Abstract
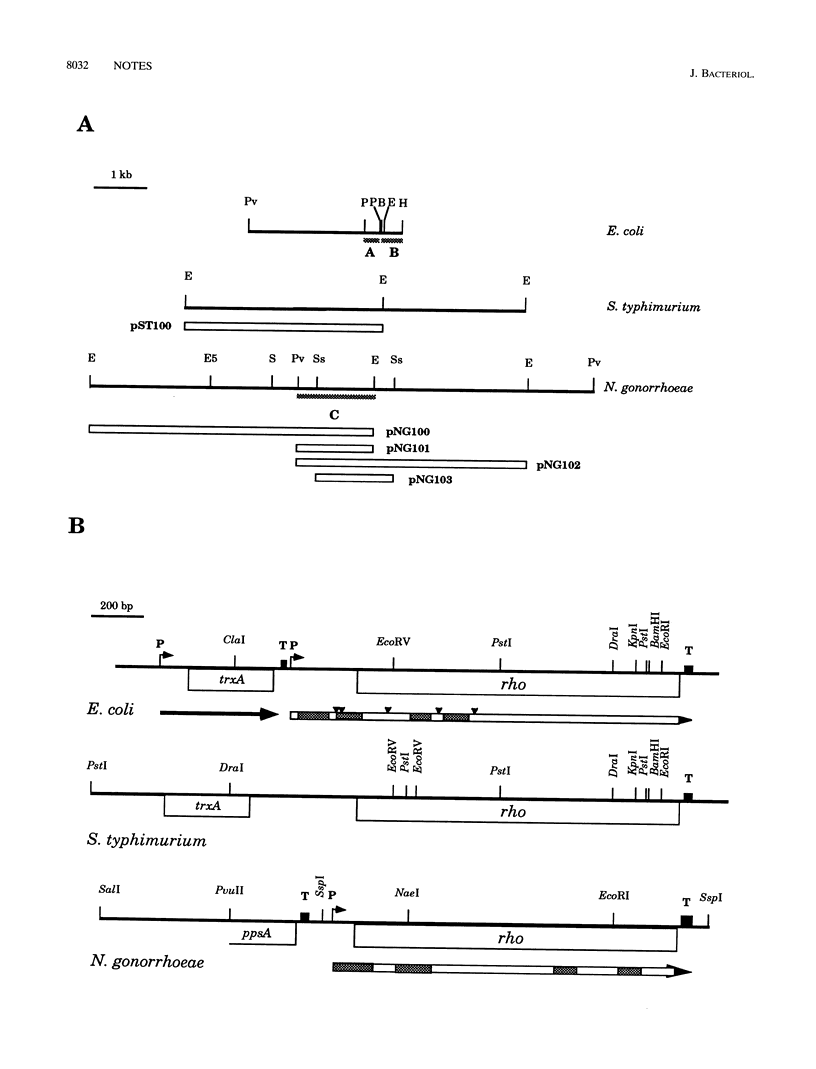
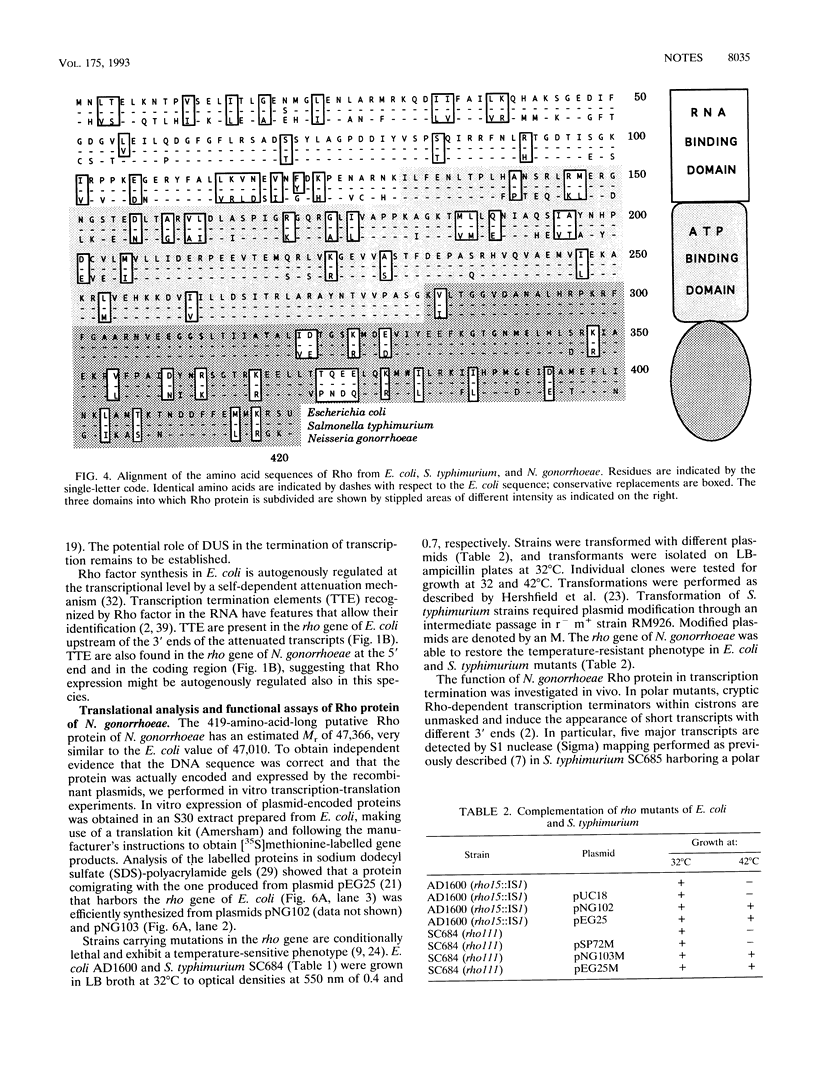
We have cloned and sequenced the genomic regions encompassing the rho genes of Neisseria gonorrhoeae and Salmonella typhimurium. Rho factor of S. typhimurium has only three amino acid differences with respect to the Escherichia coli homolog. Northern (RNA) blots and primer extension experiments were used to characterize the N. gonorrhoeae rho transcript and to identify the transcription initiation and termination elements of this cistron. The function of the Rho factor of N. gonorrhoeae was investigated by complementation assays of rho mutants of E. coli and S. typhimurium and by in vivo transcription assays in polar mutants of S. typhimurium.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Alifano P., Rivellini F., Limauro D., Bruni C. B., Carlomagno M. S. A consensus motif common to all Rho-dependent prokaryotic transcription terminators. Cell. 1991 Feb 8;64(3):553–563. doi: 10.1016/0092-8674(91)90239-u. [DOI] [PubMed] [Google Scholar]
- Argos P., Leberman R. Homologies and anomalies in primary structural patterns of nucleotide binding proteins. Eur J Biochem. 1985 Nov 4;152(3):651–656. doi: 10.1111/j.1432-1033.1985.tb09244.x. [DOI] [PubMed] [Google Scholar]
- Biville F., Guiso N. Evidence for the presence of cAMP, cAMP receptor and transcription termination factor rho in different gram-negative bacteria. J Gen Microbiol. 1985 Nov;131(11):2953–2960. doi: 10.1099/00221287-131-11-2953. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Hildebrandt J. F. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981 Jun;32(3):985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlomagno M. S., Chiariotti L., Alifano P., Nappo A. G., Bruni C. B. Structure and function of the Salmonella typhimurium and Escherichia coli K-12 histidine operons. J Mol Biol. 1988 Oct 5;203(3):585–606. doi: 10.1016/0022-2836(88)90194-5. [DOI] [PubMed] [Google Scholar]
- Carlomagno M. S., Riccio A., Bruni C. B. Convergently functional, Rho-independent terminator in Salmonella typhimurium. J Bacteriol. 1985 Jul;163(1):362–368. doi: 10.1128/jb.163.1.362-368.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampi M. S., Alifano P., Nappo A. G., Bruni C. B., Carlomagno M. S. Features of the rho-dependent transcription termination polar element within the hisG cistron of Salmonella typhimurium. J Bacteriol. 1989 Aug;171(8):4472–4478. doi: 10.1128/jb.171.8.4472-4478.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan J. W., Marshall N. F., Richardson J. P. Transcription termination factor rho has three distinct structural domains. J Biol Chem. 1990 Apr 5;265(10):5747–5754. [PubMed] [Google Scholar]
- Dombroski A. J., Brennan C. A., Spear P., Platt T. Site-directed alterations in the ATP-binding domain of rho protein affect its activities as a termination factor. J Biol Chem. 1988 Dec 15;263(35):18802–18809. [PubMed] [Google Scholar]
- Duncan T. M., Parsonage D., Senior A. E. Structure of the nucleotide-binding domain in the beta-subunit of Escherichia coli F1-ATPase. FEBS Lett. 1986 Nov 10;208(1):1–6. doi: 10.1016/0014-5793(86)81519-8. [DOI] [PubMed] [Google Scholar]
- Elkins C., Thomas C. E., Seifert H. S., Sparling P. F. Species-specific uptake of DNA by gonococci is mediated by a 10-base-pair sequence. J Bacteriol. 1991 Jun;173(12):3911–3913. doi: 10.1128/jb.173.12.3911-3913.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farewell A., Brazas R., Davie E., Mason J., Rothfield L. I. Suppression of the abnormal phenotype of Salmonella typhimurium rfaH mutants by mutations in the gene for transcription termination factor Rho. J Bacteriol. 1991 Aug;173(16):5188–5193. doi: 10.1128/jb.173.16.5188-5193.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. H., Wray L. V., Jr Regulation of glutamine synthetase in Streptomyces coelicolor. J Bacteriol. 1989 May;171(5):2378–2383. doi: 10.1128/jb.171.5.2378-2383.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. C., Kuby S. A., Mildvan A. S. ATP-binding site of adenylate kinase: mechanistic implications of its homology with ras-encoded p21, F1-ATPase, and other nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):907–911. doi: 10.1073/pnas.83.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. D., Scocca J. J. Factors influencing the specific interaction of Neisseria gonorrhoeae with transforming DNA. J Bacteriol. 1991 Sep;173(18):5921–5923. doi: 10.1128/jb.173.18.5921-5923.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. D., Scocca J. J. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6982–6986. doi: 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisolia V., Carlomagno M. S., Bruni C. B. Cloning and expression of the distal portion of the histidine operon of Escherichia coli K-12. J Bacteriol. 1982 Aug;151(2):692–700. doi: 10.1128/jb.151.2.692-700.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulletta E., Spagnuolo G., Adhya S. Cloning and expression of the Escherichia coli rho gene in a plasmid vector. Microbiologica. 1985 Oct;8(4):303–312. [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley P. R., Leavitt A. D., Whitfield H. J. Genetic analysis of a temperature-sensitive Salmonella typhimurium rho mutant with an altered rho-associated polycytidylate-dependent adenosine triphosphatase activity. J Bacteriol. 1981 Jul;147(1):13–24. doi: 10.1128/jb.147.1.13-24.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley P. R., Whitfield H. J. Transcription termination factor rho from wild type and rho-111 strains of Salmonella typhimurium. J Biol Chem. 1982 Mar 10;257(5):2569–2577. [PubMed] [Google Scholar]
- Hwang J. Y., Doi R. H. Transcription-termination factor Rho from Bacills subtilis. Eur J Biochem. 1980 Feb;104(1):313–320. doi: 10.1111/j.1432-1033.1980.tb04430.x. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol. 1985 Jan;2(1):13–34. doi: 10.1093/oxfordjournals.molbev.a040335. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Pan Z. X., Honzatko R. B., Ke H. M., Lipscomb W. N. Structural asymmetry in the CTP-liganded form of aspartate carbamoyltransferase from Escherichia coli. J Mol Biol. 1987 Aug 20;196(4):853–875. doi: 10.1016/0022-2836(87)90410-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavitola A., Vanni M., Martin P. M., Bruni C. B. Cloning and characterization of a Neisseria gene homologous to hisJ and argT of Escherichia coli and Salmonella typhimurium. Res Microbiol. 1992 Mar-Apr;143(3):295–305. doi: 10.1016/0923-2508(92)90021-f. [DOI] [PubMed] [Google Scholar]
- Lowery C., Richardson J. P. Characterization of the nucleoside triphosphate phosphohydrolase (ATPase) activity of RNA synthesi termination factor p. I. Enzymatic properties and effects of inhibitors. J Biol Chem. 1977 Feb 25;252(4):1375–1380. [PubMed] [Google Scholar]
- Matsumoto Y., Shigesada K., Hirano M., Imai M. Autogenous regulation of the gene for transcription termination factor rho in Escherichia coli: localization and function of its attenuators. J Bacteriol. 1986 Jun;166(3):945–958. doi: 10.1128/jb.166.3.945-958.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niersbach M., Kreuzaler F., Geerse R. H., Postma P. W., Hirsch H. J. Cloning and nucleotide sequence of the Escherichia coli K-12 ppsA gene, encoding PEP synthase. Mol Gen Genet. 1992 Jan;231(2):332–336. doi: 10.1007/BF00279808. [DOI] [PubMed] [Google Scholar]
- Pannekoek Y., van Putten J. P., Dankert J. Identification and molecular analysis of a 63-kilodalton stress protein from Neisseria gonorrhoeae. J Bacteriol. 1992 Nov;174(21):6928–6937. doi: 10.1128/jb.174.21.6928-6937.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham J. L., Platt T. The nucleotide sequence of the rho gene of E. coli K-12. Nucleic Acids Res. 1983 Jun 11;11(11):3531–3545. doi: 10.1093/nar/11.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk P. G., Dunkley E. A., Jr, Lee P., Krulwich T. A. Identification of a putative Bacillus subtilis rho gene. J Bacteriol. 1993 Feb;175(3):647–654. doi: 10.1128/jb.175.3.647-654.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechler M. M., Bruni C. B., Martin R. G., Terry W. An intercistronic region in the histidine operon of Salmonella typhimurium. J Mol Biol. 1972 Aug 28;69(3):427–452. doi: 10.1016/0022-2836(72)90256-2. [DOI] [PubMed] [Google Scholar]
- Rivellini F., Alifano P., Piscitelli C., Blasi V., Bruni C. B., Carlomagno M. S. A cytosine- over guanosine-rich sequence in RNA activates rho-dependent transcription termination. Mol Microbiol. 1991 Dec;5(12):3049–3054. doi: 10.1111/j.1365-2958.1991.tb01864.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha M. K., So M., Seifert H. S., Billyard E., Marchal C. Pilin expression in Neisseria gonorrhoeae is under both positive and negative transcriptional control. EMBO J. 1988 Dec 20;7(13):4367–4378. doi: 10.1002/j.1460-2075.1988.tb03335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Clark V. L. Genetic loci and linkage associations in Neisseria gonorrhoeae and Neisseria meningitidis. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S92–103. doi: 10.1128/cmr.2.suppl.s92. [DOI] [PMC free article] [PubMed] [Google Scholar]