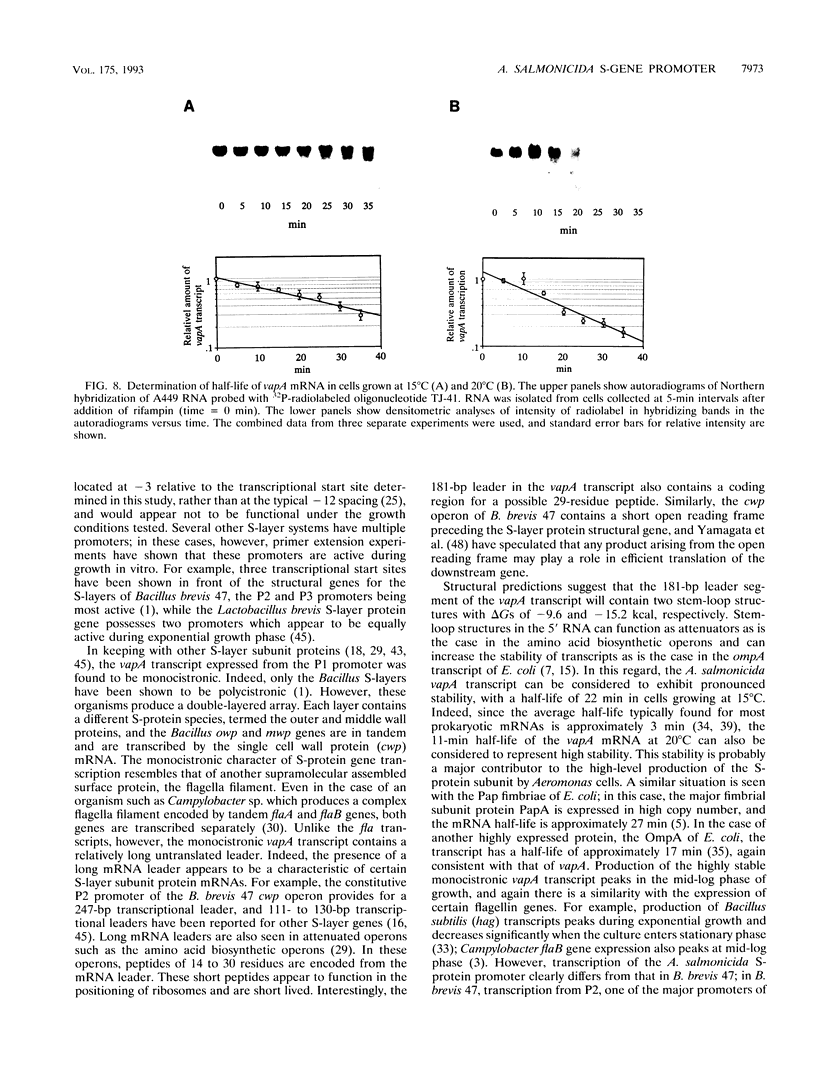
Abstract
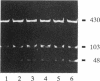
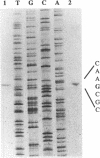
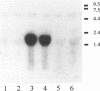
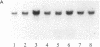
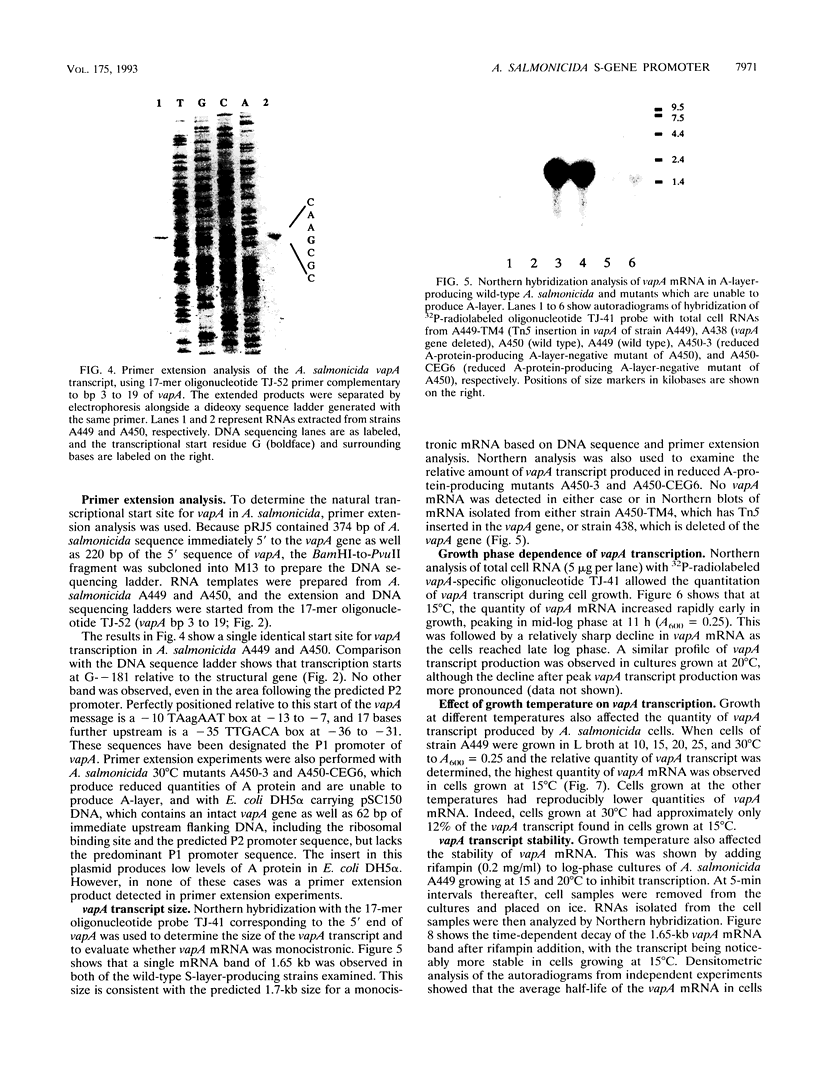
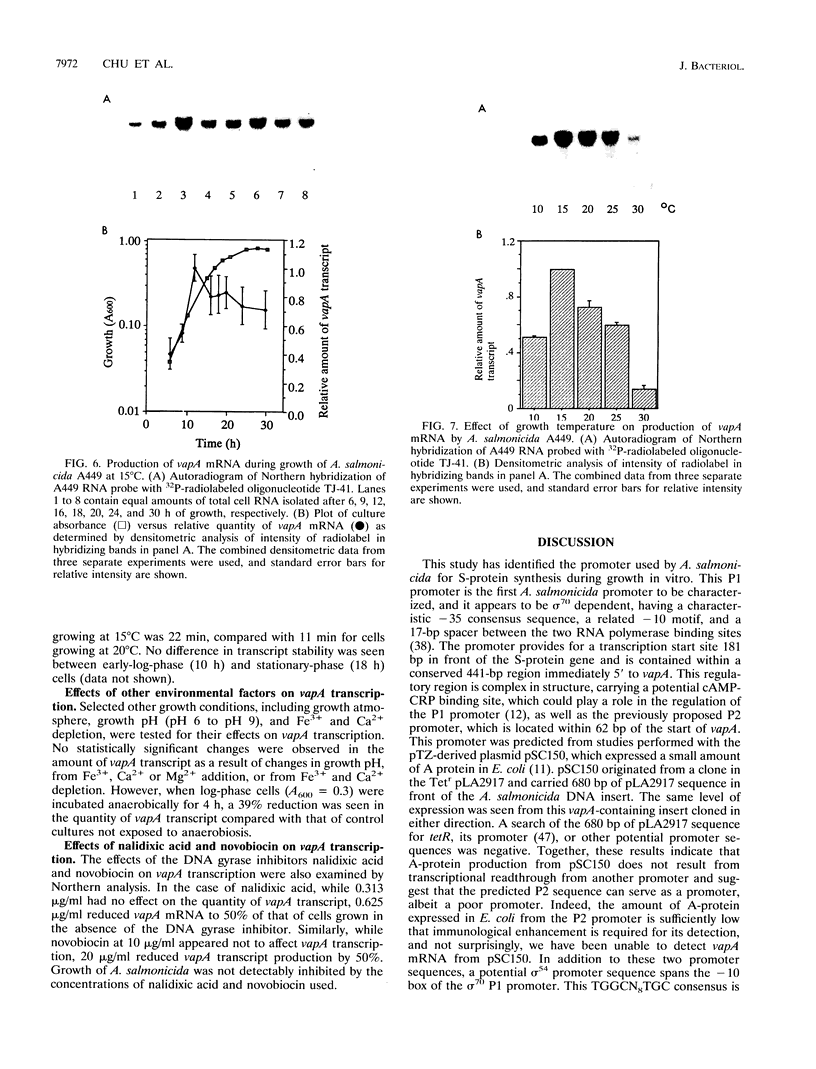
The vapA gene of Aeromonas salmonicida encodes the subunit of the surface protein array known as A-layer. Nucleotide sequence analysis of the 374 bp of DNA immediately upstream of vapA revealed two potential promoter sequences and other possible regulatory sequences. Sequencing and polymerase chain reaction analysis showed that the region was conserved in wild-type A. salmonicida. Primer extension and Northern (RNA) blot analysis showed that vapA transcription in A. salmonicida was directed predominantly by a distal promoter, P1, resulting in a 1.7-kb unit-length mRNA with an untranslated 181-nucleotide leader sequence which contained two predicted low-free-energy stem-loop structures. Northern analysis of cells grown at 15 degrees C showed that vapA transcript production peaked during the mid-log phase of growth (A600 = 0.25). At 15 degrees C, the half-life of the vapA mRNA was 22 min, while at 20 degrees C, the half-life was significantly shorter, 11 min. The amount of vapA transcript produced was reduced by growth in the presence of the DNA gyrase inhibitors nalidixic acid and novobiocin. Environmental factors such as growth temperature and atmospheric oxygen tension also affected the quantity of vapA mRNA. vapA transcript could not be detected in mutants which produced either low levels of full-length or truncated A protein or no detectable A protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Yamagata H., Tsukagoshi N., Udaka S. Multiple and tandemly arranged promoters of the cell wall protein gene operon in Bacillus brevis 47. J Bacteriol. 1989 Feb;171(2):1010–1016. doi: 10.1128/jb.171.2.1010-1016.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi T., Yamagata H., Tsukagoshi N., Udaka S. Repression of the cell wall protein gene operon in Bacillus brevis 47 by magnesium and calcium ions. J Bacteriol. 1991 Jul;173(13):4243–4245. doi: 10.1128/jb.173.13.4243-4245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm R. A., Guerry P., Trust T. J. The Campylobacter sigma 54 flaB flagellin promoter is subject to environmental regulation. J Bacteriol. 1993 Jul;175(14):4448–4455. doi: 10.1128/jb.175.14.4448-4455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Wildhaber I., Phipps B. M. Principles of organization in eubacterial and archaebacterial surface proteins. Can J Microbiol. 1989 Jan;35(1):215–227. doi: 10.1139/m89-034. [DOI] [PubMed] [Google Scholar]
- Belasco J. G., Nilsson G., von Gabain A., Cohen S. N. The stability of E. coli gene transcripts is dependent on determinants localized to specific mRNA segments. Cell. 1986 Jul 18;46(2):245–251. doi: 10.1016/0092-8674(86)90741-5. [DOI] [PubMed] [Google Scholar]
- Belland R. J., Trust T. J. Cloning of the gene for the surface array protein of Aeromonas salmonicida and evidence linking loss of expression with genetic deletion. J Bacteriol. 1987 Sep;169(9):4086–4091. doi: 10.1128/jb.169.9.4086-4091.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belland R. J., Trust T. J. Synthesis, export, and assembly of Aeromonas salmonicida A-layer analyzed by transposon mutagenesis. J Bacteriol. 1985 Sep;163(3):877–881. doi: 10.1128/jb.163.3.877-881.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Båga M., Göransson M., Normark S., Uhlin B. E. Processed mRNA with differential stability in the regulation of E. coli pilin gene expression. Cell. 1988 Jan 29;52(2):197–206. doi: 10.1016/0092-8674(88)90508-9. [DOI] [PubMed] [Google Scholar]
- Chu S., Cavaignac S., Feutrier J., Phipps B. M., Kostrzynska M., Kay W. W., Trust T. J. Structure of the tetragonal surface virulence array protein and gene of Aeromonas salmonicida. J Biol Chem. 1991 Aug 15;266(23):15258–15265. [PubMed] [Google Scholar]
- Collado-Vides J., Magasanik B., Gralla J. D. Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev. 1991 Sep;55(3):371–394. doi: 10.1128/mr.55.3.371-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman C. J., Ni Bhriain N., Higgins C. F. DNA supercoiling and environmental regulation of virulence gene expression in Shigella flexneri. Nature. 1990 Apr 19;344(6268):789–792. doi: 10.1038/344789a0. [DOI] [PubMed] [Google Scholar]
- Ebright R. H., Cossart P., Gicquel-Sanzey B., Beckwith J. Mutations that alter the DNA sequence specificity of the catabolite gene activator protein of E. coli. Nature. 1984 Sep 20;311(5983):232–235. doi: 10.1038/311232a0. [DOI] [PubMed] [Google Scholar]
- Emory S. A., Bouvet P., Belasco J. G. A 5'-terminal stem-loop structure can stabilize mRNA in Escherichia coli. Genes Dev. 1992 Jan;6(1):135–148. doi: 10.1101/gad.6.1.135. [DOI] [PubMed] [Google Scholar]
- Faraldo M. M., de Pedro M. A., Berenguer J. Sequence of the S-layer gene of Thermus thermophilus HB8 and functionality of its promoter in Escherichia coli. J Bacteriol. 1992 Nov;174(22):7458–7462. doi: 10.1128/jb.174.22.7458-7462.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher J. A., Smit J., Agabian N. Transcriptional analysis of the major surface array gene of Caulobacter crescentus. J Bacteriol. 1988 Oct;170(10):4706–4713. doi: 10.1128/jb.170.10.4706-4713.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Curtiss R., 3rd Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun. 1990 Jun;58(6):1879–1885. doi: 10.1128/iai.58.6.1879-1885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garduño R. A., Kay W. W. Interaction of the fish pathogen Aeromonas salmonicida with rainbow trout macrophages. Infect Immun. 1992 Nov;60(11):4612–4620. doi: 10.1128/iai.60.11.4612-4620.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garduño R. A., Lee E. J., Kay W. W. S-layer-mediated association of Aeromonas salmonicida with murine macrophages. Infect Immun. 1992 Oct;60(10):4373–4382. doi: 10.1128/iai.60.10.4373-4382.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson C. E., Thomas C. J., Trust T. J. Detection of Aeromonas salmonicida from fish by using polymerase chain reaction amplification of the virulence surface array protein gene. Appl Environ Microbiol. 1992 Dec;58(12):3816–3825. doi: 10.1128/aem.58.12.3816-3825.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göransson M., Sondén B., Nilsson P., Dagberg B., Forsman K., Emanuelsson K., Uhlin B. E. Transcriptional silencing and thermoregulation of gene expression in Escherichia coli. Nature. 1990 Apr 12;344(6267):682–685. doi: 10.1038/344682a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Helmann J. D. Alternative sigma factors and the regulation of flagellar gene expression. Mol Microbiol. 1991 Dec;5(12):2875–2882. doi: 10.1111/j.1365-2958.1991.tb01847.x. [DOI] [PubMed] [Google Scholar]
- Hovmöller S., Sjögren A., Wang D. N. The structure of crystalline bacterial surface layers. Prog Biophys Mol Biol. 1988;51(2):131–163. doi: 10.1016/0079-6107(88)90012-0. [DOI] [PubMed] [Google Scholar]
- Ishiguro E. E., Kay W. W., Ainsworth T., Chamberlain J. B., Austen R. A., Buckley J. T., Trust T. J. Loss of virulence during culture of Aeromonas salmonicida at high temperature. J Bacteriol. 1981 Oct;148(1):333–340. doi: 10.1128/jb.148.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Yanofsky C. Attenuation in amino acid biosynthetic operons. Annu Rev Genet. 1982;16:113–134. doi: 10.1146/annurev.ge.16.120182.000553. [DOI] [PubMed] [Google Scholar]
- Lechner J., Sumper M. The primary structure of a procaryotic glycoprotein. Cloning and sequencing of the cell surface glycoprotein gene of halobacteria. J Biol Chem. 1987 Jul 15;262(20):9724–9729. [PubMed] [Google Scholar]
- Logan S. M., Trust T. J., Guerry P. Evidence for posttranslational modification and gene duplication of Campylobacter flagellin. J Bacteriol. 1989 Jun;171(6):3031–3038. doi: 10.1128/jb.171.6.3031-3038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Messner P., Sleytr U. B. Crystalline bacterial cell-surface layers. Adv Microb Physiol. 1992;33:213–275. doi: 10.1016/s0065-2911(08)60218-0. [DOI] [PubMed] [Google Scholar]
- Mirel D. B., Chamberlin M. J. The Bacillus subtilis flagellin gene (hag) is transcribed by the sigma 28 form of RNA polymerase. J Bacteriol. 1989 Jun;171(6):3095–3101. doi: 10.1128/jb.171.6.3095-3101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G., Belasco J. G., Cohen S. N., von Gabain A. Growth-rate dependent regulation of mRNA stability in Escherichia coli. Nature. 1984 Nov 1;312(5989):75–77. doi: 10.1038/312075a0. [DOI] [PubMed] [Google Scholar]
- Parsot C., Mekalanos J. J. Structural analysis of the acfA and acfD genes of Vibrio cholerae: effects of DNA topology and transcriptional activators on expression. J Bacteriol. 1992 Aug;174(16):5211–5218. doi: 10.1128/jb.174.16.5211-5218.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff W. S., Siegele D. A., Cowing D. W., Gross C. A. The regulation of transcription initiation in bacteria. Annu Rev Genet. 1985;19:355–387. doi: 10.1146/annurev.ge.19.120185.002035. [DOI] [PubMed] [Google Scholar]
- Romeo J. M., Zusman D. R. Determinants of an unusually stable mRNA in the bacterium Myxococcus xanthus. Mol Microbiol. 1992 Oct;6(20):2975–2988. doi: 10.1111/j.1365-2958.1992.tb01756.x. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Tummuru M. K., Blaser M. J. Characterization of the Campylobacter fetus sapA promoter: evidence that the sapA promoter is deleted in spontaneous mutant strains. J Bacteriol. 1992 Sep;174(18):5916–5922. doi: 10.1128/jb.174.18.5916-5922.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidgrén G., Palva I., Pakkanen R., Lounatmaa K., Palva A. S-layer protein gene of Lactobacillus brevis: cloning by polymerase chain reaction and determination of the nucleotide sequence. J Bacteriol. 1992 Nov;174(22):7419–7427. doi: 10.1128/jb.174.22.7419-7427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Waters S. H., Rogowsky P., Grinsted J., Altenbuchner J., Schmitt R. The tetracycline resistance determinants of RP1 and Tn1721: nucleotide sequence analysis. Nucleic Acids Res. 1983 Sep 10;11(17):6089–6105. doi: 10.1093/nar/11.17.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Adachi T., Tsuboi A., Takao M., Sasaki T., Tsukagoshi N., Udaka S. Cloning and characterization of the 5' region of the cell wall protein gene operon in Bacillus brevis 47. J Bacteriol. 1987 Mar;169(3):1239–1245. doi: 10.1128/jb.169.3.1239-1245.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P. M., Hulsebos T. J., Schoenmakers J. G. Nucleotide sequence of the filamentous bacteriophage M13 DNA genome: comparison with phage fd. Gene. 1980 Oct;11(1-2):129–148. doi: 10.1016/0378-1119(80)90093-1. [DOI] [PubMed] [Google Scholar]