A. R., Schaefer M. R., Chiang G. G., Collier J. L. Environmental effects on the light-harvesting complex of cyanobacteria. J Bacteriol. 1993 Feb;175(3):575–582. doi: 10.1128/jb.175.3.575-582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D., Wolk C. P. Identification and characterization of hetA, a gene that acts early in the process of morphological differentiation of heterocysts. J Bacteriol. 1990 Jun;172(6):3131–3137. doi: 10.1128/jb.172.6.3131-3137.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Schwarz R., Lieman-Hurwitz J., Reinhold L. Physiological and molecular aspects of the inorganic carbon-concentrating mechanism in cyanobacteria. Plant Physiol. 1991 Nov;97(3):851–855. doi: 10.1104/pp.97.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenbach D. E., Grossman A. R. Characterization and mutagenesis of sulfur-regulated genes in a cyanobacterium: evidence for function in sulfate transport. J Bacteriol. 1991 May;173(9):2739–2750. doi: 10.1128/jb.173.9.2739-2750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenbach D. E., Herbert S. K., McDowell C., Fork D. C., Grossman A. R., Straus N. A. Cytochrome c-553 is not required for photosynthetic activity in the cyanobacterium Synechococcus. Plant Cell. 1990 Sep;2(9):913–924. doi: 10.1105/tpc.2.9.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. Regulation of cell division in E. coli. Trends Genet. 1990 Jan;6(1):22–25. doi: 10.1016/0168-9525(90)90045-8. [DOI] [PubMed] [Google Scholar]
- Nixon P. J., Diner B. A. Aspartate 170 of the photosystem II reaction center polypeptide D1 is involved in the assembly of the oxygen-evolving manganese cluster. Biochemistry. 1992 Jan 28;31(3):942–948. doi: 10.1021/bi00118a041. [DOI] [PubMed] [Google Scholar]
- Omata T., Ohmori M., Arai N., Ogawa T. Genetically engineered mutant of the cyanobacterium Synechococcus PCC 7942 defective in nitrate transport. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6612–6616. doi: 10.1073/pnas.86.17.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi H. B., De Ciechi P., Whitmarsh J. Site directed mutagenesis of the heme axial ligands of cytochrome b559 affects the stability of the photosystem II complex. EMBO J. 1991 Jul;10(7):1619–1627. doi: 10.1002/j.1460-2075.1991.tb07684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Ray J. M., Bhaya D., Block M. A., Grossman A. R. Isolation, transcription, and inactivation of the gene for an atypical alkaline phosphatase of Synechococcus sp. strain PCC 7942. J Bacteriol. 1991 Jul;173(14):4297–4309. doi: 10.1128/jb.173.14.4297-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M., Schaechter M. Control of cell division in bacteria. Bacteriol Rev. 1974 Jun;38(2):199–221. doi: 10.1128/br.38.2.199-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R. V., Zhou J., Leary J. A., Williams T., de Lorimier R., Bryant D. A., Glazer A. N. Characterization of phycocyanin produced by cpcE and cpcF mutants and identification of an intergenic suppressor of the defect in bilin attachment. J Biol Chem. 1992 Aug 15;267(23):16146–16154. [PubMed] [Google Scholar]
- Tandeau de Marsac N., Borrias W. E., Kuhlemeier C. J., Castets A. M., van Arkel G. A., van den Hondel C. A. A new approach for molecular cloning in cyanobacteria: cloning of an Anacystis nidulans met gene using a Tn901-induced mutant. Gene. 1982 Nov;20(1):111–119. doi: 10.1016/0378-1119(82)90092-0. [DOI] [PubMed] [Google Scholar]
- Thiel T., Leone M. Effect of glutamine on growth and heterocyst differentiation in the cyanobacterium Anabaena variabilis. J Bacteriol. 1986 Nov;168(2):769–774. doi: 10.1128/jb.168.2.769-774.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]