Abstract
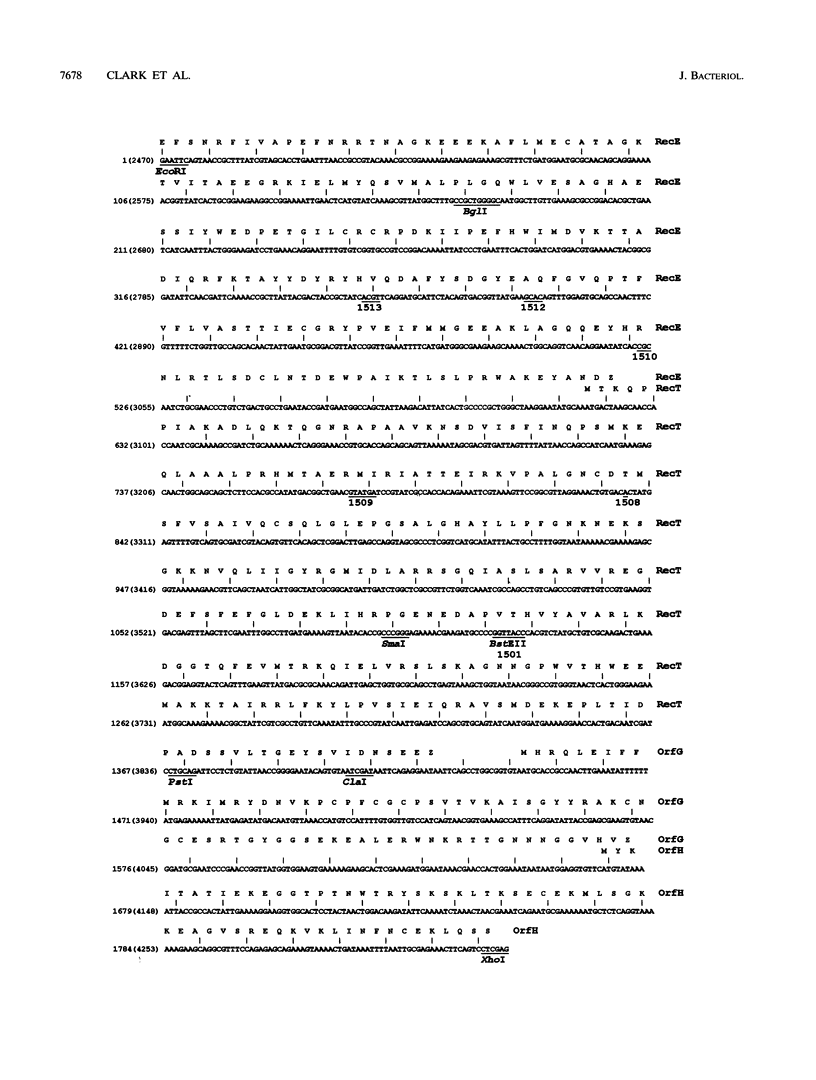
The nucleotide sequence of the C-terminal region of the recE gene of the Rac prophage of Escherichia coli K-12 reveals the presence of a partially overlapping reading frame we call recT. Deletion mutations show that recT is required for the RecE pathway of conjugational recombination. By cloning recT with a plasmid vector compatible with pBR322, we showed by cis-trans tests that the portion of the recE gene encoding ExoVIII DNA nuclease activity is also required for RecE pathway conjugational recombination. The recT gene can replace the redB gene of lambda for recA-independent plasmid recombination. A Tn10 insertion mutation previously thought to be in recE is located in recT and is renamed recT101::Tn10. Discrepancies between the molecular mass estimates of wild-type ExoVIII protein determined from mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and calculated from the predicted amino acid sequence are discussed. The hypothesis that wild-type ExoVIII protein results from fusion of RecE and RecT proteins is disproved genetically, thus supporting a previous hypothesis that the discrepancies are due to abnormal protein mobility in SDS-PAGE. A computer-performed scan of the bacteriophage nucleotide sequence data base of GenBank revealed substantial similarity between most of recE and a 2.5-kb portion of the b2 region of lambda. This suggests interesting speculations concerning the evolutionary relationship of lambda and Rac prophages.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Nagaishi H., Templin A., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. II. Rec+ revertants caused by indirect suppression of rec- mutations. Proc Natl Acad Sci U S A. 1970 Sep;67(1):128–135. doi: 10.1073/pnas.67.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger I., Cohen A. Suppression of recA deficiency in plasmid recombination by bacteriophage lambda beta protein in RecBCD- ExoI- Escherichia coli cells. J Bacteriol. 1989 Jun;171(6):3523–3529. doi: 10.1128/jb.171.6.3523-3529.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J. Genetic analysis of a "double male" strain of Escherichia coli K-12. Genetics. 1963 Jan;48:105–120. doi: 10.1093/genetics/48.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calos M. P., Lebkowski J. S., Botchan M. R. High mutation frequency in DNA transfected into mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3015–3019. doi: 10.1073/pnas.80.10.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
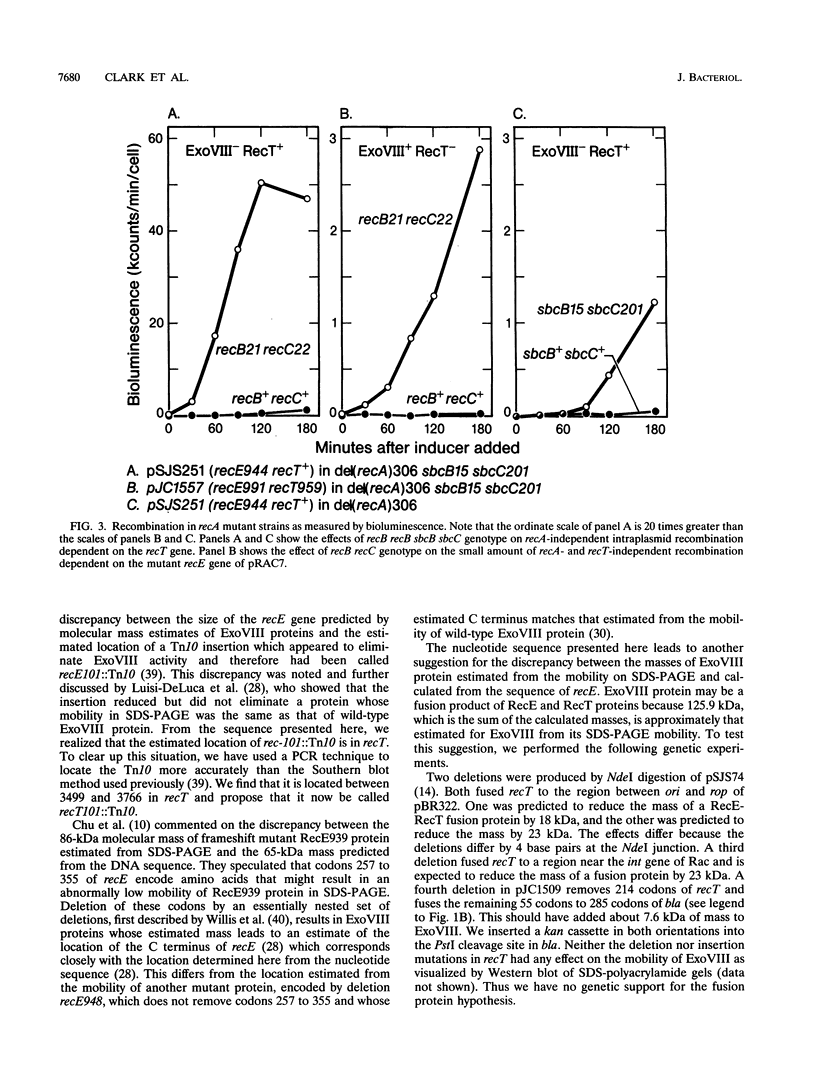
- Chu C. C., Templin A., Clark A. J. Suppression of a frameshift mutation in the recE gene of Escherichia coli K-12 occurs by gene fusion. J Bacteriol. 1989 Apr;171(4):2101–2109. doi: 10.1128/jb.171.4.2101-2109.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Sandler S. J., Willis D. K., Chu C. C., Blanar M. A., Lovett S. T. Genes of the RecE and RecF pathways of conjugational recombination in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1984;49:453–462. doi: 10.1101/sqb.1984.049.01.051. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts K. E., Wasie-Gilbert T., Willis D. K., Clark A. J., Barbour S. D. Genetic analysis of transposon-induced mutations of the Rac prophage in Escherichia coli K-12 which affect expression and function of recE. J Bacteriol. 1983 Nov;156(2):718–726. doi: 10.1128/jb.156.2.718-726.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen J. R., Karu A. E., Nagaishi H., Clark A. J. Characterization of the deoxyribonuclease determined by lambda reverse as exonuclease VIII of Escherichia coli. J Mol Biol. 1977 Jun 15;113(1):27–41. doi: 10.1016/0022-2836(77)90039-0. [DOI] [PubMed] [Google Scholar]
- Hall S. D., Kane M. F., Kolodner R. D. Identification and characterization of the Escherichia coli RecT protein, a protein encoded by the recE region that promotes renaturation of homologous single-stranded DNA. J Bacteriol. 1993 Jan;175(1):277–287. doi: 10.1128/jb.175.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling S. M., Simons R. W., Way J. C., Walsh R. B., Kleckner N. DNA sequence organization of IS10-right of Tn10 and comparison with IS10-left. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2608–2612. doi: 10.1073/pnas.79.8.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Joseph J. W., Kolodner R. Exonuclease VIII of Escherichia coli. I. Purification and physical properties. J Biol Chem. 1983 Sep 10;258(17):10411–10417. [PubMed] [Google Scholar]
- Kmiec E., Holloman W. K. Beta protein of bacteriophage lambda promotes renaturation of DNA. J Biol Chem. 1981 Dec 25;256(24):12636–12639. [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Clark A. J. Isolation of exonuclease VIII: the enzyme associated with sbcA indirect suppressor. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3593–3597. doi: 10.1073/pnas.71.9.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi-DeLuca C., Clark A. J., Kolodner R. D. Analysis of the recE locus of Escherichia coli K-12 by use of polyclonal antibodies to exonuclease VIII. J Bacteriol. 1988 Dec;170(12):5797–5805. doi: 10.1128/jb.170.12.5797-5805.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi-DeLuca C., Lovett S. T., Kolodner R. D. Genetic and physical analysis of plasmid recombination in recB recC sbcB and recB recC sbcA Escherichia coli K-12 mutants. Genetics. 1989 Jun;122(2):269–278. doi: 10.1093/genetics/122.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S. K., Chu C. C., Willis D. K., Templin A., Clark A. J. Physical analysis of spontaneous and mutagen-induced mutants of Escherichia coli K-12 expressing DNA exonuclease VIII activity. Genetics. 1990 Jun;125(2):261–273. doi: 10.1093/genetics/125.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Nussbaum A., Cohen A. Use of a bioluminescence gene reporter for the investigation of red-dependent and gam-dependent plasmid recombination in Escherichia coli K12. J Mol Biol. 1988 Sep 20;203(2):391–402. doi: 10.1016/0022-2836(88)90007-1. [DOI] [PubMed] [Google Scholar]
- Radding C. M. The role of exonuclease and beta protein of bacteriophage lambda in genetic recombination. I. Effects of red mutants on protein structure. J Mol Biol. 1970 Sep 28;52(3):491–499. doi: 10.1016/0022-2836(70)90415-8. [DOI] [PubMed] [Google Scholar]
- Sandler S. J., Clark A. J. Factors affecting expression of the recF gene of Escherichia coli K-12. Gene. 1990 Jan 31;86(1):35–43. doi: 10.1016/0378-1119(90)90111-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M. J., Hallick L. M., Echols H., Signer E. R. Properties of recombination-deficient mutants of bacteriophage lambda. J Mol Biol. 1970 Sep 28;52(3):501–520. doi: 10.1016/0022-2836(70)90416-x. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Willis D. K., Fouts K. E., Barbour S. D., Clark A. J. Restriction nuclease and enzymatic analysis of transposon-induced mutations of the Rac prophage which affect expression and function of recE in Escherichia coli K-12. J Bacteriol. 1983 Nov;156(2):727–736. doi: 10.1128/jb.156.2.727-736.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. K., Satin L. H., Clark A. J. Mutation-dependent suppression of recB21 recC22 by a region cloned from the Rac prophage of Escherichia coli K-12. J Bacteriol. 1985 Jun;162(3):1166–1172. doi: 10.1128/jb.162.3.1166-1172.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


