Abstract
Caveolae are vesicular invaginations of the plasma membrane. Caveolin-3 is the principal structural component of caveolae in skeletal muscle cells in vivo. We have recently generated caveolin-3 transgenic mice and demonstrated that overexpression of wild-type caveolin-3 in skeletal muscle fibers is sufficient to induce a Duchenne-like muscular dystrophy phenotype. In addition, we have shown that caveolin-3 null mice display mild muscle fiber degeneration and T-tubule system abnormalities. These data are consistent with the mild phenotype observed in Limb-girdle muscular dystrophy-1C (LGMD-1C) in humans, characterized by a ∼95% reduction of caveolin-3 expression. Thus, caveolin-3 transgenic and null mice represent valid mouse models to study Duchenne muscular dystrophy (DMD) and LGMD-1C, respectively, in humans. Here, we derived conditionally immortalized precursor skeletal muscle cells from caveolin-3 transgenic and null mice. We show that overexpression of caveolin-3 inhibits myoblast fusion to multinucleated myotubes and lack of caveolin-3 enhances the fusion process. M-cadherin and microtubules have been proposed to mediate the fusion of myoblasts to myotubes. Interestingly, we show that M-cadherin is downregulated in caveolin-3 transgenic cells and upregulated in caveolin-3 null cells. For the first time, variations of M-cadherin expression have been linked to a muscular dystrophy phenotype. In addition, we demonstrate that microtubules are disorganized in caveolin-3 null myotubes, indicating the importance of the cytoskeleton network in mediating the phenotype observed in these cells. Taken together, these results propose caveolin-3 as a key player in myoblast fusion and suggest that defects of the fusion process may represent additional molecular mechanisms underlying the pathogenesis of DMD and LGMD-1C in humans.
INTRODUCTION
Caveolae are vesicular invaginations of the plasma membrane. Caveolins are the structural components of caveolae. It has been proposed that caveolins participate in vesicular trafficking events and signal transduction processes (Lisanti et al., 1994; Sargiacomo et al., 1994; Scherer et al., 1996; Song et al., 1996a, 1997; Couet et al., 1997; Okamoto et al., 1998) by acting as scaffolding proteins (Sargiacomo et al., 1995) to organize, concentrate, and regulate specific lipids (cholesterol and glyco-sphingolipids; Murata et al., 1995; Li et al., 1996) and lipid-modified signaling molecules (Src-like kinases, H-Ras, eNOS, components of the p42/44 MAP kinase pathway, and G-proteins) within caveolae membranes (Scherer et al., 1994, 1995; Smart et al., 1994; Li et al., 1995, 1996; Moldovan et al., 1995; Garcia-Cardena et al., 1996; Song et al., 1996a). The mammalian caveolin gene family consists of caveolin-1, 2, and 3 (Scherer et al., 1996; Tang et al., 1996; Okamoto et al., 1998). Caveolin-3 is muscle specific and is found in both cardiac and skeletal muscle as well as smooth muscle cells (Smart et al., 1994; Moldovan et al., 1995; Scherer et al., 1995; Song et al., 1996b; Minetti et al., 1998; Galbiati et al., 1999b, 2000b, 2000c, 2001a). The expression of caveolin-3 is induced during the differentiation of skeletal myoblasts and caveolin-3 is localized to the muscle cell plasma membrane (sarcolemma) where it forms a complex with dystrophin and its associated glycoproteins (Song et al., 1996b). However, under certain conditions caveolin-3 can be physically separated from the dystrophin complex (Crosbie et al., 1998). This indicates that although caveolin-3 is dystrophin-associated, it is not absolutely required for the biogenesis of the dystrophin complex (Crosbie et al., 1998).
Several morphological and biochemical observations seemingly implicate caveolae and caveolin-3 in the pathogenesis of Duchenne muscular dystrophy (DMD). Studies using electron microscopy and freeze-fracture techniques have shown that there is an increased number of caveolae in the skeletal muscle of DMD patients that is associated with overexpression of caveolin-3 (Bonilla et al., 1981). Similar results have been obtained with mdx mice, an animal model of DMD, with a dystrophin deficiency. We have recently demonstrated that overexpression of wild-type caveolin-3 in skeletal muscle fibers as a transgene, was sufficient to induce a Duchenne-like muscular dystrophy phenotype. These mice showed a number of pathological changes that often are associated with muscular dystrophy, including regenerating and immature fibers with central nuclei, necrotic fibers, and an increase in the connective tissue component within the muscle (Galbiati et al., 2000b). Importantly, overexpression of caveolin-3 was associated with a severe reduction in the expression of dystrophin and dystrophin-associated glycoproteins (Galbiati et al., 2000b).
A dramatic reduction in the expression of caveolin-3 in humans results in limb-girdle muscular dystrophy-1C (LGMD-1C; Minetti et al., 1998). Recently, we demonstrated that caveolin-3 deficiency in mice results in loss of caveolae at the sarcolemma and in muscle fiber degeneration (Galbiati et al., 2001a). The degenerative process was mild compared with DMD patients or caveolin-3–overexpressing mice. Similar results were reported by Hagiwara and colleagues (Hagiwara et al., 2000). These data are consistent with the mild phenotype observed in LGMD-1C in humans. Interestingly, we showed that loss of caveolin-3 expression results in the exclusion of the dystrophin complex from lipid raft domains (Galbiati et al., 2001a). However, the level of expression of dystrophin and its associated glycoproteins was not affected in caveolin-3 null mice (Galbiati et al., 2001a).
Satellite cells play a key role in skeletal muscle regeneration. In response to muscle damage, satellite cells become activated, proliferate, differentiate, and eventually fuse to each other to form new muscle or fuse with existing myofibers to repair damaged segments (Schultz and McCormick, 1994; Grounds, 1998; Cooper et al., 1999). Muscular dystrophies are progressive, genetically determined, and degenerative myopathies, where repetitive cycles of degeneration and regeneration occur. In one of the most severe form of muscular dystrophy, Duchenne muscular dystrophy, the extensive cycles of degeneration and regeneration are not effective. In fact, despite numerous fibers with central nuclei, typical of regenerating fibers, the muscle cannot overcome the progressive degeneration (Cossu and Mavilio, 2000; Heslop et al., 2000). The molecular mechanisms responsible for poor regeneration in dystrophic muscle are not completely understood.
Here, we characterize precursor skeletal muscle cells derived from caveolin-3 transgenic and null mice. We demonstrate that overexpression of caveolin-3 in precursor skeletal muscle cells inhibits myoblast fusion to multinucleated myotubes. In contrast, lack of caveolin-3 results in the formation of larger myotubes, compared with control cells. Also, we show that overexpression or lack of caveolin-3 affects myotube formation in vivo as well.
Cadherins, Ca2+-dependent cell adhesion molecules, constitute a family of transmembrane proteins that play important roles in morphogenesis (Geiger and Ayalon, 1992; Huber et al., 1996). The family member M-cadherin is highly upregulated in activated satellite cells of regenerating muscle, but is downregulated in mature myotubes, indicating a possible role of M-cadherin in muscle repair mechanisms (Irintchev et al., 1994). We demonstrate here that M-cadherin expression is transiently upregulated after 1 d of differentiation in control skeletal muscle cells. In contrast, M-cadherin expression is significantly reduced in caveolin-3 transgenic cells after 1 d of differentiation, whereas it remains highly expressed later in the differentiation in caveolin-3 null cells. These results contribute to explain the fusion defects observed in these cells. For the first time, variations of M-cadherin expression have been linked to a muscular dystrophy phenotype.
Alignment of myoblasts before and during the fusion to myotubes is a fundamental step mediated by microtubules (Saitoh et al., 1988; Gundersen et al., 1989; Mangan and Olmsted, 1996; Kaufmann et al., 1999). In this report, we show that microtubules are disorganized in caveolin-3 null myotubes, indicating that lack of caveolin-3 leads to alterations of the cytoskeleton network, which may contribute to the fusion defect observed in these cells.
Taken together, these data indicate that caveolin-3 overexpression or lack of caveolin-3 is sufficient to severely affect myoblast fusion to myotubes. In addition, they suggest that caveolin-3–dependent defects of myoblast fusion may represent important molecular mechanisms underlying the muscle damage observed in DMD and LGMD-1C in humans. Thus, these investigations have direct consequences at furthering our understanding of skeletal muscle differentiation and regeneration in DMD and LGMD-1C and will be helpful for developing new therapeutic strategies.
MATERIALS AND METHODS
Materials
Antibodies and their sources were as follows: anti–caveolin-3 IgG (mouse mAb 26; BD Transduction Laboratories (Lexington, KY)); anti–caveolin-1 IgG (mouse mAb 2297; BD Tranduction Laboratories); antimyosin heavy chain IgG (mouse mAb; Sigma, St. Louis, MO); anti–troponin-T IgG (mouse mAb; Sigma); anticadherin IgGs (M-, and N-cadherin; mouse mAbs; BD Transduction Laboratories); anticatenin IgGs (α-, β-, γ-catenin; mouse mAbs; BD Tranduction Laboratories); anti–α-tubulin IgG (mouse mAb; Santa Cruz Biotechnology, Santa Cruz, CA). Fibroblast growth factor-2/basic FGF was from Upstate Biotechnology (Lake Placis, NY); γ-interferon (murine, recombinant) was from Gibco Invitrogen (Carlsbad, CA). All other biochemicals used were of the highest purity available and were obtained from regular commercial sources.
Generation and Screening of “Immortomice” Overexpressing or Lacking Caveolin-3
Transgenic mice overexpressing caveolin-3 (with a C57Bl6 background) and caveolin-3 knock-out mice (with a C57Bl6 background) were crossed with the “immortomouse” (with a C57Bl6 background, from Charles River Laboratories, Wilmington, MA). The presence of the temperature-sensitive SV40 large T-antigene gene (tsA58), under the control of an interferon-γ–sensitive promoter (H-2Kb), was detected by PCR analysis using the following pair of oligos: i) agc gct tgt gtc gcc att gta ttc; ii) gtc aca cca cag aag taa ggt tcc. Caveolin-3 transgenic and knock-out mice were screened by Western blotting analysis. Caveolin-3 transgenic and knock-out mice harboring the temperature-sensitive SV40 large T-antigene gene (tsA58), under the control of an interferon-γ–sensitive promoter (H-2Kb), were sacrificed to generate conditionally immortalized skeletal muscle cells. Mice harboring only the temperature-sensitive SV40 large T-antigene gene (tsA58), under the control of an interferon-γ–sensitive promoter (H-2Kb), which express normal levels of endogenous caveolin-3, were used as controls.
Generation of Conditionally Immortalized Skeletal Muscle Cells
Mice (8–10 months old) were sacrificed and limb muscle was isolated and collected in a Petri dish containing PBS + penicillin and streptomycin. Limb muscle was minced to very fine pieces and transferred to a tube containing 0.5% collagenase type II (Gibco, Invitrogen) and 1% dispase (Gibco, Invitrogen) diluted in PBS + penicillin and streptomycin. Muscle was incubated for 30 min at 37°C and mixed during the incubation. The supernatant was collected and an equal volume of complete medium was added (Ham's F-10 with 20% FBS). Samples were filtered through a cell strainer and centrifuged at 900 × g for 10 min. The pellet, containing skeletal muscle cells, was resuspended in complete medium. Myoblasts were grown under permissive conditions: Ham's F-10 with 20% FBS in the presence of γ-interferon (50 U/ml) at 33°C. Differentiation of myoblasts to multinucleated myotubes was achieved by growing the cells for different periods of time under nonpermissive conditions (DMEM with 4% horse serum in the absence of γ-interferon at 37°C).
Immunoblotting
Cells were collected in boiling sample buffer and homogenized using a 26-gauge needle. Cellular proteins were resolved by SDS-PAGE (12.5% acrylamide) and transferred to BA83 nitrocellulose membranes (Schleicher & Schuell, Keene, NH). Blots were incubated for 2 h in TBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.2% Tween 20) containing 2% powdered skim milk and 1% bovine serum albumin (BSA). After three washes with TBST, membranes were incubated for 2 h with the primary antibody (∼1000-fold diluted in TBST) and for 1 h with horseradish peroxidase–conjugated goat anti-rabbit/mouse IgG (∼5000-fold diluted). Bound antibodies were detected using an ECL detection kit (Pierce, Rockford, IL).
Preparation of Caveolae-enriched Membrane Fractions
Cells were scraped into 2 ml of Mes-buffered saline containing 1% (vol/vol) Triton X-100. Homogenization was carried out with 10 strokes of a loose fitting Dounce homogenizer. The homogenate was adjusted to 40% sucrose by the addition of 2 ml of 80% sucrose prepared in Mes-buffered saline and placed at the bottom of an ultracentrifuge tube. A 5–30% linear sucrose gradient was formed above the homogenate and centrifuged at 39,000 rpm for 16–20 h in a SW41 rotor (Beckman Coulter, Fullerton, CA). A light-scattering band confined to the 15–20% sucrose region was observed that contained endogenous caveolin-3, but excluded most of other cellular proteins. From the top of each gradient, 1-ml gradient fractions were collected to yield a total of 12 fractions. Fractions 4–6, representing caveolar fractions, and fractions 9–12, representing noncaveolar fractions, were pooled together. An equal amount of protein from each of the two groups was separated by SDS-PAGE and subjected to immunoblot analysis.
Immunofluorescence Microscopy
Cells grown on glass coverslips were washed three times with PBS and fixed for 30 min at room temperature with 2% paraformaldehyde in PBS. Fixed cells were rinsed with PBS and permeabilized with 0.1% Triton X-100, 0.2% bovine serum albumin for 10 min. Then cells were treated with 25 mM NH4Cl in PBS for 10 min at room temperature to quench free aldehyde groups. Cells were rinsed with PBS and incubated with the primary antibody (diluted in PBS with 0.1% Triton X-100, 0.2% bovine serum albumin) for 1 h at room temperature. After three washes with PBS (10 min each), cells were incubated with the secondary antibody for 1 h at room temperature: lissamine rhodamine B sulfonyl chloride–conjugated goat anti-rabbit antibody (5 μg/ml) and/or fluorescein isothiocyanate–conjugated goat anti-mouse antibody (5 μg/ml). Finally, cells were washed three times with PBS (10 min each wash) and slides were mounted with slow-Fade antifade reagent (Molecular Probes, Inc., Eugene, OR) and observed using an Olympus Provis fluorescent microscope.
Coimmunoprecipitation
Cells were washed twice with PBS and lysed for 30 min at 4°C in a buffer containing 10 mM Tris, pH 8.0, 0.15 M NaCl, 5 mM EDTA, 1% Triton X-100, and 60 mM octyl glucoside. Samples were precleared for 1 h at 4°C using protein A-Sepharose (20 μl; slurry, 1:1) and subjected to overnight immunoprecipitation at 4°C using the intended antibody and protein A-Sepharose (30 μl; slurry, 1:1). As an internal control, immunoprecipitations with unrelated IgG were performed (unpublished data). After three washes with the immunoprecipitation buffer, samples were separated by SDS-PAGE (12.5% acrylamide) and transferred to nitrocellulose. Then, blots were probed with the intended antibody.
DAPI Staining
Control (CTL), caveolin-3 transgenic (Cav-3), and caveolin-3 null (KO) skeletal muscle cells were differentiated for 3 d. Cells were washed twice with PBS and fixed with 2% paraformaldehyde at room temperature for 30 min. Then, cells were incubated with RNAse A (10 μg/ml in PBS) for 10 min and with DAPI (1 μg/ml in PBS) for 10 min. Cells were examined with an Olympus Provis fluorescent microscope.
RESULTS
Generation of Conditionally Immortalized Precursor Skeletal Muscle Cells from Normal Control, Caveolin-3 Transgenic, and Caveolin-3 Null Mice
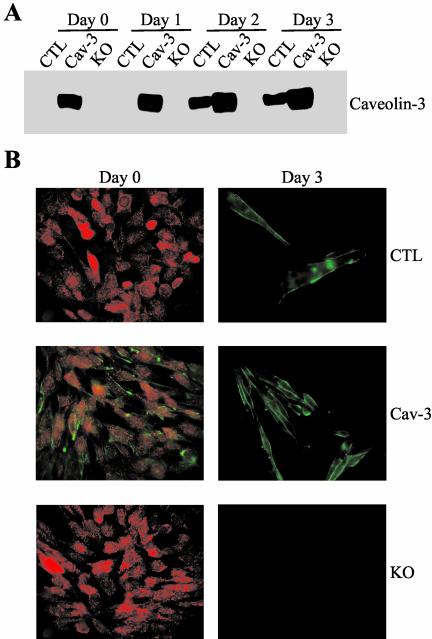
To generate conditionally immortalized skeletal muscle cells, we crossed normal control, caveolin-3 transgenic, and caveolin-3 null mice with the immortomouse, a transgenic mouse that harbor a temperature-sensitive SV40 large T-antigene gene (tsA58) under the control of an interferon-γ–sensitive promoter (H-2Kb) in the genome (Jat et al., 1991; Morgan et al., 1994; Ehler et al., 1995; Noble et al., 1995; Kanda et al., 1996). The immortomouse allows the generation of conditionally immortalized cell lines from different tissues. Normal control, caveolin-3 transgenic, and caveolin-3 null mice harboring the temperature-sensitive SV40 large T-antigene gene (tsA58) were identified by PCR analysis (see MATERIALS AND METHODS for details). Then, skeletal muscle cells harboring the temperature-sensitive SV40 large T-antigene gene (tsA58) were derived from these mice (see MATERIALS AND METHODS for details). Conditionally immortalized skeletal muscle cells were routinely propagated at 33°C with 20% fetal bovine serum in the presence of γ-interferon (permissive condition; Morgan et al., 1994). Differentiation of myoblasts to myotubes was achieved by switching confluent cell populations to a nonpermissive condition, that is growth at 37°C with 4% horse serum in the absence of γ-interferon for different periods of time (Morgan et al., 1994). Figure 1A demonstrates that we successfully generated conditionally immortalized skeletal muscle cells expressing normal levels of caveolin-3 (CTL), overexpressing caveolin-3 (Cav-3), or lacking caveolin-3 protein expression (KO). More specifically, when cells were grown in permissive conditions (day 0), or differentiated for 1 d, caveolin-3 was expressed only in cells harboring the caveolin-3 transgene (Cav-3). Caveolin-3 was expressed in both CTL and Cav-3 myotubes after differentiation for 2 or 3 d. Importantly, caveolin-3 expression in myotubes harboring the caveolin-3 transgene (Cav-3) was significantly higher than that in myotubes lacking the caveolin-3 transgene (CTL), confirming our previous results with skeletal muscle tissues derived from normal control and caveolin-3 transgenic mice (Galbiati et al., 2000b). As expected, caveolin-3 was not expressed in myoblasts and myotubes derived from caveolin-3 null mice (KO). In addition, myosin heavy chain and troponin-T, two muscle-specific marker proteins, were equally expressed in differentiated CTL, Cav-3, and KO cells (our unpublished observations). These results are consistent with previous observations showing that caveolin-3 is expressed only in differentiated skeletal muscle fibers (Song et al., 1996b). Also, caveolin-1 and caveolin-2 were not expressed in differentiated skeletal muscle cells, indicating that caveolin-3 is the only caveolin isoform expressed in these cells (our unpublished observations).
Figure 1.
Generation of conditionally immortalized skeletal muscle cells. (A) Immunoblotting. Conditionally immortalized skeletal muscle cells were derived from control (CTL), caveolin-3 transgenic (Cav-3), and caveolin-3 null mice (KO). Cells were differentiated for different periods of time (0, 1, 2, and 3 d). Cell lysates were subjected to immunoblotting analysis using an antibody probe specific for caveolin-3. Note that caveolin-3 is upregulated after 2 d of differentiation in control cells. However, Cav-3 transgenic cells overexpress caveolin-3 before and after differentiation. Caveolin-3 is not expressed in caveolin-3 null cells (KO). Each lane contains an equal amount of total protein. (B) Immunofluorescence. Conditionally immortalized skeletal muscle cells were derived from control (CTL), caveolin-3 transgenic (Cav-3), and caveolin-3 null mice (KO). Cells were differentiated for different periods of time (0 and 3 d). Then, cells were fixed with 2% paraformaldheyde for 30 min and immunostained with anti–caveolin-3 IgG and anti–c-Met IgG. Note that caveolin-3 (green) is mainly localized at the plasma membrane only after differentiation in control cells (top panels). In contrast, caveolin-3 is localized at the sarcolemma before and after differentiation in caveolin-3 transgenic cells (middle panels). Caveolin-3 null cells (KO) do not express caveolin-3 (bottom panels). In addition, c-Met (red) is only expressed before differentiation in precursor skeletal muscle cells (myoblasts) but not in differentiated myotubes. Moreover, caveolin-3 and c-Met do not colocalize in caveolin-3 transgenic myoblasts (middle left panel).
c-Met, a Marker of Satellite Cells, Is Expressed Only in Conditionally Immortalized Myoblasts
The use of conditionally immortalized skeletal muscle cells may represent a valid model for studying the differentiation of skeletal muscle satellite cells that occurs during muscle regeneration. To prove the validity of this approach, we investigated the expression of c-Met (the receptor for hepatocyte growth factor, HGF), a marker of satellite cells, in conditionally immortalized skeletal muscle cells before and after differentiation. Satellite cells, but not differentiated myotubes, have been shown to express c-Met (Cornelison and Wold, 1997). Control, caveolin-3 transgenic, and caveolin-3 null cells were grown in permissive conditions (day 0) or differentiated for 3 d (day 3). Then, cells were subjected to double immunostaining with anti–c-met and anti–caveolin-3 antibodies. Figure 1B illustrates that c-Met (red) was expressed only in undifferentiated myoblasts but not in differentiated myotubes. In contrast, caveolin-3 (green) was expressed at the plasma membrane only after differentiation in control cells. Caveolin-3 transgenic cells expressed caveolin-3 before and after differentiation. Caveolin-3 was not expressed in caveolin-3 null cells. These data directly support the results of Figure 1A. Thus, these findings confirm that the differentiation of conditionally immortalized myoblasts to myotubes may resemble the muscle regeneration process that occurs in skeletal muscle tissues.
Overexpression or Lack of Caveolin-3 Affects Myoblast Fusion to Myotubes
We have previously demonstrated that transgenic overexpression of caveolin-3 in mice promotes a Duchenne-like muscular dystrophy phenotype in skeletal muscle tissues (Galbiati et al., 2000b) and that lack of caveolin-3 expression resembles the phenotype observed in Limbgirdle muscular dystrophy-1C (Galbiati et al., 2001a). Thus, the differentiation program might be impaired in skeletal muscle cells derived from these mice. As a consequence, we decided to investigate whether overexpression or lack of caveolin-3 may affect myoblast differentiation to myotubes using conditionally immortalized skeletal muscle cells.
Figure 2A illustrates the morphology of conditionally immortalized skeletal muscle cells (derived from normal control [CTL], caveolin-3 transgenic [Cav-3], and caveolin-3 null mice [KO]) after 3 d of differentiation. Interestingly, caveolin-3–overexpressing cells failed to form large multinucleated myotubes after differentiation, in contrast to control cells (Figure 2A). In fact, caveolin-3–overexpressing myotubes appeared significantly smaller in diameter. Importantly, Figure 2A indicates that the alignment of caveolin-3 cells before fusion occurred normally; however, these cells failed to fuse and form bigger myotubes. These observations suggest that a defect in the fusion process may be responsible for the inability of caveolin-3–overexpressing cells to generate normal myotubes. Next, we observed the ability of skeletal muscle cells derived from caveolin-3 null mice to differentiate into multinucleated myotubes. As shown in Figure 2A, lack of caveolin-3 expression resulted in the formation of dramatically larger multinucleated myotubes, compared with control myotubes.
Figure 2.
Overexpression or lack of caveolin-3 affects myoblast fusion to myotubes in vitro. (A) Cell morphology. Control (CTL), caveolin-3 transgenic (Cav-3), and caveolin-3 null (KO) skeletal muscle cells were differentiated for 3 d. Then, cells were fixed in 2% paraformaldheyde for 30 min and examined using a BX61 Fluoview confocal microscope (Olympus). Note that control skeletal muscle cells form large multinucleated myotubes. In contrast, caveolin-3 transgenic myotubes appear smaller in diameter. In addition, caveolin-3 null cells form dramatically larger myotubes than control cells. (B) DAPI staining. Control (CTL), caveolin-3 transgenic (Cav-3), and caveolin-3 null (KO) skeletal muscle cells were differentiated for 3 d. Then, cells were fixed in 2% paraformaldheyde for 30 min and nuclei were stained with DAPI. The number of nuclei per myotube was recorded. At least 50 myotubes for each cell type were scored.
Differentiation of myoblasts results in the formation of multinucleated myotubes. Thus, to further characterize the myoblast fusion defect observed in caveolin-3 transgenic and null cells, we counted the number of nuclei per myotube after 3 d of differentiation. Cells were fixed with 2% paraformaldheyde, and nuclei were stained with DAPI. Figure 2B illustrates that control cells had 15 ± 3 nuclei per myotube. In contrast, caveolin-3 transgenic cells had only 5 ± 1.5 nuclei per myotube, whereas 38 ± 5 nuclei were counted in caveolin-3 null myotubes. These results directly support the data of Figure 2A. It is important to point out that virtually identical results were obtained using three independent preparations of conditionally immortalized control, caveolin-3 transgenic, and caveolin-3 null cells. Moreover, CTL, Cav-3, and KO myoblasts showed similar fibroblast-like cell morphology before differentiation (our unpublished observations).
To investigate whether the fusion defects observed in conditionally immortalized skeletal muscle cells occur in vivo, longitudinal sections of extensor digitorum longus (EDL) muscle from 8- to 10-month-old control, caveolin-3 transgenic, and caveolin-3 null mice were subjected to hematoxylin and eosin (H&E) staining. Representative results are shown in Figure 3A. Caveolin-3–overexpressing skeletal muscle fibers appeared smaller in diameter than fibers derived form normal control mice. Arrows indicate regions where thin fibers are next to each other, but fail to fuse and form bigger myotubes. In contrast, skeletal muscle fibers derived from caveolin-3 null mice appeared significantly larger in diameter (Figure 3A). Quantitation is shown in Figure 3B. These results directly support our data obtained with conditionally immortalized skeletal muscle cells (Figure 2, A and B). The muscle degeneration observed in the caveolin-3 transgenic sections (degenerating regions are indicated by asterisks) was expected, in fact we have previously demonstrated that overexpression of caveolin-3 in mice induces muscle degeneration (Galbiati et al., 2000b).
Figure 3.
Overexpression or lack of caveolin-3 affects myoblast fusion to myotubes in vivo. (A) H&E staining. Longitudinal sections of extensor digitorum longus (EDL) muscle from 8- to 10-month-old control (CTL), caveolin-3 transgenic (Cav-3), and caveolin-3 null (KO) mice were stained with hematoxylin and eosin (H&E). A representative field is shown. Note that skeletal muscle fibers overexpressing caveolin-3 are significantly smaller in diameter than control fibers. In addition, lack of caveolin-3 results in abnormally larger myofibers. Degenerating regions are indicated by asterisks. (B) Quantitation of the measurements of fiber diameters in control (CTL), caveolin-3 transgenic (Cav-3), and caveolin-3 null (KO) mice is shown. At least 50 fibers from three different mice were counted. Values represent means ± SEM; *p < 0.05.
Interestingly, overexpression or lack of caveolin-3 promoted two totally opposite phenotypes: overexpression of caveolin-3 inhibited myoblast fusion to myotubes, whereas lack of caveolin-3 expression enhanced the fusion process. Taken together, these data suggest that caveolin-3 may play a key role in mediating myoblast fusion and that variations of caveolin-3 expression affect the ability of myoblasts to generate fully differentiated myotubes.
M-cadherin and N-cadherin Are Reduced in Caveolin-3 Transgenic Cells, and M-cadherin Remains Elevated in Caveolin-3 Null Cells Late in the Differentiation
M-cadherin has been demonstrated to play an important role in the fusion of myoblasts to multinucleated myofibers (Kuch et al., 1997; Kaufmann et al., 1999). We observed that overexpression or lack of caveolin-3 has dramatic consequences on the fusion of myoblasts to myotubes (Figures 2 and 3). Thus, we decided to investigate whether M-cadherin expression is affected in caveolin-3 transgenic and null cells. Control, caveolin-3 transgenic, and caveolin-3 null cells were differentiated for different periods of time (0, 1, 2, and 3 d). Then, cells were collected and subjected to immunoblot analysis with an antibody probe specific for M-cadherin. Figure 4A illustrates that M-cadherin expression was transiently upregulated after 1 d of differentiation in control skeletal muscle cells (CTL). This result is consistent with previous data showing that M-cadherin expression is greatly induced in activated satellite cells of regenerating muscle (Kuch et al., 1997; Kaufmann et al., 1999). Interestingly, caveolin-3–overexpressing cells (Cav-3) failed to upregulate M-cadherin after 1 d of differentiation (Figure 4A). In contrast, caveolin-3 null cells (KO) upregulated M-cadherin, like control cells, after 1 d of differentiation, but failed to downregulate its expression as the differentiation continued (Figure 4A). Quantitative analysis from three independent experiments is shown in Figure 4B. We speculate that the abnormal pattern of M-cadherin expression observed in caveolin-3 transgenic and null cells may represent one of the molecular mechanisms underlying the fusion defect observed in these cells.
Figure 4.
Expression of cadherins in conditionally immortalized skeletal muscle cells derived from control, caveolin-3 transgenic, and caveolin-3 null mice. Cells were differentiated for different periods of time (0, 1, 2, and 3 d). Then, cell lysates were subjected to SDS-PAGE and Western blotting analysis using antibody probes specific for M-cadherin (A), and N-cadherin (C). Interestingly, M-cadherin and N-cadherin expression is reduced in caveolin-3 transgenic (Cav-3) cells after 1 d of differentiation. In addition, M-cadherin protein level remains elevated in caveolin-3 null (KO) cells after 3 d of differentiation, compared with control cells. Quantitation of M-cadherin and N-cadherin protein expression, from three independent experiments, is illustrated in B and D, respectively.
Besides M-cadherin, skeletal muscle cells have been shown to express N-cadherin. Thus, we decided to investigate whether overexpression or lack of caveolin-3 affects the expression levels of N-cadherin as well. Figure 4C indicates that, similarly to M-cadherin, N-cadherin expression was upregulated after 1 d of differentiation in control cells. However, although M-cadherin expression was downregulated later during the differentiation in control cells (see M-cadherin expression in cells differentiated for 2 and 3 d), N-cadherin expression remained elevated. This result suggests that these two cadherin family members may potentially have distinct temporal functions during skeletal muscle differentiation. Interestingly, N-cadherin expression was significantly reduced in caveolin-3 transgenic cells, compared with control and caveolin-3 null cells (Figure 4C). Thus, downregulation of both M- and N-cadherin early during the differentiation may contribute to the fusion defect observed in caveolin-3 transgenic cells. On the other hand, N-cadherin expression does not seem to contribute to the enhanced fusion process occurring in caveolin-3 null cells. In fact, contrarily to M-cadherin, N-cadherin expression was identical in control and caveolin-3 null cells after 3 d of differentiation (Figure 4C). Quantitative analysis from three independent experiments is shown in Figure 4D.
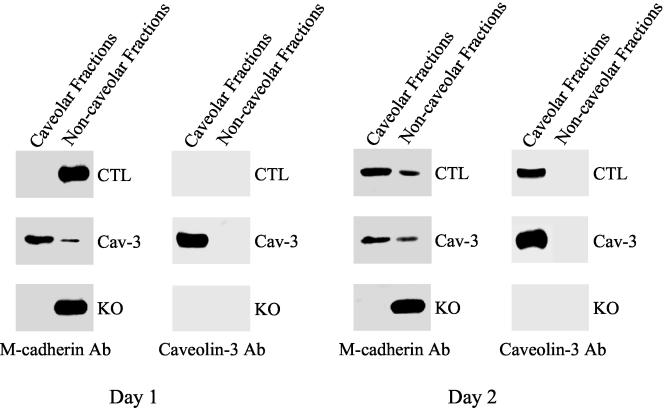
M-cadherin Is Enriched into Caveolae Membranes in Skeletal Muscle Cells Only When Caveolin-3 Is Expressed
To investigate whether caveolin-3 is involved in mediating M-cadherin signaling, it is important to demonstrate that M-cadherin is enriched into caveolae membranes in skeletal muscle cells. Control, caveolin-3 transgenic, and caveolin-3 null precursor skeletal muscle cells were differentiated for 1 and 2 d. Then, caveolae-enriched membrane fractions were separated from the bulk of cellular membranes and cytosolic proteins by taking advantage of the known biophysical properties of caveolae domains. Caveolae membranes are resistant to extraction at 4°C by nonionic detergents such as Triton X-100 and float on bottom-loaded sucrose density gradients because of their high content of cholesterol and sphingolipids. We used a well-established procedure based on the detergent resistance and low buoyant density of caveolae. Caveolin-3, the principal structural protein of caveolae in skeletal muscle tissue, was used to track the position of caveolae-derived membranes in this fractionation scheme. The distribution of M-cadherin was followed by Western blot analysis. Figure 5 illustrates that, early in the differentiation (1 d of differentiation), control skeletal muscle cells lacked caveolin-3, and M-cadherin was not found in cholesterol-enriched microdomains of the sarcolemma. After 2 d of differentiation, caveolin-3 expression was upregulated and M-cadherin moved into caveolae membranes in control cells (similar results were obtained after 3 d of differentiation; our unpublished observations). In contrast, we observed M-cadherin into sarcolemmal caveolae after either 1 or 2 d of differentiation in caveolin-3 transgenic cells, which constitutively express caveolin-3. M-cadherin was never found into cholesterol-enriched microdomains in caveolin-3 null cells. Because myoblast fusion is inhibited in caveolin-3–overexpressing myotubes and enhanced in caveolin-3 null myotubes, these results suggest that M-cadherin promotes myoblast fusion only when localized outside caveolae membranes.
Figure 5.
Cofractionation of caveolin-3 with M-cadherin in skeletal muscle cells. Control (CTL), caveolin-3 transgenic (Cav-3), and caveolin-3 null (KO) skeletal muscle cells were differentiated for 1 or 2 d. Caveolae membranes were separated from the bulk of cellular membranes and cytosolic proteins by equilibrium sucrose density gradient centrifugation (see MATERIALS AND METHODS for details). In this fractionation scheme, immunoblotting with anti–caveolin-3 IgG can be used to track the position of caveolae-derived membranes within these bottom-loaded sucrose gradient. Fractions 4–6, caveolar membranes; fractions 9–12, noncaveolar membranes. Note that M-cadherin is enriched into caveolae membranes only when caveolin-3 is expressed.
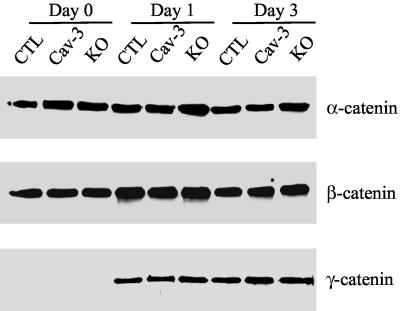
α-, β-, and γ-catenin Expression in Conditionally Immortalized Skeletal Muscle Cells
In skeletal muscle cells, cadherins have been proposed to interact with β-catenin or γ-catenin (plakoglobin) and, via these proteins, with α-catenin (Kuch et al., 1997; Cifuentes-Diaz et al., 1998). Because we demonstrated that overexpression or lack of caveolin-3 affects the expression of M- and N-cadherin, we evaluated, by Western blotting analysis, the expression levels of α-, β-, and γ-catenin in CTL, Cav-3, and KO skeletal muscle cells. Cells were differentiated for different periods of time (0, 1, and 3 d) and subjected to immunoblotting analysis using monoclonal antibodies specific for these catenins. Figure 6 indicates that α- and β-catenin were the major catenin isoforms expressed before differentiation in control cells. After differentiation, α-catenin expression did not change, whereas β-catenin was slightly transiently upregulated after 1 d of differentiation. In contrast, γ-catenin was dramatically upregulated after 1 d of differentiation and its expression remained unchanged as the differentiation proceeded. In addition, Figure 6 shows that the expression levels of α-, β-, and γ-catenin were not affected by overexpression or lack of caveolin-3. Taken together, these results indicate that either reduced levels of M- and N-cadherin in caveolin-3 transgenic cells or increased M-cadherin expression in caveolin-3 null cells did not affect the expression of α-, β-, and γ-catenin. Thus, the primary defect of the cadherin/catenin complex in caveolin-3 transgenic and null cells is limited to cadherin members.
Figure 6.
Expression of catenins in conditionally immortalized skeletal muscle cells derived from control, caveolin-3 transgenic, and caveolin-3 null mice. Cells were differentiated for different periods of time (0, 1, and 3 d). Then, cell lysates were subjected to SDS-PAGE and Western blotting analysis using antibody probes specific for α-, β-, and γ-catenin. Note that caveolin-3 overexpression or lack of caveolin-3 does not affect α-, β-, and γ-catenin protein expression.
α-tubulin Interacts and Cofractionates with Caveolin-3 in Control and Caveolin-3 Transgenic Myotubes
The dystrophin glicoprotein complex, which links the extracellular matrix to the intracellular cytoskeleton network, is enriched into caveolae membranes in skeletal muscle cells (Stahlhut and van Deurs, 2000). We have previously demonstrated that dystrophin, α-sarcoglycan, and β-dystroglycan are downregulated in skeletal muscle tissue of caveolin-3 transgenic mice (Galbiati et al., 2000b) and are excluded from lipid raft domains in skeletal muscle tissue of caveolin-3 null mice (Galbiati et al., 2001a). Thus, it is possible to speculate that overexpression or lack of caveolin-3 may affect the proper organization of the cytoskeleton network in conditionally immortalized skeletal muscle cells.
Microtubules are key components of the cytoskeleton network. Microtubules have been shown to mediate myotube formation by modulating the alignment of myoblasts, which is necessary to generate multinucleated myotubes (Saitoh et al., 1988; Gundersen et al., 1989; Mangan and Olmsted, 1996; Kaufmann et al., 1999). Because we demonstrated fusion defects in caveolin-3 transgenic and null cells, we asked whether overexpression or lack of caveolin-3 affects the organization of microtubules in these cells.
To investigate a possible role of caveolin-3 in the organization of microtubules, we decided first to verify whether α-tubulin interacts with caveolin-3. α-tubulin is a major cytoskeleton component that multimerizes with β-tubulin to form microtubule filaments. Skeletal muscle cells were differentiated for 3 d and solubilized with octyl glucoside, which is known to solubilize caveolae membranes, and cell lysates were immunoprecipitated with anti–caveolin-3 IgG. Then, immunoprecipitates were subjected to Western blotting analysis with an antibody probe specific for α-tubulin. Figure 7A shows that caveolin-3 IgG coimmunoprecipitated α-tubulin in control and caveolin-3 transgenic myotubes. The amount of α-tubulin immunoprecipitated by caveolin-3 IgG was slightly higher in caveolin-3 transgenic cells, compared with control cells. This may reflect the higher level of endogenous caveolin-3 in caveolin-3–overexpressing myotubes. Importantly, α-tubulin was not coimmunoprecipitated in caveolin-3 null myotubes, which do not express caveolin-3 (Figure 7A).
Figure 7.
α-tubulin interacts with caveolin-3 and is partially localized into caveolae membranes in control and caveolin-3 transgenic myotubes. (A) Coimmunoprecipitation. Control (CTL), caveolin-3 transgenic (Cav-3), and caveolin-3 null (KO) skeletal muscle cells were differentiated for 3 d. Cells were solubilized with octyl glucoside, and cell lysates were immunoprecipitated with anti–caveolin-3 IgG. Then, immunoprecipitates were subjected to immunoblot analysis with an antibody probe specific for α-tubulin (top panel). Interestingly, α-tubulin was coimmunoprecipitated by caveolin-3 IgG in control and caveolin-3 transgenic myotubes. As an important internal control, caveolin-3 IgG did not coimmunoprecipitate α-tubulin in caveolin-3 null myotubes, which do not express caveolin-3. The two lower panels illustrate the expression of α-tubulin and caveolin-3 before immunoprecipitation. (B) Sucrose gradient. Control (CTL), caveolin-3 transgenic (Cav-3), and caveolin-3 null (KO) skeletal muscle cells were differentiated for 3 d. Caveolae membranes were separated from the bulk of cellular membranes and cytosolic proteins by equilibrium sucrose density gradient centrifugation (see MATERIALS AND METHODS for details). In this fractionation scheme, immunoblotting with anti–caveolin-3 IgG can be used to track the position of caveolae-derived membranes within these bottom-loaded sucrose gradient. Fractions 4–6, caveolar membranes; fractions 9–12, noncaveolar membranes. Note that α-tubulin partially cofractionates with caveolin-3 in control and caveolin-3 transgenic cells.
To further characterize the interaction between α-tubulin and caveolin-3, we examined the possible caveolar localization of α-tubulin in conditionally immortalized myotubes. Toward this end, we separated caveolae-enriched membrane fractions from the bulk of cellular membranes and cytosolic proteins by using the sucrose density gradient centrifugation protocol described above. We used caveolin-3 to track the position of caveolae-derived membranes. The distribution of α-tubulin was followed by Western blotting analysis. Figure 7B shows that α-tubulin was partially localized into caveolae fractions in control and caveolin-3 transgenic myotubes after 3 d of differentiation. In contrast, α-tubulin was completely excluded from Triton-insoluble microdomains in caveolin-3 null myotubes, which lack caveolin-3 protein expression (Figure 7B). Taken together, these data indicate that caveolin-3 and α-tubulin may coexist within the same cellular compartment.
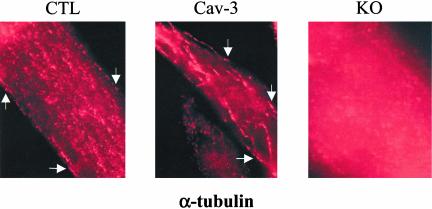
Microtubules Appear Disorganized in Caveolin-3 Null Myotubes
The results of Figure 7, A and B, suggest that caveolae may play a pivotal role in anchoring microtubules to the plasma membrane in skeletal muscle cells. To further investigate this hypothesis, we studied the cellular localization of α-tubulin in differentiated conditionally immortalized skeletal muscle cells. Cells were differentiated for 3 d at 37°C with 4% horse serum in the absence of γ-interferon. Then, cells were fixed in 2% paraformaldheyde for 30 min and subjected to immunostaining with a mAb probe that recognizes only α-tubulin. Microtubules appeared as filaments that occasionally reached the plasma membrane in control and caveolin-3 transgenic myotubes (see arrows in Figure 8, left and middle panels). These results are consistent with the partial localization of α-tubulin in caveolar membranes in control and caveolin-3 transgenic myotubes (Figure 7B). Interestingly, α-tubulin was not localized at the plasma membrane and appeared diffused into the cytoplasm in caveolin-3 null myotubes (Figure 8, right panel), suggesting that caveolin-3 is necessary to anchor microtubules to the plasma membrane.
Figure 8.
Microtubules appear disorganized in caveolin-3 null myotubes. Immunofluorescence. Control (CTL), caveolin-3 transgenic (Cav-3), and caveolin-3 null (KO) skeletal muscle cells were differentiated for 3 d. Then, cells were fixed in 2% paraformaldheyde for 30 min and immunostained with anti–α-tubulin IgG. Cells were observed using a Provis fluorescent microscope (Olympus). Interestingly, microtubules appear as filaments reaching the plasma membrane only in control and caveolin-3 transgenic myotubes. α-tubulin staining is diffused in caveolin-3 null myotubes.
Given the importance of microtubules in modulating myoblast fusion to myotubes, these data suggest that lack of caveolin-3 presumably affects microtubule functions, which may partially contribute to the fusion defect that we observed in caveolin-3 null cells.
Actin Localization in Caveolin-3 Transgenic and Null Myotubes
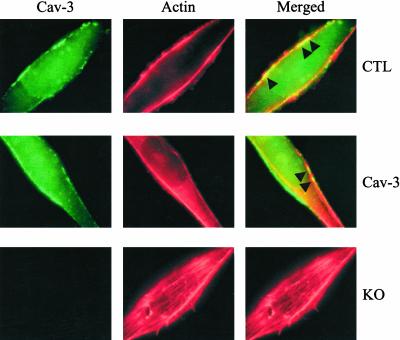
To further understand how overexpression or lack of caveolin-3 may affect the organization of the cytoskeleton network, we decided to investigate the localization of actin in conditionally immortalized skeletal muscle cells. Cells were differentiated for 3 d and fixed in 2% paraformaldheyde for 30 min. Then, cells were subjected to immunostaining with a monoclonal specific antibody probe that recognizes only caveolin-3 and with Alexa 568–labeled phalloidin to identify actin. Cells were observed under a Provis fluorescence microscope (Olympus). As expected, caveolin-3 was mainly localized at the plasma membrane in control and caveolin-3 transgenic myotubes (Figure 9). In addition, Figure 9 shows that Alexa 568-labeled phalloidin gave rise to two major string-like staining in the proximity of the plasma membrane in control and caveolin-3–overexpressing myotubes. Interestingly, the merged image clearly indicates that caveolin-3 and actin partially colocalized at the plasma membrane in both control and caveolin-3 transgenic myotubes (see arrowheads in the upper and middle right panels). In contrast, several actin filaments, which resemble stress-fiber like structures, appeared in the middle of caveolin-3 null myotubes, far from the plasma membrane (Figure 9, bottom panels). Because stress-fiber like structures have been observed during myotube formation in culture (van der Ven et al., 1993; van der Loop et al., 1996), these data are consistent with the idea that myoblast fusion remains constantly elevated in caveolin-3 null myotubes. Taken together, these data indicate that lack of caveolin-3 results in significant rearrangements of actin filaments. As microtubules and actin filaments are both part of the cytoskeleton network, these results are consistent with the mislocalization of α-tubulin in caveolin-3 null myotubes (Figure 8).
Figure 9.
Actin localization in conditionally immortalized skeletal muscle myotubes. Conditionally immortalized skeletal muscle cells derived from control (CTL), caveolin-3 transgenic (Cav-3), and caveolin-3 null (KO) mice, were differentiated for 3 d. Then, cells were subjected to double staining with anti–caveolin-3 IgG (green) and Alexa 568-labeled phalloidin (red). Note that string-like actin filaments partially colocalize with caveolin-3 at the sarcolemma in control and caveolin-3 transgenic cells. In contrast, actin filaments appear as stress-fiber like structures, which run in the middle of the myotube, in caveolin-3 null cells.
DISCUSSION
The inability of satellite cells to properly differentiate, fuse, and form new myotubes during the regeneration process represents an attractive potential mechanism underlying the progressive muscle degeneration observed in muscular dystrophies. Interestingly, satellite cells have been demonstrated to exhibit reduced proliferative capacity with increasing age of the donor and are rapidly depleted in Duchenne muscular dystrophy, presumably because of high levels of ongoing regeneration (Cossu and Mavilio, 2000; Heslop et al., 2000; Renault et al., 2000). In the present study, we further investigated the role of satellite cells in the pathogenesis of DMD and LGMD-1C. Toward this end, we generated conditionally immortalized precursor skeletal muscle cells from caveolin-3 transgenic and null mice. Importantly, promoting the differentiation of precursor skeletal muscle cells in culture is a valid model for investigating skeletal muscle development and the muscle regeneration process that occur in vivo. We demonstrated that myotubes originated from precursor caveolin-3 transgenic myoblasts are smaller, whereas caveolin-3 null myotubes are larger than control myotubes. These observations suggest that overexpression of caveolin-3 inhibits myoblast fusion to multinucleated myotubes and that lack of caveolin-3 enhances the fusion process.
Why do caveolin-3 transgenic and null cells exhibit severe fusion defects? Our data indicate that overexpression or lack of caveolin-3 may affect M-cadherin functions. M-cadherin has been demonstrated to play an important role in the fusion of myoblasts to multinucleate myofibers (Kuch et al., 1997; Kaufmann et al., 1999). In fact, prevention of cell-cell interactions through the use of synthetic peptides that bind to the extracellular interaction domain of M-cadherin inhibited myoblast fusion without affecting the differentiation process (Zeschnigk et al., 1995). In addition, overexpression of M-cadherin in cadherin-deficient cells that fail to adhere to each other, promoted cell adhesion, an essential step to achieve cell fusion (Kuch et al., 1997). We demonstrated here that caveolin-3 transgenic cells fail to upregulate M-cadherin early in the differentiation and that caveolin-3 null cells fail to downregulate M-cadherin late in the differentiation. Thus, these results directly indicate that perturbation of the M-cadherin signaling may represent one of the molecular mechanisms behind the fusion defect observed in these cells.
Recently, Hollnagel, Arnold, and colleagues, have demonstrated that M-cadherin null satellite cells exhibit normal growth and fusion potential in culture (Hollnagel et al., 2002). However, they reported a delay in the upregulation of Myf-5 and myogenin, two myogenic regulatory factors, in regenerating muscle of M-cadherin null mice. In addition, they showed that E-cadherin and N-cadherin, two additional members of this family of adhesion molecules are expressed at normal levels in these cells. They concluded that E-cadherin and N-cadherin can largely compensate for M-cadherin during skeletal muscle differentiation (Hollnagel et al., 2002). Thus, in order to investigate whether overexpression or lack of caveolin-3 may affect the expression levels of additional cadherin members, we evaluated the expression level of N-cadherin in control, caveolin-3 transgenic, and caveolin-3 null precursor skeletal muscle cells. We demonstrated that N-cadherin, similarly to M-cadherin, is downregulated in caveolin-3 transgenic skeletal muscle cells after differentiation, compared with control cells. These results suggest that overexpression of caveolin-3 may affect the expression of different members of the cadherin family, i.e., M-cadherin and N-cadherin. Thus, consistently with the results of Hollnagel, Arnold, and colleagues, the defect of myoblast fusion that we observed in Cav-3 skeletal muscle cells may be partially explained by the lack of a compensatory mechanism. In addition, in contrast to M-cadherin, we found that N-cadherin expression after 3 d of differentiation was similar in control and caveolin-3 null cells. These data suggest that elevated expression of M-cadherin in caveolin-3 null cells, but not other members of the cadherin family, may enhance skeletal muscle fusion. Taken together, these data support the hypothesis that M-cadherin is a key element in skeletal muscle fusion. Because caveolin-3 transgenic and null cells are derived from mouse models of DMD and LGMD-1C, respectively, these data link for the first time M-cadherin dysfunctions to a muscular dystrophy phenotype.
Interestingly, M-cadherin downregulation after 2 d of differentiation in control skeletal muscle cells temporally matches the upregulation of caveolin-3. In addition, we demonstrated that after 2 d of differentiation, M-cadherin is enriched into caveolae membranes in control cells. Thus, we may speculate that the movement of M-cadherin into these microdomains of the plasma membrane inhibits M-cadherin–mediated signaling and, as a consequence, myoblast fusion. This may represent a molecular mechanism, adopted by skeletal muscle cells, to tightly regulate myofiber size within mature skeletal muscle tissue. In caveolin-3 transgenic cells, M-cadherin is constantly enriched into caveolae and the myoblast fusion process is inhibited early in the differentiation program. In contrast, the enhanced myoblast fusion observed in caveolin-3 null cells may be explained by the absence of the negative regulation of caveolin-3 on M-cadherin signaling. These results are schematically summarized in Figure 10.
Figure 10.
Schematic diagram summarizing the modulation of myoblast fusion by caveolin-3. M-cadherin stimulates myoblast fusion in normal control skeletal muscle cells (CTL) as long as caveolin-3 is not expressed. After 2 d of differentiation, upregulation of caveolin-3 leads to sequestration of M-cadherin into caveolae membranes and, as a consequence, inhibition of myoblast fusion. Caveolin-3 is always expressed in caveolin-3 transgenic cells (Cav-3) and M-cadherin is constantly enriched into caveolae. As a result, myoblast fusion is inhibited by overexpression of caveolin-3. In contrast, inhibition of myoblast fusion after 2 d of differentiation does not occur in caveolin-3 null myotubes (KO), which do not express caveolin-3.
The fusion defect observed in caveolin-3 null cells may be further explained by the disorganization of microtubules. In support of this hypothesis, microtubules have been proposed to modulate the alignment of myoblasts before and during the fusion to myotubes (Saitoh et al., 1988; Gundersen et al., 1989; Mangan and Olmsted, 1996; Kaufmann et al., 1999). We found that α-tubulin staining is diffused in caveolin-3 null myotubes, indicating a severe defect in the organization of microtubules associated with lack of caveolin-3. We also demonstrated that α-tubulin coimmunoprecipitates with caveolin-3 and is partially localized into caveolae membranes, suggesting that microtubules may be linked to the plasma membrane at the level of caveolae. As a consequence, lack of caveolin-3 may have a dramatic negative impact on the anchoring of microtubules to the sarcolemma in skeletal muscle cells and, as a consequence, a direct effect on the fusion ability of caveolin-3 null myotubes. Because caveolin-3 null myotubes keep fusing as the differentiation continues, we may speculate that microtubules, at least late in the differentiation program, contribute to mediate signaling events important to avoid excessive myoblast fusion.
Surprisingly, the enhanced fusion phenotype that we observed in conditionally immortalized caveolin-3 null cells was in contrast to our previous observations that C2C12 cells, in which caveolin-3 expression was kept low by a caveolin-3 antisense cDNA, were unable to form myotubes in culture (Galbiati et al., 1999a). However, this discrepancy can be explained by the fact that C2C12 cells express caveolin-1 and caveolin-2 in addition to caveolin-3 (Galbiati et al., 1999a). In contrast, skeletal muscle tissue and conditionally immortalized myotubes express only caveolin-3. Because caveolin-1 expression is sufficient to form caveolae, C2C12 cells harboring a caveolin-3 antisense construct presumably possess caveolae membranes. In this report, we demonstrate that plasma membrane caveolae are important for the organization of the cytoskeleton and the proper localization of M-cadherin at the plasma membrane. In addition, we show that lack of caveolae membranes in caveolin-3 null myotubes enhances myoblast fusion. Caveolin-1 and -3 are 65% identical and 85% similar (Tang et al., 1996) and have been shown to share similar functional properties. In fact, both caveolin-1 and -3 i) promote cell cycle arrest in the G0/G1 phase of the cell cycle (Galbiati et al., 2001b); ii) inhibit Raf, MEK, and ERK-mediated signal transduction in vivo (Engelman et al., 1998a, 1998b, 1999); iii) inhibit the enzymatic activity of nitric oxide synthase (NOS) in vitro and in vivo (Garcia-Cardena et al., 1997; Engelman et al., 1998a; Galbiati et al., 2001b); and iv) stimulate the ability of the insulin-receptor kinase to phosphorylate IRS-1 in vitro (Yamamoto et al., 1998). In addition, we have previously demonstrated that E-cadherin is localized into caveolae membranes in MDCK cells, where caveolin-1, but not caveolin-3, is expressed (Galbiati et al., 2000a). Also, caveolin-1 has been shown to bind filamin, suggesting a link between caveolin-1 and the actin cytoskeleton (Stahlhut and van Deurs, 2000). Given the similarity between caveolin-1 and -3, one may reasonably assume that the presence of caveolin-1 in caveolin-3 antisense C2C12 cells may overcome the dramatic disorganization of M-cadherin and the cytoskeleton observed in conditionally immortalized caveolin-3 null cells. Most importantly, we show in the present report that lack of caveolin-3 enhances myoblast fusion in vivo. In fact, skeletal muscle fibers derived from caveolin-3 null mice appear larger in diameter (Figure 3). As a consequence, these in vivo results directly support the in vitro data of conditionally immortalized caveolin-3 null myotubes.
Lack of a link between intracellular cytoskeleton elements and the extracellular matrix, due to downregulation of the dystrophin complex, is considered one of the primary causes of muscle degeneration in Duchenne muscular dystrophy. In fact, in the mdx mouse, a mouse model of DMD with a dystrophin deficiency, the muscle demonstrated an enhanced susceptibility to injury during repetitive lengthening activation (Petrof et al., 1993; Deconinck et al., 1996). In addition, such membrane destabilization leads to the activation of multiple pathophysiological processes, many of which converge on alterations in intracellular calcium handling, which eventually leads to cell death (Turner et al., 1988). However, the precise molecular mechanisms underlying Duchenne muscular dystrophy are not completely understood. We have previously shown that overexpression of caveolin-3 in mice results in downregulation of dystrophin and its associated glycoproteins, and in severe muscle degeneration, as seen in Duchenne muscular dystrophy patients (Galbiati et al., 2000b). Interestingly, caveolin-3 expression is upregulated in Duchenne patients and in the mdx mouse (by ∼2- to 3-fold; Vaghy et al., 1998; Repetto et al., 1999). Thus, one possibility is that overexpression of wild-type caveolin-3 disrupts the normal processing or stoichiometry of the dystrophin complex, leading to the degradation of its components and, as a consequence, contributes to the degeneration process. In support of this hypothesis, a novel WW-like domain within caveolin-3 has been shown to directly recognize the extreme C terminus of β-dystroglycan that contains a PPXY motif (Sotgia et al., 2000). Because the WW domain of dystrophin recognizes the same site within β-dystroglycan, caveolin-3 can effectively block the interaction of dystrophin with β-dystroglycan in vitro (Sotgia et al., 2000), suggesting competitive regulation of the recruitment of dystrophin to the sarcolemma in vivo.
We speculate here that a defect in the fusion of satellite cells to form new myotubes, during the regeneration process, may represent an additional molecular mechanism underlying the progressive muscle degeneration observed in DMD. These results directly contribute at furthering our understanding of the molecular mechanisms underlying Duchenne muscular dystrophy in humans.
Acknowledgments
We thank Dr. Michael P. Lisanti for generously providing caveolin-3 transgenic and knock-out mice. We thank Dr. Simon Watkins and Sean Alber for help with confocal and fluorescent microscopy. This work was supported by grants from the American Heart Association (AHA) and the American Cancer Society (IRG-60–002-40) and start-up funds from the Department of Pharmacology (to F.G.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–03–0161. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-03-0161.
References
- Bonilla, E., Fishbeck, K., and Schotland, D. (1981). Freeze-fracture studies of muscle caveolae in human muscular dystrophy. Am. J. Pathol. 104, 167–173. [PMC free article] [PubMed] [Google Scholar]
- Cifuentes-Diaz, C., Goudou, D., Mege, R.M., Velasco, E., Nicolet, M., Herren-knecht, K., Rubin, L., and Rieger, F. (1998). Distinct location and prevalence of alpha-, beta-catenins and gamma-catenin/plakoglobin in developing and denervated skeletal muscle. Cell Adhes. Commun. 5, 161–176. [DOI] [PubMed] [Google Scholar]
- Cooper, R.N., Tajbakhsh, S., Mouly, V., Cossu, G., Buckingham, M., and Butler-Browne, G.S. (1999). In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J. Cell Sci. 112(Pt 17), 2895–2901. [DOI] [PubMed] [Google Scholar]
- Cornelison, D.D., and Wold, B.J. (1997). Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 191, 270–283. [DOI] [PubMed] [Google Scholar]
- Cossu, G., and Mavilio, F. (2000). Myogenic stem cells for the therapy of primary myopathies: wishful thinking or therapeutic perspective? J. Clin. Invest. 105, 1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couet, J., Li, S., Okamoto, T., Scherer, P.S., and Lisanti, M.P. (1997). Molecular and cellular biology of caveolae: paradoxes and plasticities. Trends Cardiovasc. Med. 7, 103–110. [DOI] [PubMed] [Google Scholar]
- Crosbie, R.H., Yamada, H., Venzke, D.P., Lisanti, M.P., and Campbell, K.P. (1998). Caveolin-3 is not an essential component of the dystrophin glycoprotein complex. FEBS Lett. 427, 279–282. [DOI] [PubMed] [Google Scholar]
- Deconinck, N., Ragot, T., Marechal, G., Perricaudet, M., and Gillis, J.M. (1996). Functional protection of dystrophic mouse (mdx) muscles after adenovirus-mediated transfer of a dystrophin minigene. Proc. Natl. Acad. Sci. USA 93, 3570–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehler, E., Jat, P.S., Noble, M.D., Citi, S., and Draeger, A. (1995). Vascular smooth muscle cells of H-2Kb-tsA58 transgenic mice. Characterization of cell lines with distinct properties. Circulation 92, 3289–3296. [DOI] [PubMed] [Google Scholar]
- Engelman, J.A., Chu, C., Lin, A., Jo, H., Ikezu, T., Okamoto, T., Kohtz, D.S., and Lisanti, M.P. (1998a). Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 428, 205–211. [DOI] [PubMed] [Google Scholar]
- Engelman, J.A. et al. (1998b). Reciprocal regulation of Neu tyrosine kinase activity and caveolin-1 protein expression in vitro and in vivo. Implications for mammary tumorigenesis. J. Biol. Chem. 273, 20448–20455. [DOI] [PubMed] [Google Scholar]
- Engelman, J.A., Zhang, X.L., Razani, B., Pestell, R.G., and Lisanti, M.P. (1999). p42/44 MAP kinase-dependent and -independent signaling pathways regulate caveolin-1 gene expression. Activation of Ras-MAP kinase and protein kinase a signaling cascades transcriptionally down-regulates caveolin-1 promoter activity. J. Biol. Chem. 274, 32333–32341. [DOI] [PubMed] [Google Scholar]
- Galbiati, F. et al. (2001a). Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J. Biol. Chem. 276, 21425–21433. [DOI] [PubMed] [Google Scholar]
- Galbiati, F., Volonte, D., Brown, A.M., Weinstein, D.E., Ben-Ze'ev, A., Pestell, R.G., and Lisanti, M.P. (2000a). Caveolin-1 expression inhibits Wnt/beta-catenin/Lef-1 signaling by recruiting beta-catenin to caveolae membrane domains. J. Biol. Chem. 275, 23368–23377. [DOI] [PubMed] [Google Scholar]
- Galbiati, F. et al. (2000b). Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc. Natl. Acad. Sci. USA 97, 9689–9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati, F., Volonte, D., Engelman, J.A., Scherer, P.E., and Lisanti, M.P. (1999a). Targeted down-regulation of caveolin-3 is sufficient to inhibit myotube formation in differentiating C2C12 myoblasts. Transient activation of p38 mitogen-activated protein kinase is required for induction of caveolin-3 expression and subsequent myotube formation. J. Biol. Chem. 274, 30315–30321. [DOI] [PubMed] [Google Scholar]
- Galbiati, F., Volonte, D., Liu, J., Capozza, F., Frank, P.G., Zhu, L., Pestell, R.G., and Lisanti, M.P. (2001b). Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Mol. Biol. Cell 12, 2229–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati, F., Volonte, D., Minetti, C., Bregman, D.B., and Lisanti, M.P. (2000c). Limb-girdle muscular dystrophy (LGMD-1C) mutants of caveolin-3 undergo ubiquitination and proteasomal degradation. Treatment with proteasomal inhibitors blocks the dominant negative effect of LGMD-1C mutants and rescues wild-type caveolin-3. J. Biol. Chem. 275, 37702–37711. [DOI] [PubMed] [Google Scholar]
- Galbiati, F., Volonte, D., Minetti, C., Chu, J.B., and Lisanti, M.P. (1999b). Phenotypic behavior of caveolin-3 mutations that cause autosomal dominant limb girdle muscular dystrophy (LGMD-1C). Retention of LGMD-1C caveolin-3 mutants within the golgi complex. J. Biol. Chem. 274, 25632–25641. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena, G., Martasek, P., Masters, B.S., Skidd, P.M., Couet, J., Li, S., Lisanti, M.P., and Sessa, W.C. (1997). Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J. Biol. Chem. 272, 25437–25440. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena, G., Oh, P., Liu, J., Schnitzer, J.E., and Sessa, W.C. (1996). Targeting of nitric oxide synthase to endothelilal cell caveolae via palmitoylation: implications for caveolae localization. Proc. Natl. Acad. Sci. USA 93, 6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger, B., and Ayalon, O. (1992). Cadherins. Annu. Rev. Cell Biol. 8, 307–332. [DOI] [PubMed] [Google Scholar]
- Grounds, M.D. (1998). Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann. NY Acad. Sci. 854, 78–91. [DOI] [PubMed] [Google Scholar]
- Gundersen, G.G., Khawaja, S., and Bulinski, J.C. (1989). Generation of a stable, posttranslationally modified microtubule array is an early event in myogenic differentiation. J. Cell Biol. 109, 2275–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara, Y., Sasaoka, T., Araishi, K., Imamura, M., Yorifuji, H., Nonaka, I., Ozawa, E., and Kikuchi, T. (2000). Caveolin-3 deficiency causes muscle degeneration in mice. Hum. Mol. Genet. 9, 3047–3054. [DOI] [PubMed] [Google Scholar]
- Heslop, L., Morgan, J.E., and Partridge, T.A. (2000). Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J. Cell Sci. 113(Pt 12), 2299–2308. [DOI] [PubMed] [Google Scholar]
- Hollnagel, A., Grund, C., Franke, W.W., and Arnold, H.H. (2002). The cell adhesion molecule M-cadherin is not essential for muscle development and regeneration. Mol. Cell Biol. 22, 4760–4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, O., Bierkamp, C., and Kemler, R. (1996). Cadherins and catenins in development. Curr. Opin. Cell Biol. 8, 685–691. [DOI] [PubMed] [Google Scholar]
- Irintchev, A., Zeschnigk, M., Starzinski-Powitz, A., and Wernig, A. (1994). Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev. Dyn. 199, 326–337. [DOI] [PubMed] [Google Scholar]
- Jat, P.S., Noble, M.D., Ataliotis, P., Tanaka, Y., Yannoutsos, N., Larsen, L., and Kioussis, D. (1991). Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc. Natl. Acad. Sci. USA 88, 5096–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda, S., Landgren, E., Ljungstrom, M., and Claesson-Welsh, L. (1996). Fibroblast growth factor receptor 1-induced differentiation of endothelial cell line established from tsA58 large T transgenic mice. Cell Growth Differ. 7, 383–395. [PubMed] [Google Scholar]
- Kaufmann, U., Kirsch, J., Irintchev, A., Wernig, A., and Starzinski-Powitz, A. (1999). The M-cadherin catenin complex interacts with microtubules in skeletal muscle cells: implications for the fusion of myoblasts. J. Cell Sci. 112(Pt 1), 55–68. [DOI] [PubMed] [Google Scholar]
- Kuch, C., Winnekendonk, D., Butz, S., Unvericht, U., Kemler, R., and Starzinski-Powitz, A. (1997). M-cadherin-mediated cell adhesion and complex formation with the catenins in myogenic mouse cells. Exp. Cell Res. 232, 331–338. [DOI] [PubMed] [Google Scholar]
- Li, S., Okamoto, T., Chun, M., Sargiacomo, M., Casanova, J.E., Hansen, S.H., Nishimoto, I., and Lisanti, M.P. (1995). Evidence for a regulated interaction of hetero-trimeric G proteins with caveolin. J. Biol. Chem. 270, 15693–15701. [DOI] [PubMed] [Google Scholar]
- Li, S., Song, K.S., and Lisanti, M.P. (1996). Expression and characterization of recombinant caveolin: purification by poly-histidine tagging and cholesterol-dependent incorporation into defined lipid membranes. J. Biol. Chem. 271, 568–573. [PubMed] [Google Scholar]
- Lisanti, M.P., Scherer, P., Tang, Z.-L., and Sargiacomo, M. (1994). Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 4, 231–235. [DOI] [PubMed] [Google Scholar]
- Mangan, M.E., and Olmsted, J.B. (1996). A muscle-specific variant of microtubule-associated protein 4 (MAP4) is required in myogenesis. Development 122, 771–781. [DOI] [PubMed] [Google Scholar]
- Minetti, C. et al. (1998). Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat. Genet. 18, 365–368. [DOI] [PubMed] [Google Scholar]
- Moldovan, N., Heltianu, C., Simionescu, N., and Simionescu, M. (1995). Ultrastructural evidence of differential solubility in Triton X-100 of endothelial vesicles and plasma membrane. Exp. Cell Res. 219, 309–313. [DOI] [PubMed] [Google Scholar]
- Morgan, J.E., Beauchamp, J.R., Pagel, C.N., Peckham, M., Ataliotis, P., Jat, P.S., Noble, M.D., Farmer, K., and Partridge, T.A. (1994). Myogenic cell lines derived from transgenic mice carrying a thermolabile T antigen: a model system for the derivation of tissue-specific and mutation-specific cell lines. Dev. Biol. 162, 486–498. [DOI] [PubMed] [Google Scholar]
- Murata, M., Peranen, J., Schreiner, R., Weiland, F., Kurzchalia, T., and Simons, K. (1995). VIP21/caveolin is a cholesterol-binding protein. Proc. Natl. Acad. Sci. USA 92, 10339–10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble, M., Groves, A.K., Ataliotis, P., Ikram, Z., and Jat, P.S. (1995). The H-2KbtsA58 transgenic mouse: a new tool for the rapid generation of novel cell lines. Transgenic Res. 4, 215–225. [DOI] [PubMed] [Google Scholar]
- Okamoto, T., Schlegel, A., Scherer, P.E., and Lisanti, M.P. (1998). Caveolins, a family of scaffolding proteins for organizing “pre-assembled signaling complexes”at the plasma membrane (Mini-review). J. Biol. Chem. 273, 5419–5422. [DOI] [PubMed] [Google Scholar]
- Petrof, B.J., Shrager, J.B., Stedman, H.H., Kelly, A.M., and Sweeney, H.L. (1993). Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. USA 90, 3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault, V., Piron-Hamelin, G., Forestier, C., DiDonna, S., Decary, S., Hentati, F., Saillant, G., Butler-Browne, G.S., and Mouly, V. (2000). Skeletal muscle regeneration and the mitotic clock. Exp. Gerontol. 35, 711–719. [DOI] [PubMed] [Google Scholar]
- Repetto, S. et al. (1999). Increased number of caveolae and caveolin-3 overexpression in Duchenne muscular dystrophy. Biochem. Biophys Res. Commun. 261, 547–550. [DOI] [PubMed] [Google Scholar]
- Saitoh, O., Arai, T., and Obinata, T. (1988). Distribution of microtubules and other cytoskeletal filaments during myotube elongation as revealed by fluorescence microscopy. Cell Tissue Res. 252, 263–273. [DOI] [PubMed] [Google Scholar]
- Sargiacomo, M., Scherer, P.E., Tang, Z.-L., Casanova, J.E., and Lisanti, M.P. (1994). In vitro phosphorylation of caveolin-rich membrane domains: identification of an associated serine kinase activity as a casein kinase II-like enzyme. Oncogene 9, 2589–2595. [PubMed] [Google Scholar]
- Sargiacomo, M., Scherer, P.E., Tang, Z.-L., Kubler, E., Song, K.S., Sanders, M.C., and Lisanti, M.P. (1995). Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc. Natl. Acad. Sci. USA 92, 9407–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer, P.E., Lisanti, M.P., Baldini, G., Sargiacomo, M., Corley-Mastick, C., and Lodish, H.F. (1994). Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J. Cell Biol. 127, 1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer, P.E., Okamoto, T., Chun, M., Nishimoto, I., Lodish, H.F., and Lisanti, M.P. (1996). Identification, sequence and expression of caveolin-2 defines a caveolin gene family. Proc. Natl. Acad. Sci. USA 93, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer, P.E., Tang, Z.-L., Chun, M.C., Sargiacomo, M., Lodish, H.F., and Lisanti, M.P. (1995). Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution: identification and epitope mapping of an isoform-specific monoclonal antibody probe. J. Biol. Chem. 270, 16395–16401. [DOI] [PubMed] [Google Scholar]
- Schultz, E., and McCormick, K.M. (1994). Skeletal muscle satellite cells. Rev. Physiol. Biochem. Pharmacol. 123, 213–257. [DOI] [PubMed] [Google Scholar]
- Smart, E., Ying, Y.-S., Conrad, P., and Anderson, R.G.W. (1994). Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J. Cell Biol. 127, 1185–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, K.S., Li, S., Okamoto, T., Quilliam, L., Sargiacomo, M., and Lisanti, M.P. (1996a). Copurification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent free purification of caveolae membranes. J. Biol. Chem. 271, 9690–9697. [DOI] [PubMed] [Google Scholar]
- Song, K.S., Sargiacomo, M., Galbiati, F., Parenti, M., and Lisanti, M.P. (1997). Targeting of a G alpha subunit (Gi1 alpha) and c-Src tyrosine kinase to caveolae membranes: clarifying the role of N-myristoylation. Cell. Mol. Biol. (Noisy-Le-Grand) 43, 293–303. [PubMed] [Google Scholar]
- Song, K.S., Scherer, P.E., Tang, Z.-L., Okamoto, T., Li, S., Chafel, M., Chu, C., Kohtz, D.S., and Lisanti, M.P. (1996b). Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J. Biol. Chem. 271, 15160–15165. [DOI] [PubMed] [Google Scholar]
- Sotgia, F. et al. (2000). Caveolin-3 directly interacts with the C-terminal tail of beta-dystroglycan. identification of a central ww-like domain within caveolin family members. J. Biol. Chem. 275, 38048–38058. [DOI] [PubMed] [Google Scholar]
- Stahlhut, M., and van Deurs, B. (2000). Identification of filamin as a novel ligand for caveolin-1, evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol. Biol. Cell 11, 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Z.-L., Scherer, P.E., Okamoto, T., Song, K., Chu, C., Kohtz, D.S., Nishimoto, I., Lodish, H.F., and Lisanti, M.P. (1996). Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem. 271, 2255–2261. [DOI] [PubMed] [Google Scholar]
- Turner, P.R., Westwood, T., Regen, C.M., and Steinhardt, R.A. (1988). Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature 335, 735–738. [DOI] [PubMed] [Google Scholar]
- Vaghy, P.L., Fang, J., Wu, W., and Vaghy, L.P. (1998). Increased caveolin-3 levels in mdx mouse muscles. FEBS Lett. 431, 125–127. [DOI] [PubMed] [Google Scholar]
- van der Loop, F.T., van der Ven, P.F., Furst, D.O., Gautel, M., van Eys, G.J., and Ramaekers, F.C. (1996). Integration of titin into the sarcomeres of cultured differentiating human skeletal muscle cells. Eur J. Cell Biol. 69, 301–307. [PubMed] [Google Scholar]
- van der Ven, P.F., Schaart, G., Croes, H.J., Jap, P.H., Ginsel, L.A., and Ramaekers, F.C. (1993). Titin aggregates associated with intermediate filaments align along stress fiber-like structures during human skeletal muscle cell differentiation. J. Cell Sci. 106(Pt 3), 749–759. [DOI] [PubMed] [Google Scholar]
- Yamamoto, M., Toya, Y., Schwencke, C., Lisanti, M.P., Myers, M., and Ishikawa, Y. (1998). Caveolin is an activator of insulin receptor signaling. J. Biol. Chem. 273, 26962–26968. [DOI] [PubMed] [Google Scholar]
- Zeschnigk, M., Kozian, D., Kuch, C., Schmoll, M., and Starzinski-Powitz, A. (1995). Involvement of M-cadherin in terminal differentiation of skeletal muscle cells. J. Cell Sci. 108(Pt 9), 2973–2981. [DOI] [PubMed] [Google Scholar]