Abstract
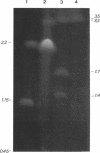
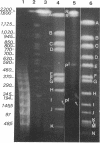
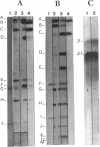
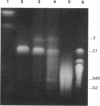
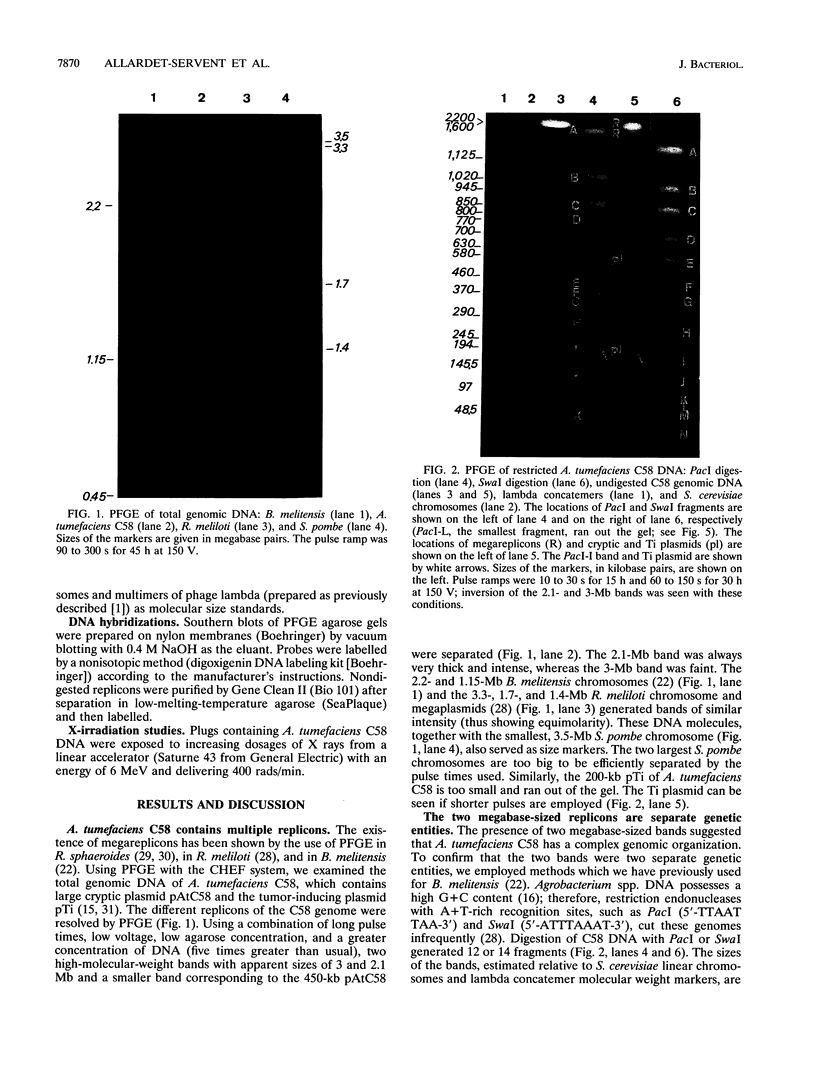
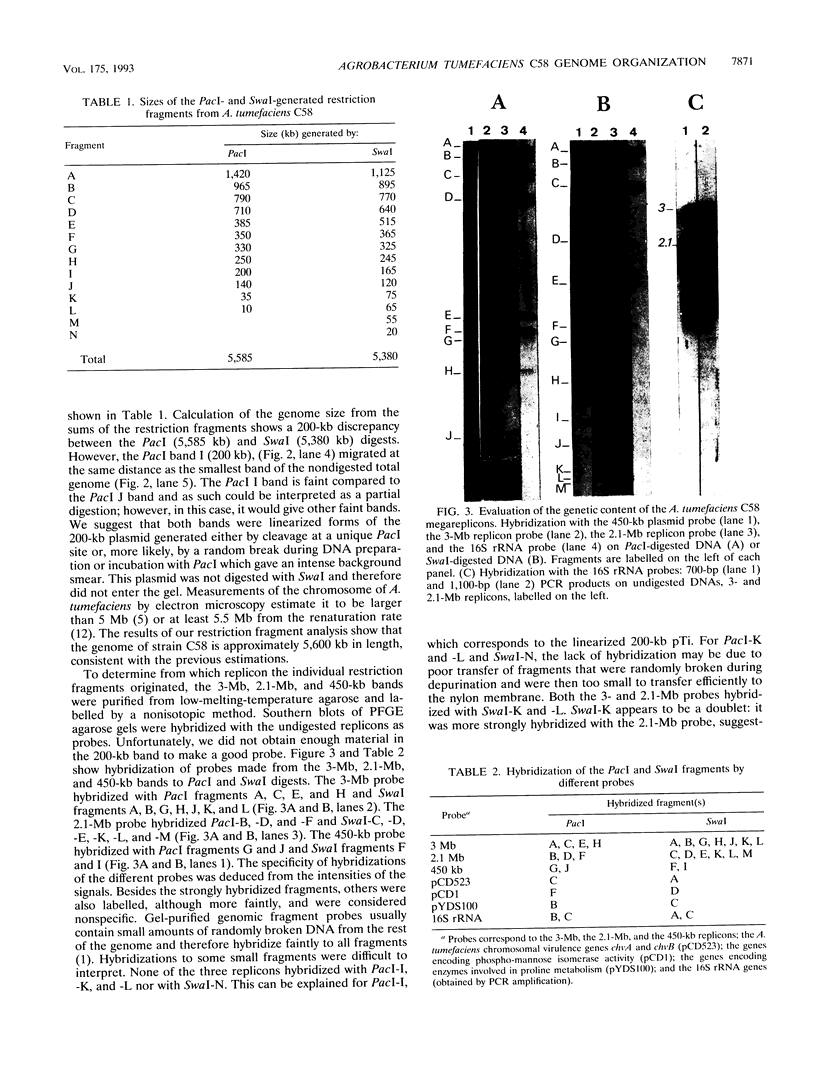
Analysis of the entire Agrobacterium tumefaciens C58 genome by pulsed-field gel electrophoresis (PFGE) reveals four replicons: two large molecules of 3,000 and 2,100 kb, the 450-kb cryptic plasmid, and the 200-kb Ti plasmid. Digestion by PacI or SwaI generated 12 or 14 fragments, respectively. The two megabase-sized replicons, used as probes, hybridize with different restriction fragments, showing that these replicons are two independent genetic entities. A 16S rRNA probe and genes encoding functions essential to the metabolism of the organism were found to hybridize with both replicons, suggesting their chromosomal nature. In PFGE, megabase-sized circular DNA does not enter the gel. The 2.1-Mb chromosome always generated an intense band, while the 3-Mb band was barely visible. After linearization of the DNA by X-irradiation, the intensity of the 3-Mb band increased while that of the 2.1-Mb remained constant. This suggests that the 3-Mb chromosome is circular and that the 2.1-Mb chromosome is linear. To confirm this hypothesis, genomic DNA, trapped in an agarose plug, was first submitted to PFGE to remove any linear DNA present. The plug was then recovered, and the remaining DNA was digested with either PacI or SwaI and then separated by PFGE. The fragments corresponding to the small chromosome were found to be absent, while those corresponding to the circular replicon remained, further proof of the linear nature of the 2.1-Mb chromosome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Carles-Nurit M. J., Bourg G., Michaux S., Ramuz M. Physical map of the Brucella melitensis 16 M chromosome. J Bacteriol. 1991 Apr;173(7):2219–2224. doi: 10.1128/jb.173.7.2219-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F. Biology of Borrelia species. Microbiol Rev. 1986 Dec;50(4):381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkardt B., Schillik D., Pühler A. Physical characterization of Rhizobium meliloti megaplasmids. Plasmid. 1987 Jan;17(1):13–25. doi: 10.1016/0147-619x(87)90004-7. [DOI] [PubMed] [Google Scholar]
- Crespi M., Messens E., Caplan A. B., van Montagu M., Desomer J. Fasciation induction by the phytopathogen Rhodococcus fascians depends upon a linear plasmid encoding a cytokinin synthase gene. EMBO J. 1992 Mar;11(3):795–804. doi: 10.1002/j.1460-2075.1992.tb05116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B. E., MacDougall J., Saint Girons I. Physical map of the linear chromosome of the bacterium Borrelia burgdorferi 212, a causative agent of Lyme disease, and localization of rRNA genes. J Bacteriol. 1992 Jun;174(11):3766–3774. doi: 10.1128/jb.174.11.3766-3774.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J., Staneloni R. J., Rubin R. A., Nester E. W. Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J Bacteriol. 1985 Mar;161(3):850–860. doi: 10.1128/jb.161.3.850-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonstein M., Zheng S., Haselkorn R. Physical map of the genome of Rhodobacter capsulatus SB 1003. J Bacteriol. 1992 Jun;174(12):4070–4077. doi: 10.1128/jb.174.12.4070-4077.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frothingham R., Allen R. L., Wilson K. H. Rapid 16S ribosomal DNA sequencing from a single colony without DNA extraction or purification. Biotechniques. 1991 Jul;11(1):40–44. [PubMed] [Google Scholar]
- Hinnebusch J., Barbour A. G. Linear plasmids of Borrelia burgdorferi have a telomeric structure and sequence similar to those of a eukaryotic virus. J Bacteriol. 1991 Nov;173(22):7233–7239. doi: 10.1128/jb.173.22.7233-7239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. F., Simon R., Pühler A. The development of plasmid-free strains of Agrobacterium tumefaciens by using incompatibility with a Rhizobium meliloti plasmid to eliminate pAtC58. Plasmid. 1985 Mar;13(2):99–105. doi: 10.1016/0147-619x(85)90062-9. [DOI] [PubMed] [Google Scholar]
- Krawiec S., Riley M. Organization of the bacterial chromosome. Microbiol Rev. 1990 Dec;54(4):502–539. doi: 10.1128/mr.54.4.502-539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kündig C., Hennecke H., Göttfert M. Correlated physical and genetic map of the Bradyrhizobium japonicum 110 genome. J Bacteriol. 1993 Feb;175(3):613–622. doi: 10.1128/jb.175.3.613-622.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond P., Redenbach M., Cullum J. Physical map of the Streptomyces lividans 66 genome and comparison with that of the related strain Streptomyces coelicolor A3(2). J Bacteriol. 1993 Jun;175(11):3422–3429. doi: 10.1128/jb.175.11.3422-3429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene S. D., Zimm B. H. Separations of open-circular DNA using pulsed-field electrophoresis. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4054–4057. doi: 10.1073/pnas.84.12.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaux S., Paillisson J., Carles-Nurit M. J., Bourg G., Allardet-Servent A., Ramuz M. Presence of two independent chromosomes in the Brucella melitensis 16M genome. J Bacteriol. 1993 Feb;175(3):701–705. doi: 10.1128/jb.175.3.701-705.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Stackebrandt E., Dorsch M., Wolters J., Busch M., Mayer H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J Bacteriol. 1990 Jul;172(7):3569–3576. doi: 10.1128/jb.172.7.3569-3576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old I. G., MacDougall J., Saint Girons I., Davidson B. E. Mapping of genes on the linear chromosome of the bacterium Borrelia burgdorferi: possible locations for its origin of replication. FEMS Microbiol Lett. 1992 Dec 1;78(2-3):245–250. doi: 10.1016/0378-1097(92)90034-l. [DOI] [PubMed] [Google Scholar]
- Old I. G., Margarita D., Saint Girons I. Unique genetic arrangement in the dnaA region of the Borrelia burgdorferi linear chromosome: nucleotide sequence of the dnaA gene. FEMS Microbiol Lett. 1993 Jul 15;111(1):109–114. doi: 10.1111/j.1574-6968.1993.tb06369.x. [DOI] [PubMed] [Google Scholar]
- Saint Girons I., Norris S. J., Göbel U., Meyer J., Walker E. M., Zuerner R. Genome structure of spirochetes. Res Microbiol. 1992 Jul-Aug;143(6):615–621. doi: 10.1016/0923-2508(92)90119-9. [DOI] [PubMed] [Google Scholar]
- Sobral B. W., Honeycutt R. J., Atherly A. G., McClelland M. Electrophoretic separation of the three Rhizobium meliloti replicons. J Bacteriol. 1991 Aug;173(16):5173–5180. doi: 10.1128/jb.173.16.5173-5180.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Chromosome transfer in Rhodobacter sphaeroides: Hfr formation and genetic evidence for two unique circular chromosomes. J Bacteriol. 1992 Feb;174(4):1135–1145. doi: 10.1128/jb.174.4.1135-1145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: presence of two unique circular chromosomes. J Bacteriol. 1989 Nov;171(11):5850–5859. doi: 10.1128/jb.171.11.5850-5859.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabiko H. Sequence analysis of an insertion element, IS1131, isolated from the nopaline-type Ti plasmid of Agrobacterium tumefaciens. Gene. 1992 May 15;114(2):229–233. doi: 10.1016/0378-1119(92)90579-e. [DOI] [PubMed] [Google Scholar]
- Walker E. M., Arnett J. K., Heath J. D., Norris S. J. Treponema pallidum subsp. pallidum has a single, circular chromosome with a size of approximately 900 kilobase pairs. Infect Immun. 1991 Jul;59(7):2476–2479. doi: 10.1128/iai.59.7.2476-2479.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Barns S. M., Pelletier D. A., Lane D. J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991 Jan;173(2):697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelis M. L., Kandler O., Woese C. R. On the nature of global classification. Proc Natl Acad Sci U S A. 1992 Apr;89:2930–2934. doi: 10.1073/pnas.89.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi M., Yamasato K. Phylogenetic analysis of the family Rhizobiaceae and related bacteria by sequencing of 16S rRNA gene using PCR and DNA sequencer. FEMS Microbiol Lett. 1993 Feb 15;107(1):115–120. doi: 10.1111/j.1574-6968.1993.tb06014.x. [DOI] [PubMed] [Google Scholar]