Abstract
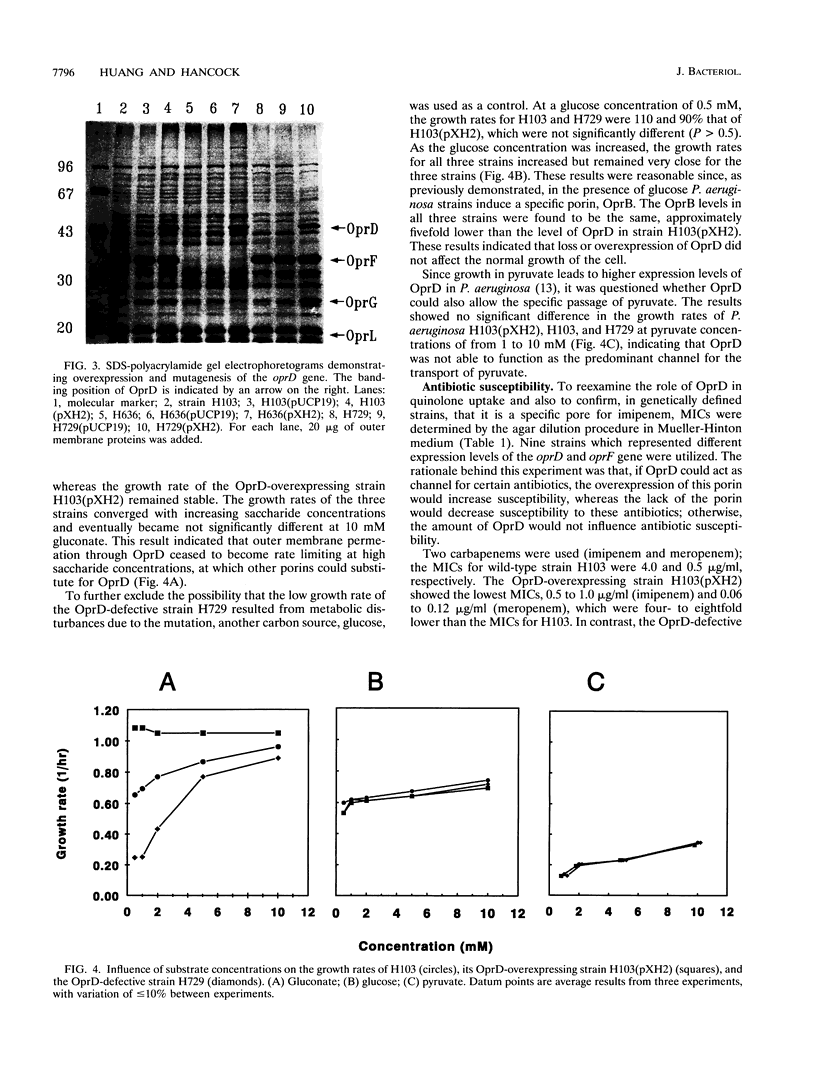
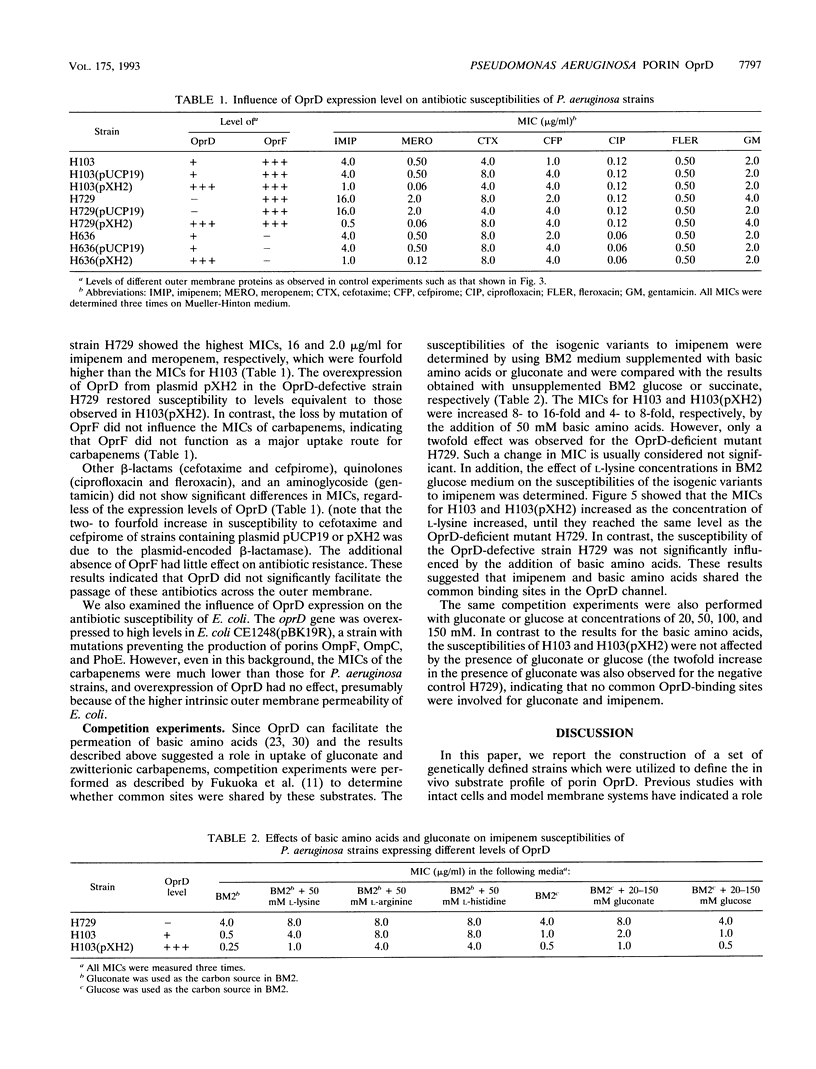
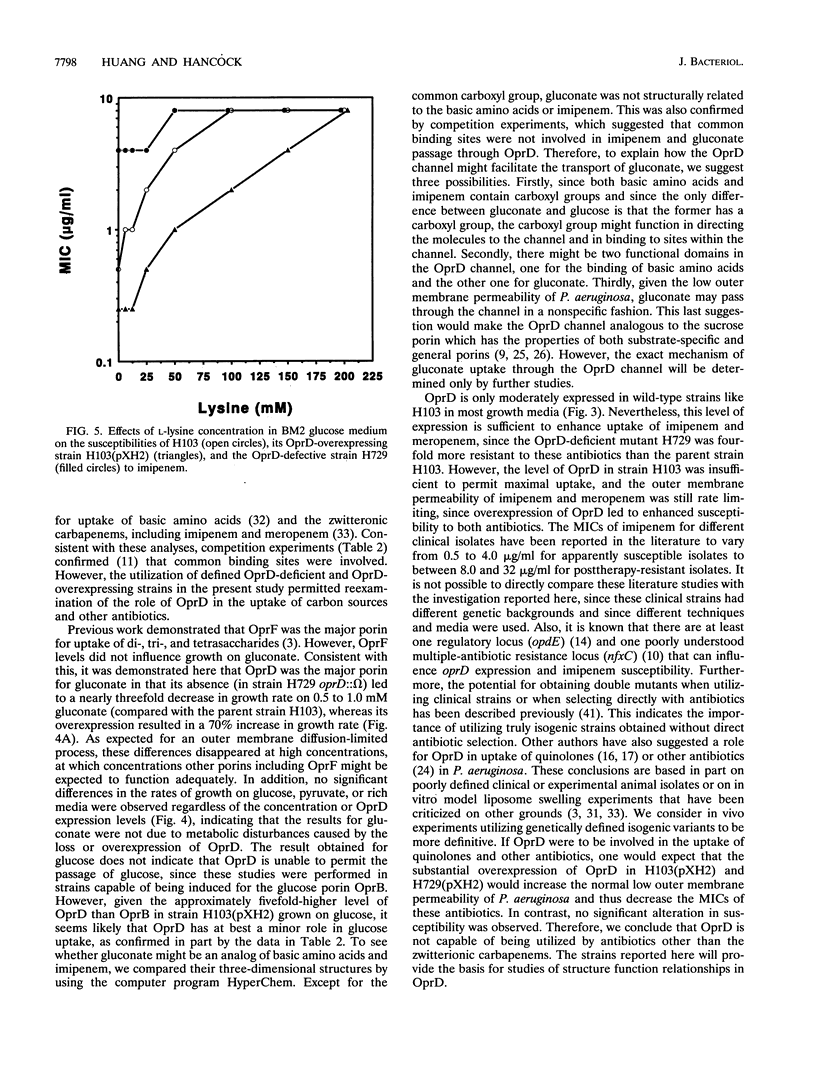
Earlier studies proved that Pseudomonas aeruginosa OprD is a specific porin for basic amino acids and imipenem. It was also considered to function as a nonspecific porin that allowed the size-dependent uptake of monosaccharides and facilitation of the uptake of quinolone and other antibiotics. In the present study, we utilized P. aeruginosa strains with genetically defined levels of OprD to characterize the in vivo substrate selectivity of this porin. An oprD::omega interposon mutant was constructed by gene replacement utilizing an in vitro mutagenized cloned oprD gene. In addition, OprD was overexpressed from the lac promoter by cloning the oprD gene into the broad-host-range plasmid pUCP19. To test the substrate selectivity, strains were grown in minimal medium with limiting concentrations of the carbon sources glucose, gluconate, or pyruvate. In minimal medium with 0.5 mM gluconate, the growth rates of the parent strain H103 and its oprD::omega mutant H729 were only 60 and 20%, respectively, of that of the OprD-overexpressing strain H103(pXH2). In contrast, no significant differences were observed in the growth rates of these three strains on glucose or pyruvate, indicating that OprD selectively facilitated the transport of gluconate. To determine the role of OprD in antibiotic uptake, nine strains representing different levels of OprD and OprF were used to determine the MICs of different antibiotics. The results clearly demonstrated that OprD could be utilized by imipenem and meropenem but that, even when substantially overexpressed, it could not be significantly utilized by other beta-lactams, quinolones, or aminoglycosides. In addition, competition experiments confirmed that imipenem had common binding sites with basic amino acids in the OprD channel, but not with gluconate or glucose.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard J., Chamberland S., Wong S., Schollaardt T., Bryan L. E. Contribution of permeability and sensitivity to inhibition of DNA synthesis in determining susceptibilities of Escherichia coli, Pseudomonas aeruginosa, and Alcaligenes faecalis to ciprofloxacin. Antimicrob Agents Chemother. 1989 Sep;33(9):1457–1464. doi: 10.1128/aac.33.9.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido F., Martin N. L., Siehnel R. J., Hancock R. E. Reevaluation, using intact cells, of the exclusion limit and role of porin OprF in Pseudomonas aeruginosa outer membrane permeability. J Bacteriol. 1992 Aug;174(16):5196–5203. doi: 10.1128/jb.174.16.5196-5203.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Hancock R. E. Mechanism of ion transport through the anion-selective channel of the Pseudomonas aeruginosa outer membrane. J Gen Physiol. 1987 Feb;89(2):275–295. doi: 10.1085/jgp.89.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büscher K. H., Cullmann W., Dick W., Opferkuch W. Imipenem resistance in Pseudomonas aeruginosa resulting from diminished expression of an outer membrane protein. Antimicrob Agents Chemother. 1987 May;31(5):703–708. doi: 10.1128/aac.31.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büscher K. H., Cullmann W., Dick W., Wendt S., Opferkuch W. Imipenem resistance in Pseudomonas aeruginosa is due to diminished expression of outer membrane proteins. J Infect Dis. 1987 Oct;156(4):681–684. doi: 10.1093/infdis/156.4.681. [DOI] [PubMed] [Google Scholar]
- Büscher K. H., Cullmann W., Opferkuch W. Resistance of Pseudomonas aeruginosa to imipenem is independent of beta-lactamase production. J Antimicrob Chemother. 1987 May;19(5):700–701. doi: 10.1093/jac/19.5.700. [DOI] [PubMed] [Google Scholar]
- Daikos G. L., Lolans V. T., Jackson G. G. Alterations in outer membrane proteins of Pseudomonas aeruginosa associated with selective resistance to quinolones. Antimicrob Agents Chemother. 1988 May;32(5):785–787. doi: 10.1128/aac.32.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst D., Schülein K., Wacker T., Diederichs K., Kreutz W., Benz R., Welte W. Crystallization and preliminary X-ray diffraction analysis of ScrY, a specific bacterial outer membrane porin. J Mol Biol. 1993 Jan 5;229(1):258–262. doi: 10.1006/jmbi.1993.1028. [DOI] [PubMed] [Google Scholar]
- Fukuda H., Hosaka M., Hirai K., Iyobe S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990 Sep;34(9):1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka T., Masuda N., Takenouchi T., Sekine N., Iijima M., Ohya S. Increase in susceptibility of Pseudomonas aeruginosa to carbapenem antibiotics in low-amino-acid media. Antimicrob Agents Chemother. 1991 Mar;35(3):529–532. doi: 10.1128/aac.35.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka T., Ohya S., Narita T., Katsuta M., Iijima M., Masuda N., Yasuda H., Trias J., Nikaido H. Activity of the carbapenem panipenem and role of the OprD (D2) protein in its diffusion through the Pseudomonas aeruginosa outer membrane. Antimicrob Agents Chemother. 1993 Feb;37(2):322–327. doi: 10.1128/aac.37.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Egli C., Benz R., Siehnel R. J. Overexpression in Escherichia coli and functional analysis of a novel PPi-selective porin, oprO, from Pseudomonas aeruginosa. J Bacteriol. 1992 Jan;174(2):471–476. doi: 10.1128/jb.174.2.471-476.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
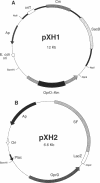
- Huang H., Siehnel R. J., Bellido F., Rawling E., Hancock R. E. Analysis of two gene regions involved in the expression of the imipenem-specific, outer membrane porin protein OprD of Pseudomonas aeruginosa. FEMS Microbiol Lett. 1992 Oct 15;76(3):267–273. doi: 10.1016/0378-1097(92)90347-q. [DOI] [PubMed] [Google Scholar]
- Lynch M. J., Drusano G. L., Mobley H. L. Emergence of resistance to imipenem in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Dec;31(12):1892–1896. doi: 10.1128/aac.31.12.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michea-Hamzehpour M., Lucain C., Pechere J. C. Resistance to pefloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991 Mar;35(3):512–518. doi: 10.1128/aac.35.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michéa-Hamzehpour M., Furet Y. X., Pechère J. C. Role of protein D2 and lipopolysaccharide in diffusion of quinolones through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991 Oct;35(10):2091–2097. doi: 10.1128/aac.35.10.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980 Aug;143(2):872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
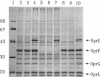
- Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989 Nov;33(11):1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., Dudek E. J., DiVincenzo C. A., Lucks D. A., Lerner S. A. Emergence of resistance to imipenem during therapy for Pseudomonas aeruginosa infections. J Infect Dis. 1986 Aug;154(2):289–294. doi: 10.1093/infdis/154.2.289. [DOI] [PubMed] [Google Scholar]
- Satake S., Yoshihara E., Nakae T. Diffusion of beta-lactam antibiotics through liposome membranes reconstituted from purified porins of the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 May;34(5):685–690. doi: 10.1128/aac.34.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Ebner R., Jahreis K., Lengeler J. W., Titgemeyer F. A sugar-specific porin, ScrY, is involved in sucrose uptake in enteric bacteria. Mol Microbiol. 1991 Apr;5(4):941–950. doi: 10.1111/j.1365-2958.1991.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Schweizer H. P. Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol Microbiol. 1992 May;6(9):1195–1204. doi: 10.1111/j.1365-2958.1992.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Schweizer H. P. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene. 1991 Jan 2;97(1):109–121. doi: 10.1016/0378-1119(91)90016-5. [DOI] [PubMed] [Google Scholar]
- Schülein K., Schmid K., Benzl R. The sugar-specific outer membrane channel ScrY contains functional characteristics of general diffusion pores and substrate-specific porins. Mol Microbiol. 1991 Sep;5(9):2233–2241. doi: 10.1111/j.1365-2958.1991.tb02153.x. [DOI] [PubMed] [Google Scholar]
- Trias J., Dufresne J., Levesque R. C., Nikaido H. Decreased outer membrane permeability in imipenem-resistant mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989 Aug;33(8):1202–1206. doi: 10.1128/aac.33.8.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Nikaido H. Outer membrane protein D2 catalyzes facilitated diffusion of carbapenems and penems through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jan;34(1):52–57. doi: 10.1128/aac.34.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Nikaido H. Protein D2 channel of the Pseudomonas aeruginosa outer membrane has a binding site for basic amino acids and peptides. J Biol Chem. 1990 Sep 15;265(26):15680–15684. [PubMed] [Google Scholar]
- Trias J., Rosenberg E. Y., Nikaido H. Specificity of the glucose channel formed by protein D1 of Pseudomonas aeruginosa. Biochim Biophys Acta. 1988 Mar 3;938(3):493–496. doi: 10.1016/0005-2736(88)90148-4. [DOI] [PubMed] [Google Scholar]
- Watanabe N. A., Nagasu T., Katsu K., Kitoh K. E-0702, a new cephalosporin, is incorporated into Escherichia coli cells via the tonB-dependent iron transport system. Antimicrob Agents Chemother. 1987 Apr;31(4):497–504. doi: 10.1128/aac.31.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. D. Activity of imipenem against Pseudomonas and Bacteroides species. Rev Infect Dis. 1985 Jul-Aug;7 (Suppl 3):S411–S416. doi: 10.1093/clinids/7.supplement_3.s411. [DOI] [PubMed] [Google Scholar]
- Woodruff W. A., Hancock R. E. Construction and characterization of Pseudomonas aeruginosa protein F-deficient mutants after in vitro and in vivo insertion mutagenesis of the cloned gene. J Bacteriol. 1988 Jun;170(6):2592–2598. doi: 10.1128/jb.170.6.2592-2598.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara E., Nakae T. Identification of porins in the outer membrane of Pseudomonas aeruginosa that form small diffusion pores. J Biol Chem. 1989 Apr 15;264(11):6297–6301. [PubMed] [Google Scholar]
- Yoshihara E., Yoneyama H., Nakae T. In vitro assembly of the functional porin trimer from dissociated monomers in Pseudomonas aeruginosa. J Biol Chem. 1991 Jan 15;266(2):952–957. [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J Bacteriol. 1982 Nov;152(2):636–642. doi: 10.1128/jb.152.2.636-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. Y., Kitzis M. D., Gutmann L. Role of cephalosporinase in carbapenem resistance of clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993 Jun;37(6):1387–1389. doi: 10.1128/aac.37.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]