Abstract
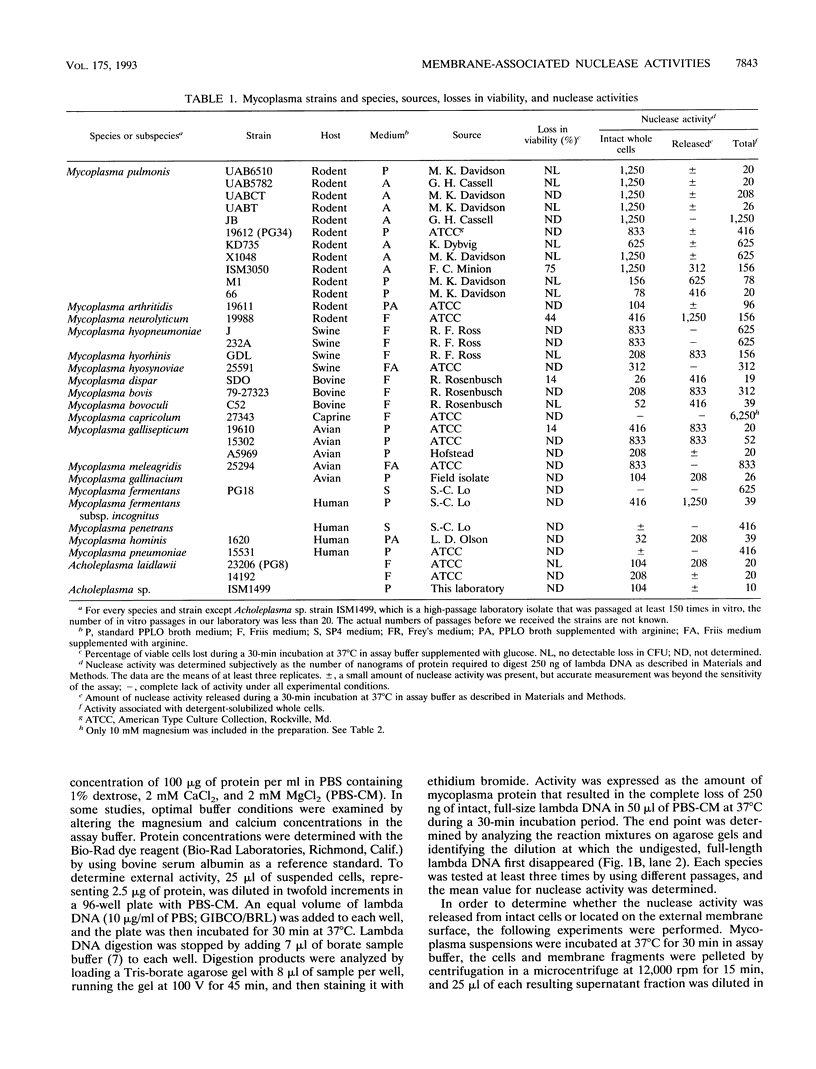
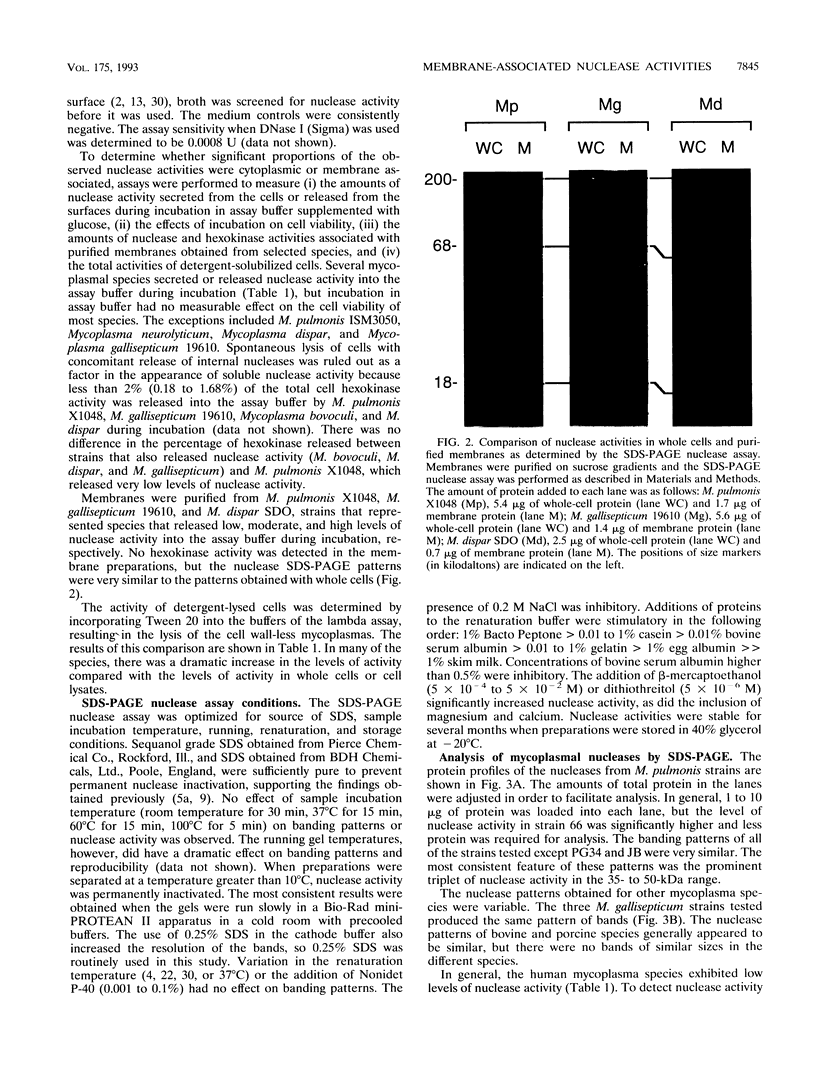
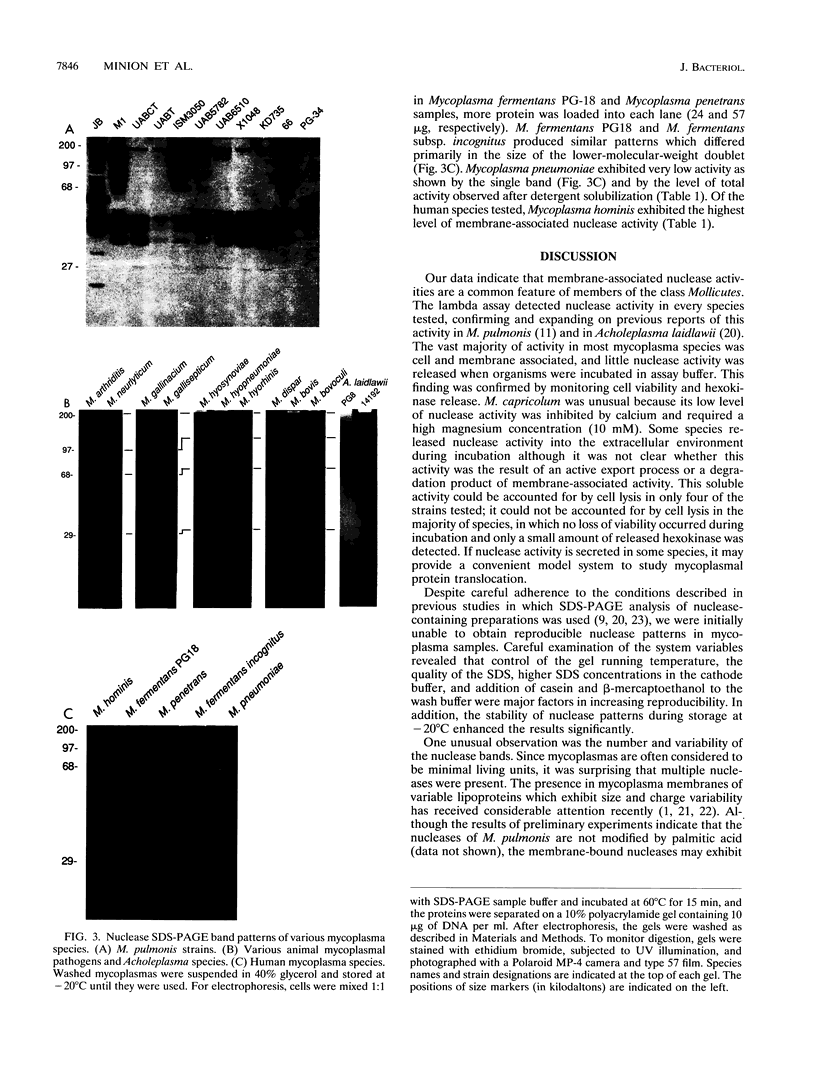
Membrane-associated nucleases of various mycoplasmal species were investigated by using two nuclease assays. A lambda DNA assay was developed to measure nuclease activity associated with whole-cell suspensions, activity released from intact cells, and activity associated with detergent-disrupted cells. In most species, nuclease activities were entirely membrane associated, and disruption by a detergent had a stimulatory effect on these activities. All mycoplasmal species contained nuclease activity, but Mycoplasma capricolum was unusual because its activity was dependent upon magnesium and was inhibited by calcium. We developed a sodium dodecyl sulfate-polyacrylamide gel electrophoresis system that produced reproducible nuclease patterns, and this system was used to determine the apparent molecular weights of the nuclease proteins. An examination of 20 mycoplasmal species failed to identify common bands in their nuclease patterns. An examination of 11 Mycoplasma pulmonis strains, however, indicated that nuclease patterns on polyacrylamide gels may provide a means for categorizing strains within a species. Our results suggest that nucleases are important constituents of mycoplasmal membranes and may be involved in the acquisition of host nucleic acids required for growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer M. J., Wise K. S. Lipid-modified surface protein antigens expressing size variation within the species Mycoplasma hyorhinis. Infect Immun. 1989 Jan;57(1):245–254. doi: 10.1128/iai.57.1.245-254.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury J. M., Jordan F. T. Studies on the adsorption of certain medium proteins to Mycoplasma gallisepticum and their influence on agglutination and haemagglutination reactions. J Hyg (Lond) 1972 Jun;70(2):267–278. doi: 10.1017/s0022172400022324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Atkin C. L. The Mycoplasma arthritidis T-cell mitogen, MAM: a model superantigen. Immunol Today. 1991 Aug;12(8):271–276. doi: 10.1016/0167-5699(91)90125-D. [DOI] [PubMed] [Google Scholar]
- Davidson M. K., Lindsey J. R., Parker R. F., Tully J. G., Cassell G. H. Differences in virulence for mice among strains of Mycoplasma pulmonis. Infect Immun. 1988 Aug;56(8):2156–2162. doi: 10.1128/iai.56.8.2156-2162.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. A., Springhorn S. S., Rosenthal A. L. Effect of the composition of sodium dodecyl sulfate preparations on the renaturation of enzymes after polyacrylamide gel electrophoresis. Anal Biochem. 1979 Dec;100(2):357–363. doi: 10.1016/0003-2697(79)90241-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Yoshida I. Mycoplasmas produce double-stranded ribonuclease. J Cell Physiol. 1990 Jun;143(3):416–419. doi: 10.1002/jcp.1041430303. [DOI] [PubMed] [Google Scholar]
- McGrew B. R., Green D. M. Enhanced removal of detergent and recovery of enzymatic activity following sodium dodecyl sulfate-polyacrylamide gel electrophoresis: use of casein in gel wash buffer. Anal Biochem. 1990 Aug 15;189(1):68–74. doi: 10.1016/0003-2697(90)90045-b. [DOI] [PubMed] [Google Scholar]
- Minion F. C. Correlation of Mycoplasma-pulmonis-mediated hemolysis with translocation of a fluorescent cholesterol probe. Isr J Med Sci. 1987 May;23(5):458–461. [PubMed] [Google Scholar]
- Minion F. C., Goguen J. D. Identification and preliminary characterization of external membrane-bound nuclease activities in Mycoplasma pulmonis. Infect Immun. 1986 Jan;51(1):352–354. doi: 10.1128/iai.51.1.352-354.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolet J., Paroz P., Kristensen B. Growth medium constituents contaminating mycoplasma preparations and their role in the study of membrane glycoproteins in porcine mycoplasmas. J Gen Microbiol. 1980 Jul;119(1):17–26. doi: 10.1099/00221287-119-1-17. [DOI] [PubMed] [Google Scholar]
- Pollack J. D., Hoffmann P. J. Properties of the nucleases of mollicutes. J Bacteriol. 1982 Oct;152(1):538–541. doi: 10.1128/jb.152.1.538-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZIN S., KNIGHT B. C. The effects of ribonucleic acid and deoxyribonucleic acid on the growth of Myeoplasma. J Gen Microbiol. 1960 Apr;22:504–519. doi: 10.1099/00221287-22-2-504. [DOI] [PubMed] [Google Scholar]
- Razin S., Gottfried L., Rottem S. Amino acid transport in Mycoplasma. J Bacteriol. 1968 May;95(5):1685–1691. doi: 10.1128/jb.95.5.1685-1691.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roganti F. S., Rosenthal A. L. DNases of Acholeplasma spp. J Bacteriol. 1983 Aug;155(2):802–805. doi: 10.1128/jb.155.2.802-805.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. Phenotypic switching in mycoplasmas: phase variation of diverse surface lipoproteins. Science. 1990 Jan 19;247(4940):315–318. doi: 10.1126/science.1688663. [DOI] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. The Vlp system of Mycoplasma hyorhinis: combinatorial expression of distinct size variant lipoproteins generating high-frequency surface antigenic variation. J Bacteriol. 1991 Aug;173(15):4782–4793. doi: 10.1128/jb.173.15.4782-4793.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. L., Lacks S. A. Nuclease detection in SDS-polyacrylamide gel electrophoresis. Anal Biochem. 1977 May 15;80(1):76–90. doi: 10.1016/0003-2697(77)90627-3. [DOI] [PubMed] [Google Scholar]
- Stuart P. M., Cassell G. H., Woodward J. G. Induction of class II MHC antigen expression in macrophages by Mycoplasma species. J Immunol. 1989 May 15;142(10):3392–3399. [PubMed] [Google Scholar]
- Takema M., Oka S., Uno K., Nakamura S., Arita H., Tawara K., Inaba K., Muramatsu S. Macrophage-activating factor extracted from mycoplasmas. Cancer Immunol Immunother. 1991;33(1):39–44. doi: 10.1007/BF01742526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunekawa H., Takagi E., Kishimoto H., Shimokata K. Depressed cellular immunity in Mycoplasma pneumoniae pneumonia. Eur J Respir Dis. 1987 May;70(5):293–299. [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Clark H. F., Williamson D. L. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science. 1977 Mar 4;195(4281):892–894. doi: 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]
- Wise K. S., Cassell G. H., Action R. T. Selective association of murine T lymphoblastoid cell surface alloantigens with Mycoplasma hyorhinis. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4479–4483. doi: 10.1073/pnas.75.9.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Minion F. C., Cheung H. C. Translocation of Thy-1 antigen and a fluorescent lipid probe during lymphoblastoid cell interaction with Mycoplasma hyorhinis. Rev Infect Dis. 1982 May-Jun;4 (Suppl):S210–S218. doi: 10.1093/clinids/4.supplement_1.s210. [DOI] [PubMed] [Google Scholar]
- Yaguzhinskaya O. E. Detection of serum proteins in the electrophoretic patterns of total proteins of mycoplasma cells. J Hyg (Lond) 1976 Oct;77(2):189–198. doi: 10.1017/s002217240002461x. [DOI] [PMC free article] [PubMed] [Google Scholar]