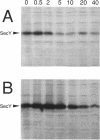
Abstract
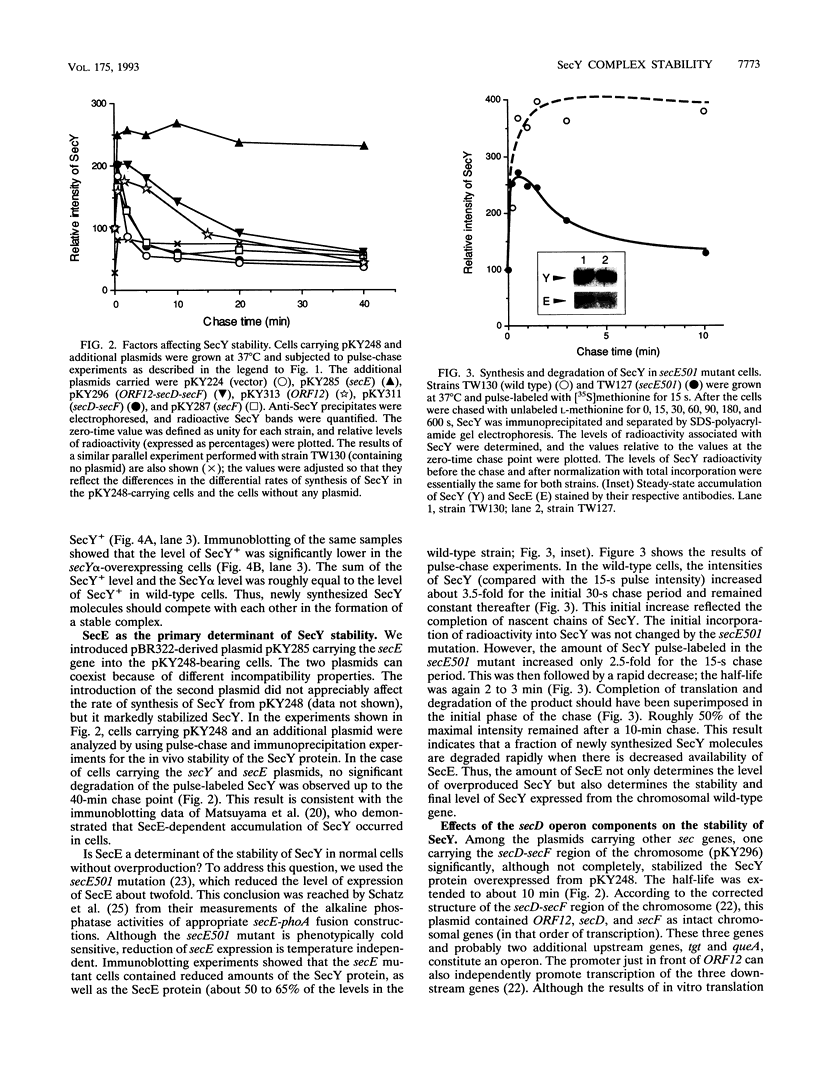
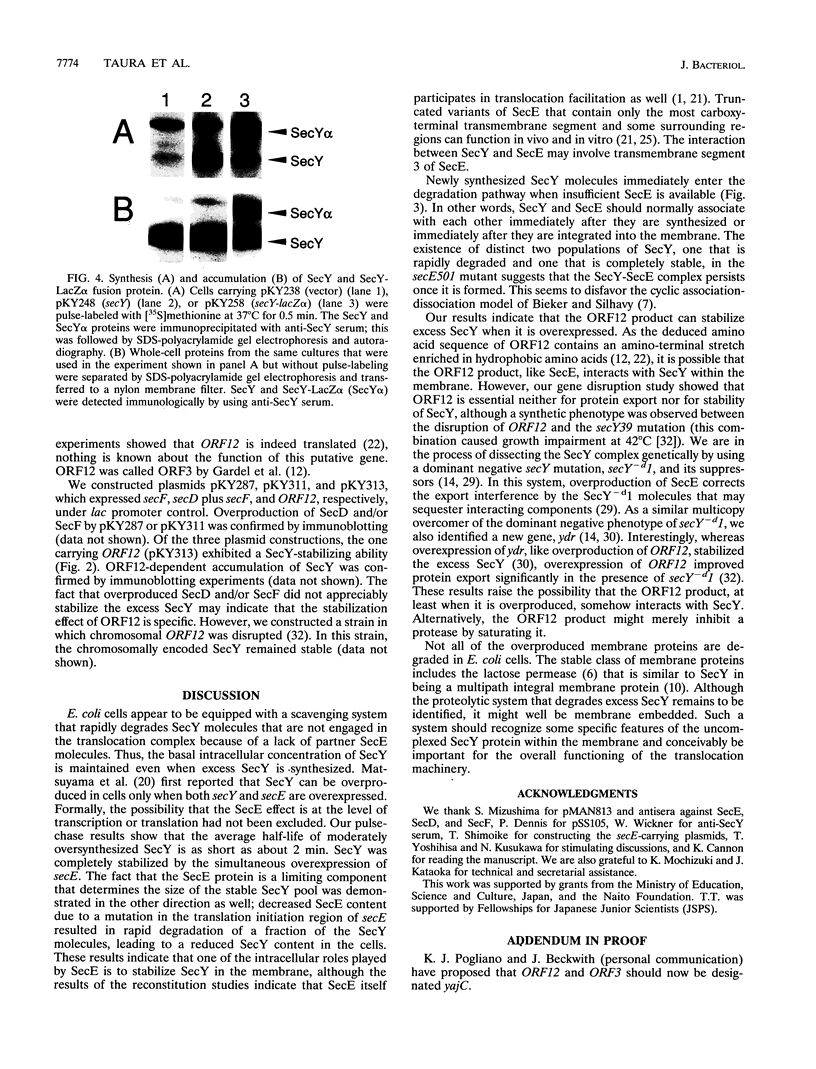
While SecY in wild-type Escherichia coli cells is stable and is complexed with other proteins within the membrane, moderately overexpressed and presumably uncomplexed SecY was degraded with a half-life of 2 min. The fact that the amount of stable SecY is strictly regulated by the degradation of excess SecY was demonstrated by competitive entry of the SecY+ protein and a SecY-LacZ alpha fusion protein into the stable pool. Simultaneous overexpression of SecE led to complete stabilization of excess SecY. Overproduced SecD and SecF did not affect the stability of SecY, but plasmids carrying ORF12 located within the secD-secF operon partially stabilized this protein. In contrast, mutational reduction of the SecE content (but not the ORF12 content) led to the appearance of two populations of newly synthesized SecY molecules, one that was immediately degraded and one that was completely stable. Thus, the E. coli cell is equipped with a system that eliminates SecY unless it is complexed with SecE, a limiting partner of SecY. Our observations implied that in wild-type cells, SecY and SecE rapidly associate with each other and remain complexed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akimaru J., Matsuyama S., Tokuda H., Mizushima S. Reconstitution of a protein translocation system containing purified SecY, SecE, and SecA from Escherichia coli. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6545–6549. doi: 10.1073/pnas.88.15.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama Y., Ito K. Overproduction, isolation and determination of the amino-terminal sequence of the SecY protein, a membrane protein involved in protein export in Escherichia coli. Eur J Biochem. 1986 Sep 1;159(2):263–266. doi: 10.1111/j.1432-1033.1986.tb09862.x. [DOI] [PubMed] [Google Scholar]
- Akiyama Y., Ito K. SecY protein, a membrane-embedded secretion factor of E. coli, is cleaved by the ompT protease in vitro. Biochem Biophys Res Commun. 1990 Mar 16;167(2):711–715. doi: 10.1016/0006-291x(90)92083-c. [DOI] [PubMed] [Google Scholar]
- Akiyama Y., Ito K. The SecY membrane component of the bacterial protein export machinery: analysis by new electrophoretic methods for integral membrane proteins. EMBO J. 1985 Dec 1;4(12):3351–3356. doi: 10.1002/j.1460-2075.1985.tb04088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama Y., Ito K. Topology analysis of the SecY protein, an integral membrane protein involved in protein export in Escherichia coli. EMBO J. 1987 Nov;6(11):3465–3470. doi: 10.1002/j.1460-2075.1987.tb02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieker K. L., Silhavy T. J. PrlA (SecY) and PrlG (SecE) interact directly and function sequentially during protein translocation in E. coli. Cell. 1990 Jun 1;61(5):833–842. doi: 10.1016/0092-8674(90)90193-i. [DOI] [PubMed] [Google Scholar]
- Brundage L., Fimmel C. J., Mizushima S., Wickner W. SecY, SecE, and band 1 form the membrane-embedded domain of Escherichia coli preprotein translocase. J Biol Chem. 1992 Feb 25;267(6):4166–4170. [PubMed] [Google Scholar]
- Brundage L., Hendrick J. P., Schiebel E., Driessen A. J., Wickner W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell. 1990 Aug 24;62(4):649–657. doi: 10.1016/0092-8674(90)90111-q. [DOI] [PubMed] [Google Scholar]
- Calamia J., Manoil C. lac permease of Escherichia coli: topology and sequence elements promoting membrane insertion. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4937–4941. doi: 10.1073/pnas.87.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing W. L., Sullivan S. L., Gottesman M. E., Dennis P. P. Sequence and transcriptional pattern of the essential Escherichia coli secE-nusG operon. J Bacteriol. 1990 Mar;172(3):1621–1627. doi: 10.1128/jb.172.3.1621-1627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel C., Johnson K., Jacq A., Beckwith J. The secD locus of E.coli codes for two membrane proteins required for protein export. EMBO J. 1990 Oct;9(10):3209–3216. doi: 10.1002/j.1460-2075.1990.tb07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Akiyama Y. In vivo analysis of integration of membrane proteins in Escherichia coli. Mol Microbiol. 1991 Sep;5(9):2243–2253. doi: 10.1111/j.1365-2958.1991.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Ito K., Akiyama Y. Membrane components of the protein secretion machinery. Methods Cell Biol. 1991;34:189–203. doi: 10.1016/s0091-679x(08)61681-7. [DOI] [PubMed] [Google Scholar]
- Ito K. Identification of the secY (prlA) gene product involved in protein export in Escherichia coli. Mol Gen Genet. 1984;197(2):204–208. doi: 10.1007/BF00330964. [DOI] [PubMed] [Google Scholar]
- Ito K. SecY and integral membrane components of the Escherichia coli protein translocation system. Mol Microbiol. 1992 Sep;6(17):2423–2428. doi: 10.1111/j.1365-2958.1992.tb01417.x. [DOI] [PubMed] [Google Scholar]
- Ito K., Wittekind M., Nomura M., Shiba K., Yura T., Miura A., Nashimoto H. A temperature-sensitive mutant of E. coli exhibiting slow processing of exported proteins. Cell. 1983 Mar;32(3):789–797. doi: 10.1016/0092-8674(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lill R., Cunningham K., Brundage L. A., Ito K., Oliver D., Wickner W. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 1989 Mar;8(3):961–966. doi: 10.1002/j.1460-2075.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Akimaru J., Mizushima S. SecE-dependent overproduction of SecY in Escherichia coli. Evidence for interaction between two components of the secretory machinery. FEBS Lett. 1990 Aug 20;269(1):96–100. doi: 10.1016/0014-5793(90)81128-b. [DOI] [PubMed] [Google Scholar]
- Nishiyama K., Mizushima S., Tokuda H. The carboxyl-terminal region of SecE interacts with SecY and is functional in the reconstitution of protein translocation activity in Escherichia coli. J Biol Chem. 1992 Apr 5;267(10):7170–7176. [PubMed] [Google Scholar]
- Reuter K., Slany R., Ullrich F., Kersten H. Structure and organization of Escherichia coli genes involved in biosynthesis of the deazaguanine derivative queuine, a nutrient factor for eukaryotes. J Bacteriol. 1991 Apr;173(7):2256–2264. doi: 10.1128/jb.173.7.2256-2264.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs P. D., Derman A. I., Beckwith J. A mutation affecting the regulation of a secA-lacZ fusion defines a new sec gene. Genetics. 1988 Apr;118(4):571–579. doi: 10.1093/genetics/118.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Schatz P. J., Bieker K. L., Ottemann K. M., Silhavy T. J., Beckwith J. One of three transmembrane stretches is sufficient for the functioning of the SecE protein, a membrane component of the E. coli secretion machinery. EMBO J. 1991 Jul;10(7):1749–1757. doi: 10.1002/j.1460-2075.1991.tb07699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Riggs P. D., Jacq A., Fath M. J., Beckwith J. The secE gene encodes an integral membrane protein required for protein export in Escherichia coli. Genes Dev. 1989 Jul;3(7):1035–1044. doi: 10.1101/gad.3.7.1035. [DOI] [PubMed] [Google Scholar]
- Shiba K., Ito K., Yura T., Cerretti D. P. A defined mutation in the protein export gene within the spc ribosomal protein operon of Escherichia coli: isolation and characterization of a new temperature-sensitive secY mutant. EMBO J. 1984 Mar;3(3):631–635. doi: 10.1002/j.1460-2075.1984.tb01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoike T., Akiyama Y., Baba T., Taura T., Ito K. SecY variants that interfere with Escherichia coli protein export in the presence of normal secY. Mol Microbiol. 1992 May;6(9):1205–1210. doi: 10.1111/j.1365-2958.1992.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Ueguchi C., Ito K. Multicopy suppression: an approach to understanding intracellular functioning of the protein export system. J Bacteriol. 1992 Mar;174(5):1454–1461. doi: 10.1128/jb.174.5.1454-1461.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wickner W., Driessen A. J., Hartl F. U. The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem. 1991;60:101–124. doi: 10.1146/annurev.bi.60.070191.000533. [DOI] [PubMed] [Google Scholar]