Abstract
The KDEL receptor is a Golgi/intermediate compartment-located integral membrane protein that carries out the retrieval of escaped ER proteins bearing a C-terminal KDEL sequence. This occurs throughout retrograde traffic mediated by COPI-coated transport carriers. The role of the C-terminal cytoplasmic domain of the KDEL receptor in this process has been investigated. Deletion of this domain did not affect receptor subcellular localization although cells expressing this truncated form of the receptor failed to retain KDEL ligands intracellularly. Permeabilized cells incubated with ATP and GTP exhibited tubular processes-mediated redistribution from the Golgi area to the ER of the wild-type receptor, whereas the truncated form lacking the C-terminal domain remained concentrated in the Golgi. As revealed with a peptide-binding assay, this domain did not interact with both coatomer and ARF-GAP unless serine 209 was mutated to aspartic acid. In contrast, alanine replacement of serine 209 inhibited coatomer/ARF-GAP recruitment, receptor redistribution into the ER, and intracellular retention of KDEL ligands. Serine 209 was phosphorylated by both cytosolic and recombinant protein kinase A (PKA) catalytic subunit. Inhibition of endogenous PKA activity with H89 blocked Golgi-ER transport of the native receptor but did not affect redistribution to the ER of a mutated form bearing aspartic acid at position 209. We conclude that PKA phosphorylation of serine 209 is required for the retrograde transport of the KDEL receptor from the Golgi complex to the ER from which the retrieval of proteins bearing the KDEL signal depends.
INTRODUCTION
In recent years, different retrograde transport routes have been described to be operative in the early secretory pathway. Together, these fulfills several important functions such as the retrieval of escaped endoplasmic reticulum (ER) proteins (Pelham, 1988; Dean and Pelham, 1990), retention of misfolded proteins (Hammond and Helenius, 1994; Vashist et al., 2001), recycling of Golgi glycosyltransferases (Storrie et al., 1998), the internalization of bacterial and plant toxins (Lord and Roberts, 1998), and the disassembly of the Golgi complex during mitosis (Zaal et al., 1999). Among these, the recycling of ER residents has been particularly well studied. During normal anterograde flow a certain number of endogenous ER proteins continuously leave the organelle and reach downstream compartments in the secretory pathway where they are recognized and returned back to their original location (Pelham, 1991). Soluble ER proteins such as chaperones and components of the control quality machinery contain a C-terminal KDEL (HDEL in yeast) sequence that is responsible for their recognition and retrieval from post-ER compartments (Munro and Pelham, 1987; Pelham et al., 1988). The evolutionary extent of this pathway is illustrated by the fact that some bacterial toxins such as cholera toxin and Pseudomonas exotoxin A also contain a C-terminal KDEL sequence that allows them to reach the ER by retrograde transport after their uptake by endocytosis (Majoul et al., 1996; Jackson et al., 1999). Throughout their association with molecular chaperones containing the KDEL signal misfolded proteins are also efficiently recovered from post-ER compartments and retained in the ER (Yamamoto et al., 2001). Many ER transmembrane proteins, on the other hand, contain a dilysine (KKXX) motif at their C-terminus cytoplasmic tail. This is also a retrieval signal that allows recognition and subsequent retrograde transport (Nilsson et al., 1989; Jackson et al., 1990, 1993).
In addition to KDEL and KKXX sorting signals displayed by ER residents, retrieval of these proteins depends on receptors that recognize the appropriate signals. ERD2, the KDEL receptor, is an integral membrane protein located at the Golgi complex and the ER-Golgi intermediate compartment (Lewis and Pelham, 1990; Semenza et al., 1990; Griffiths et al., 1994). At these locations the receptor specifically binds KDEL-bearing proteins with high affinity and mediates their uptake into transport intermediates (Lewis and Pelham, 1992). These ferry the ligand-receptor complexes to the ER where dissociation occurs. Ligands are thus released within the ER and the receptor is recycled back to the Golgi for further rounds of transport. pH differences between the ER and the Golgi have been proposed to account for the different affinities exhibited by the receptor toward ligands at both locations (Wilson et al., 1993).
COPI-coated transport intermediates, either in the form of round vesicles or as tubular processes, mediate retrograde traffic followed by both the KDEL receptor-ligand complexes and membrane proteins containing a dilysine retrieval motif (Cosson and Letourneur, 1994; Letourneur et al., 1994; Orci et al., 1997; Presley et al., 1998). Formation of these carriers depends on a highly conserved transport machinery (Wieland and Harter, 1999). An essential component of this machinery is coatomer, a heptameric protein complex that is recruited from cytosol to the membrane before budding. Coatomer recruitment, in turn, requires previous association of ARF1, a ras-like GTPase that in its GTP-bound form initiates COPI coat assembly (Barlowe, 2000; Donaldson and Lippincott-Schwartz, 2000). Thus, ARF1-GTP binds to the Golgi/intermediate compartment membranes and recruits coatomer. ARF1 activation consists in the exchange of GDP for GTP catalyzed by an ARF1-specific guanine nucleotide exchange factor (GEF; Jackson and Casanova, 2000). By contrast, hydrolysis of GTP by ARF1 gives rise to its deactivation. This reaction is regulated by a Golgi-associated GTPase-activating protein (ARF-GAP; Cukierman et al., 1995), and recent studies indicate that this activity is also required for cargo sorting and budding (Lanoix et al., 2001; Yang et al., 2002). Additional constituents of the COPI-coated transport intermediates are the p24 proteins, which are type I transmembrane proteins that have been proposed to function in both cargo selection and coat recruitment (Kaiser, 2000).
Although the KDEL recycling pathway is well established, several important questions remain unanswered. In particular, how the occupied KDEL receptor is sorted into COPI-coated transport intermediates is largely unknown. Recent studies indicate that upon ligand binding the receptor oligomerizes and interacts with components of the transport machinery such as ARF-GAP and ARF1 (Aoe et al., 1997, 1998; Majoul et al., 2001). This most likely contributes to the formation at the donor membrane of prebudding complexes that should facilitate evagination. However, it does not explain the mechanism that allows the occupied receptor to be sorted, whereas the unoccupied receptor would be excluded. In principle, sorting would take place throughout the interaction of COPI coat proteins with the cytoplasmic domains of the KDEL receptor (Bremser et al., 1999; Wieland and Harter, 1999). The latter should bear some kind of ER retrieval motif for that. However, no such a signal has been characterized for the KDEL receptor so far, and therefore the mechanism of sorting of this protein remains unresolved. In this study, we have analyzed the functional role played by the C-terminal cytoplasmic domain of the KDEL receptor. The results indicate that this protein region is phosphorylated by cAMP-dependent protein kinase A (PKA). This phosphorylation event allows the interaction of the KDEL receptor with ARF-GAP and coatomer proteins, which in turn determines both the Golgi-ER retrograde transport followed by the receptor and the retrieval of ligands containing the KDEL signal.
MATERIALS AND METHODS
Reagents
Expression in bacteria and purification of His-tagged recombinant proteins (murine RIIα and YFP-Sar1dn) was performed as described (Martín et al., 1999). Tissue culture media and antibiotics were from Life Technologies (Paisley, Scotland, United Kingdom) and restriction endonucleases from Roche Diagnostics (Mannheim, Germany). Cα and H89 were purchased from Calbiochem (La Jolla, CA). Protein G-Sepharose, ATP, GTP, DTT, and protease inhibitors were from Sigma-Aldrich (St. Louis, MO). Streptolysin O (SLO) was purchased from Dr. H. G. Meyer (University of Mainz, Germany). The following antibodies were provided by other investigators: F6.26.1 mouse mAb against hen lysozyme (M.M. Riottot, Institut Pasteur, Paris, France; Goldbaum et al., 1999), 12G5 mouse mAb against CXCR4 (Dr. A. Caruz, University of Jaen, Spain; Amara et al., 1997), and rabbit polyclonal against GMAP-210 (Dr. R. Rios, University of Seville, Spain; Infante et al., 1999). Mouse monoclonal against the C-terminal domain of the native bovine KDEL receptor was from Stressgen Biotechnologies (Victoria, BC, Canada), 9E10 mouse monoclonal against c-myc from Roche Diagnostics, goat polyclonal to ARF-GAP1 from Abcam (Cambridgeshire, United Kingdom), FITC- and HRP-conjugated secondary antibodies from Biosource (Camarillo, CA), and Texas Red–labeled secondary antibodies from Molecular Probes (Eugene, OR). Rabbit polyclonals against coatomer proteins were purchased from Dr. F. Wieland (BZH, Heidelberg, Germany).
DNA Construction and Production of Recombinant Baculoviruses
Plasmid HE24M coding a version of the human KDEL receptor containing a c-myc epitope inserted between the last transmembrane domain and the C-terminal cytoplasmic domain was generated by site-directed mutagenesis according to the overlapping extension technique (Ho et al., 1989). An EcoRV-BamHI fragment of the coding sequence present in plasmid HE24 (provided by Dr. H.R.B. Pelham, MRC, UK; Lewis and Pelham, 1990) was subcloned into pBluescript II SK (Stratagene, La Jolla, CA) and used as template. Single amino acid changes and deletion of the C-terminal domain were also carried out by this method. For expression, modified sequences were returned to the original vector by replacement. Simultaneous expression of both lysozyme-KDEL and c-myc–tagged versions of the KDEL receptor was achieved by inserting a XhoI-NcoI fragment of HE24M into HYKE4 plasmid (provided by Dr. L.M. Roberts, Warwick University, UK; Jackson et al., 1999). To generate KDEL receptor fluorescent constructs, variants of the green fluorescent protein, namely enhanced cyan fluorescent protein (CFP) and enhanced yellow protein (YFP), were fused to the C-terminus of the receptor. Sequences coding different KDEL receptor variants were subcloned into pECFPN1 and pEYFPN1 vectors (Clontech, BD Biosciences Clontech, Palo Alto, CA). The overlapping extension procedure was also used to replace the C-terminal domain of CXCR4 with that of the native KDEL receptor. Templates in this case were an expression plasmid coding wild-type CXCR4 (provided by Dr. A. Caruz; Amara et al., 1997) and the above mentioned pBluescript vector containing a C terminal fragment of the KDEL receptor. Oligonucleotides coding amino acids 307–311 of CXCR4 and amino acids 200–205 of the KDEL receptor were used as primers. The resulting chimera was subcloned into the original CXCR4 expression vector. All constructs were verified by DNA sequencing. To generate recombinant baculoviruses, EcoRI and XhoI restriction sites were inserted at the N and C ends, respectively, of the coding sequence of HE24M. This fragment was subcloned into pFastBacI (Life Technologies). Baculoviruses were obtained in bacmid-transfected Sf9 insect cells according to the instructions provided by the manufacturer. The vector pEYFPC2-Sar1pdn coding a YFP-tagged version of the dominant-negative mutant form of Sar1 (Sar1[H79G], Sar1dn) was provided by Dr. R. Pepperkok (EMBL, Heidelberg, Germany). For expression in bacteria a NcoI-BamHI fragment was subloned into pRSETB (Invitrogen, Carlsbad, CA).
Membrane and Cytosol Preparations
Cytosol was prepared from either bovine brain or rat liver as described (Hidalgo et al., 1995; Martín et al., 2000). Total microsomes were prepared from Sf9 insect cells infected with recombinant baculoviruses. Cells (5–7 × 108) were harvested 50–60 h postinfection by centrifugation at 400 × g for 10 min. They were rinsed with cold PBS and resuspended in 12–15 ml of 0.25 M sucrose in 25 mM HEPES, pH 7.2, 5 mM MgCl2 containing protease inhibitors (1 mM PMSF, 5 mM benzamidine, 100 μg/ml soybean trypsin inhibitor, 20 μg/ml aprotinin, and 10 μg/ml each pepstatin A, leupeptin, antipain). Homogenization was performed in a ball-bearing homogenizer. The homogenate was centrifuged at 12,000 × g for 10 min at 4°C to remove nuclei, mitochondria, and unbroken cells. The supernatant was centrifuged at 100,000 × g for 1 h at 4°C. Membranes were incubated on ice with 3 M KCl for 30 min. They were recovered by centrifugation as above on a 2 M sucrose cushion. Microsomes were resuspended in 25 mM HEPES-KOH, pH 7.2, 25 mM KCl, and 2.5 mM MgCl2 at 5–9 mg/ml protein concentration, snap-frozen in liquid nitrogen, and stored at –80°C.
Mammalian Cell Culture, Transient Transfection, Microinjection, and Immunofluorescence
Vero and COS-7 cells were grown in MEM and DMEM, respectively, and supplemented with 10% (vol/vol) FCS, 2 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. Cells were transfected by electroporation. Briefly, 1–2 × 106 cells were resuspended in 0.2 ml of hypoosmolar electroporation buffer (Eppendorf, Hamburg, Germany) containing 12 μg of pure plasmid DNA and 14 μg sperm DNA. The cell suspension was transferred into a 4-mm gap sterile cuvette. Electroporation was carried out in Multiporator (Eppendorf) at 600 v, τ: 100 μs. Cells were diluted in complete culture medium containing 15 mM HEPES and recovered by centrifugation at 400 × g for 10 min. They were used 24–48 h posttransfection. Cells grown on glass coverslips for 1 d were microinjected into the cytoplasm with 2 mg/ml YFP-tagged Sar1dn using an Automated Microinjection System (Eppendorf). For indirect immunofluorescence, cells were fixed for 5 min in cold methanol or, alternatively, for 20 min in 3% (wt/vol) paraformaldehyde in PBS. They were rinsed several times with plain PBS and PBS/0.5%(wt/vol) BSA/ 0.05%(wt/vol) saponin. Incubation with antibodies diluted in PBS/BSA/saponin was performed at 37°C for 30 min. Cells were rinsed with PBS and mounted with Fluoromont G (Southern Biotechnology, Birmingham, AL).
Golgi-ER Redistribution Assay
Cells cultured on glass coverslips were rinsed with ice-cold buffer (20 mM HEPES, pH 7.2, 2 mM magnesium acetate, 90 mM potassium acetate, 1 mM DTT). They were incubated on ice for 20 min with 1 μg/ml SLO. Coverslips were rinsed thoroughly with cold buffer and then incubated at 37°C with 0.3 ml buffer containing 1 mM of both ATP and GTP in a 17-mm well of a 24-well dish.
Lysozyme Secretion
After transfection, 0.5 × 106 cells were plated on each 35-mm well of a six-well dish. One day after, the cells were depleted by incubation with methionine- and cysteine-free medium for 30 min and then radiolabeled at 37°C for 10 min with 1 ml of the same medium containing 25 mCi Tran35S-label (1000 Ci/mmol). They were rinsed with ice-cold PBS and chased in 0.5 ml of complete medium containing 1.5 mg/ml both unlabeled methionine and cysteine. At each time point, the medium was collected and the cells were washed with cold PBS and lysed with 0.4 ml lysis buffer (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% [vol/vol] Triton X-100) containing protease inhibitors. Samples were cleared by centrifugation at 12,000 × g for 20 min at 4°C, and supernatants were transferred to new tubes. Media samples were mixed with 0.5 ml of 2× lysis buffer. Incubation with antibody against hen lysozyme was carried out overnight at 4°C followed by 1-h treatment with protein G-Sepharose. Immunoprecipitates were rinsed with the above buffer supplemented with 1% (wt/vol) sodium deoxycholate and 0.1% (wt/vol) SDS (RIPA buffer) and 10 mM Tris-HCl, pH 7.8, before electrophoresis.
Peptide-binding Assay
Synthetic peptides were coupled to thiopropyl Sepharose 4B (Amersham, Piscataway, NJ) according to the manufacturer's instructions. Coupled peptides (2–3 nmol) were incubated at room temperature for 5 min with 200 μg bovine brain cytosol in 0.5 ml coupling buffer (50 mM Tris-HCl, pH 7.3, 0.1–1 M NaCl). Beads were rinsed several times with coupling buffer before processing for electrophoresis.
Phosphorylation Assay
High salt–washed microsomal membranes (35 μg) were incubated at 30°C for 10 min with 0.1 μCi [γ-32P]ATP (3000 Ci/mmol) and 1 U PKA catalytic subunit (Cα) in phosphorylation buffer (20 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 1 mM DTT). Alternatively, 100 μg crude rat liver cytosol and phosphatase inhibitors (10 mM sodium pyrophosphate, 20 mM NaF) were added to the incubation medium instead of Cα. Final volume was, in all cases, 50 μl. The reaction was stopped by transferring the tubes to ice and adding 1 ml of ice-cold phosphorylation-arresting buffer (20 mM Tris-HCl, pH 7.5, 100 mM ATP, 100 mM EDTA). Membranes were pelleted at 12,000 × g for 20 min at 4°C, rinsed with phosphorylation-arresting buffer, and lysed in 0.5 ml RIPA buffer. The KDEL receptor constructs were immunoprecipitated with anti-myc antibody and protein G-Sepharose.
Electrophoresis and Immunoblot Analysis
Beads with bound proteins were resuspended in electrophoresis sample buffer, reduced with 10 mM DTT, and boiled for 5 min. SDS-PAGE, 15%, gels were used to resolve both lysozyme and KDEL receptor molecules, whereas coatomer proteins were separated on 10% SDS-PAGE gels. Proteins were transferred onto either nitrocellulose or polyvinylidene difluoride (Immobilon-P, Millipore, Bedford, MA) membranes. Blots were blocked overnight at 4°C with 5% (wt/vol) nonfat milk in TBS containing 1% (vol/vol) Tween 20. They were incubated with primary antibody for 1 h at room temperature followed by treatment with the corresponding HRP-conjugated secondary antibody. Signals were detected by ECL (SuperSignal; Pierce Chemical Co., Rockford, IL) and quantitated by scanning densitometry. Alternatively, 35S- and 32P-labeled proteins were visualized in PhosphorImager (FUJIXBas 1000; Fuji, Tokyo, Japan) using PC-BAS 2.08 software.
RESULTS
Role of the C-terminal Domain in Subcellular Localization of the KDEL Receptor and Retrieval of KDEL Ligands
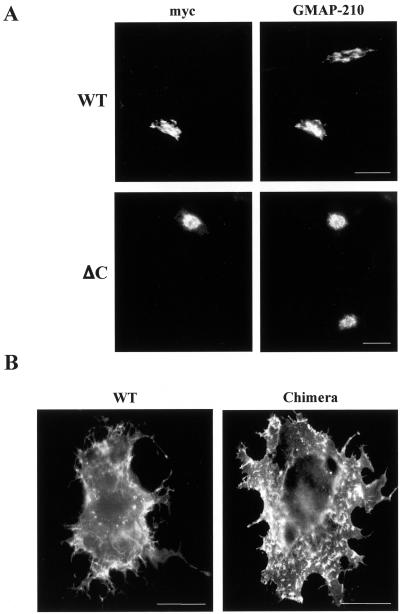
The KDEL receptor is believed to adopt a seven-transmembrane-domain topology with a short, 12–13-amino acid tail at the C terminus projecting out of the membrane toward the cytosol (Townsley et al., 1993; Scheel and Pelham, 1998). To analyze the functional role of this domain, we inserted a c-myc epitope between the last transmembrane domain and the putative cytoplasmic tail, which is between amino acids 199–200 of the native protein. When expressed in COS cells this tagged version of the receptor localized at the Golgi complex as evidenced by double immunofluorescence staining with an antibody specific for GMAP-210, a protein associated to the cis-Golgi network (Infante et al., 1999; Figure 1A). This indicated that the presence of the intercalated myc sequence did not affect the normal, steady state localization of the receptor. Deletion of the last 12 amino acids of our construct leaves the myc epitope only covered by a threonine residue at the C terminus. This truncated form of the receptor was still localized at the Golgi complex (Figure 1A), suggesting the absence of targeting signals within the C-terminal domain. To test this we investigated the consequences of replacing the C-terminal domain of a plasma membrane protein with that of the native KDEL receptor. CXCR4, a chemokine receptor, was chosen because of its structural similarity with the KDEL receptor (Amara et al., 1997). Both the chimera and the wild-type protein were able to travel throughout the exocytic pathway to the plasma membrane when expressed in COS cells (Figure 1B). Therefore, according to these results the C-terminal domain does not confer Golgi retention to a reporter protein.
Figure 1.
Immunofluorescence localization of KDEL and CXCR4 receptors. (A) COS cells were transfected with plasmid HE24M coding a version of the KDEL receptor containing a myc epitope inserted between the last transmembrane domain and the C-terminal cytoplasmic domain. Cells expressing either the intact, wild-type receptor (WT) or a truncated form lacking the last 12 amino acids (ΔC) were double-stained with anti-myc and anti-GMAP-210 antibodies. (B) COS cells were transfected with a plasmid coding either wild-type CXCR4 (WT) or a chimera resulting from the replacement of the last 41 amino acids of this protein with the C-terminal domain of the KDEL receptor. They were single-stained with anti-CXCR4 antibody. Bars, 20 μm.
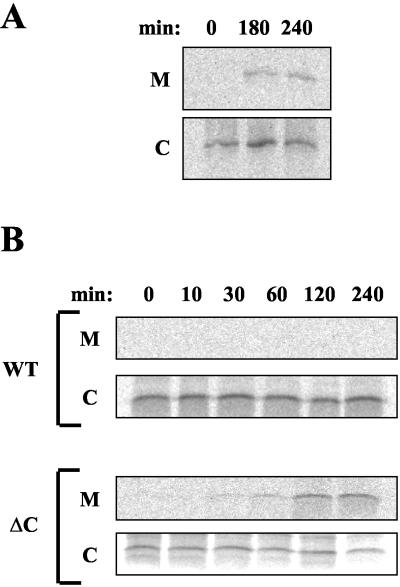
We examined the role of this domain in the retrieval from the Golgi to the ER of proteins bearing the KDEL signal at their C terminus. Cells expressing lysozyme-KDEL were pulse-labeled and chased to monitor secretion of this protein to the medium. As shown in Figure 2A, a significant amount (21%) of the initially labeled lysozyme-KDEL was secreted to the medium during a 3-h chase period, indicating saturation of the endogenous retrieval system. By contrast, secretion of lysozyme-KDEL did not occur in cells simultaneously expressing the wild-type, myc-tagged version of the KDEL receptor (Figure 2B). In these cells, lysozyme-KDEL was localized in the ER by immunofluorescence, whereas the receptor, detected with anti-myc antibody, was mostly concentrated in the perinuclear Golgi region (our unpublished results). This indicated that the efficiency of retrieval was restored after expression of the ectopic receptor that was, therefore, fully functional in terms of ligand recognition and recovery at the Golgi complex. By contrast, during a 4-h chase period 44–47% of the initially labeled lysozyme molecules were secreted to the medium by cells expressing a truncated form of the KDEL receptor lacking the C-terminal domain (Figure 2B). This suggests a severe defect in the cellular mechanisms that are responsible for the normal retention of escaped ER proteins.
Figure 2.
Secretion of lysozyme-KDEL. (A) COS cells were transfected with a plasmid coding hen lysozyme containing the KDEL signal at the C terminus. (B) Alternatively, they were transfected with a plasmid coding both lysozyme-KDEL and a particular myc-tagged version of the KDEL receptor, either the intact, wild-type receptor (WT) or a truncated form lacking the last 12 amino acids (ΔC). Cells were pulse-labeled for 10 min with [35S]methionine and -cysteine and chased for the indicated time periods. At each time point, lysozyme-KDEL was immunoprecipitated from both medium (M) and cell pellet (C) and resolved by SDS-PAGE.
The C-terminal Domain Is Required for the Transport of KDEL Receptor from the Golgi Complex to the ER
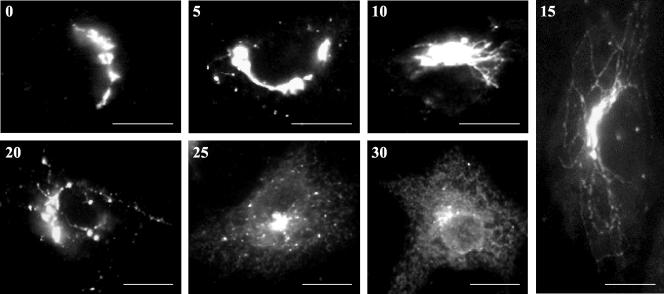
To gain insight on the role played by the C-terminal domain in retrograde transport we designed a morphological assay aimed to monitor the redistribution into the ER of the KDEL receptor. SLO-permeabilized cells were incubated at 37°C in the presence of both ATP and GTP. Under these conditions the endogenous receptor left the Golgi complex into elongated tubular processes that with time fragmented and fused with the ER (Figure 3). In addition to ATP and GTP, this process seemed to require residual cytosolic factors remaining inside the cells. Thus, permeabilized cells that were rinsed with high-salt buffer before incubation could not drive tubule formation. Addition of exogenous cytosol did not restore the cytosolic factors lost during the high salt wash (our unpublished results).
Figure 3.
Golgi-ER redistribution of the native KDEL receptor. Vero cells were permeabilized with SLO, rinsed with buffer, and incubated at 37°C for the indicated time periods (min) with 1 mM of both ATP and GTP. Cells were fixed and processed for indirect immunofluorescence with an antibody against the C-terminal domain of the KDEL receptor. Bars, 20 μm
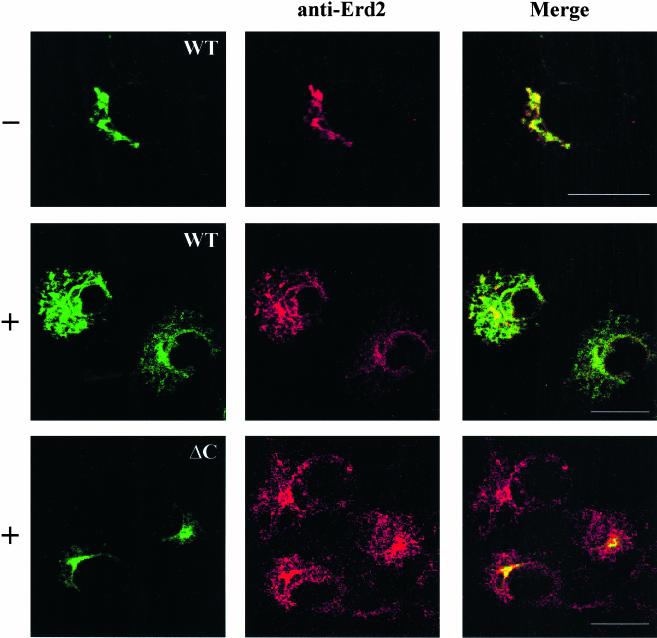
Cells expressing fluorescently tagged versions of the KDEL receptor were permeabilized and used in the redistribution assay. The wild-type receptor readily redistributed into the ER after incubation with both ATP and GTP whereas it remained localized in the Golgi in their absence (Figure 4). In contrast, a truncated form of the receptor lacking the C-terminal domain did not redistribute either in the presence or in the absence of ATP and GTP. This suggested the existence of differences in the ability of both molecular forms to participate in the Golgi-ER retrograde pathway. To reveal such differences, we used immunofluorescence staining with an antibody against the C-terminal domain. Cells expressing the truncated form of the receptor and incubated for 30–35 min with ATP and GTP showed the endogenous receptor, recognized by the antibody, redistributed into the ER, whereas the ectopic fluorescent form lacking the C-terminal domain remained retained in the Golgi (Figure 4). This clearly indicated that transport from the Golgi complex to the ER of the KDEL receptor depends on its C terminus.
Figure 4.
Golgi-ER redistribution of different fluorescent KDEL receptor constructs. COS cells were transfected with plasmid coding a CFP-tagged version of either the wild-type KDEL receptor (WT) or a truncated form lacking the last 12 amino acids (ΔC). They were permeabilized with SLO and incubated at 37°C for 35 min either in the presence (+) or in the absence (–) of both ATP and GTP. Cells were fixed and processed for indirect immunofluorescence with an antibody against the C-terminal domain of the KDEL receptor. Bars, 16 μm
Role of the C-terminal Domain in the Interaction of the KDEL Receptor with Coatomer and ARF-GAP
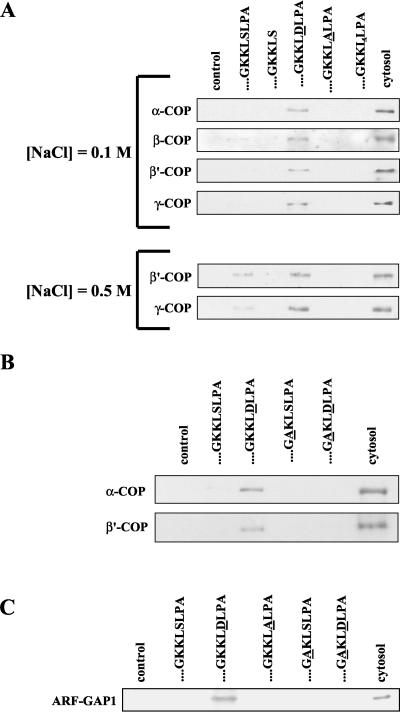
In the Golgi-ER retrograde pathway, sorting of membrane proteins occurs throughout the interaction of coat proteins with their cytoplasmic domains. The latter typically bear some kind of ER retrieval motif such as a dilysine (KKXX) motif at their C-terminus. Although apparently the KDEL receptor lacks such a signal, it is well established that it is transported from the Golgi complex to the ER in COPI-coated transport intermediates (Orci et al., 1997; Girod et al., 1999). Therefore, in order to be sorted into this kind of carriers the KDEL receptor must interact with components of the COP I coat. To examine this, we carried out pull-down experiments of cytosolic coatomer proteins with synthetic peptides corresponding to the C-terminal domain of the KDEL receptor coupled to beads (Figure 5, A and B). On incubation with cytosol the beads were rinsed and processed by SDS-PAGE before protein detection by immunoblotting. Recruitment was dependent on peptide because control beads with no peptide coupled did not bind any coatomer protein. At 0.1 M NaCl a 21-amino acid peptide covering the complete C-terminal domain of the KDEL receptor did not recruit coatomer. However, a small amount of some coatomer proteins such as β′-COP and γ-COP could be bound at higher, 0.5–1 M, NaCl concentration (Figure 5A). Interestingly, this peptide sequence contains at positions –6 and –7 from the C terminus two consecutive lysine residues. Although located far away from the C terminus, these residues could be part of a hidden ER retrieval motif that under certain circumstances would be exposed for interaction with coatomer. To investigate this possibility a similar peptide lacking the last three amino acids was also assayed. Despite the presence of a potential retrieval dilysine motif at the C terminus coatomer proteins did not interact either with this peptide sequence (Figure 5A). Therefore, other factors different from the classic dilysine retrieval motif would be responsible for the interaction of the C-terminal domain of the KDEL receptor with coatomer proteins. Thus, a serine residue located at position 209 in the native protein was shown to be critical for interaction. Even at high salt concentration no coatomer protein bound to a peptide sequence in which this particular amino acid was omitted. We reasoned that serine 209 could be a potential phosphorylation target. Accordingly, serine was replaced by either alanine, which cannot be phosphorylated, or alternatively, by aspartic acid, which mimics a phosphorylated residue. The latter peptide sequence was able to recruit coatomer under all conditions assayed. By contrast, alanine replacement abolished coatomer binding (Figure 5A). These results suggested that phosphorylation of serine 209 would determine the interaction of the C-terminal domain of the KDEL receptor with cytosolic coatomer proteins.
Figure 5.
Interaction of native coatomer proteins and ARF-GAP with peptides. Synthetic peptides were covalently coupled to thiopropyl Sepharose beads. They were incubated with crude bovine brain cytosol for 5 min at room temperature, rinsed with buffer, and processed by SDS-PAGE and immunoblotting. The same Western blot membrane was reused to detect several coatomer proteins. Incubation with cytosol and rinses with buffer were carried out at the indicated NaCl concentrations (A) or, alternatively, at 0.1 M NaCl (B and C). Peptide... GKKLSLPA corresponds to the entire C-terminal domain of the native KDEL receptor. Changes on this sequence are indicated as underlined residues and the caret indicates deletion). As a negative control, beads with no peptide coupled were similarly processed (control). Also, 20 μg of crude cytosol was processed and loaded on gels as a positive control (cytosol).
Although phosphorylation of serine 209 could be a relevant event, additional determinants might be also involved in coatomer binding. As mentioned above, a putative dilysine motif is present within the C-terminal domain of the KDEL receptor. In principle, this motif should not be functional in retrieval because it is located far away from the C terminus. Figure 5B shows that peptides in which this motif was altered by alanine replacement did not bind coatomer proteins. Apparently, this situation could not be overcome by phosphorylation of serine 209. Thus, a peptide lacking an intact dilysine motif and simultaneously containing an aspartic acid residue at a position equivalent to 209 in the native protein could not bind coatomer (Figure 5B). Therefore, in addition to phosphorylation of serine 209, a functional dilysine retrieval motif must be present within the C-terminal domain of the KDEL receptor for coatomer interaction to occur.
The KDEL receptor, on the other hand, has been shown to interact with ARF-GAP in a ligand-stimulated process (Aoe et al., 1997, 1998). This interaction is thought to promote ARF-GAP recruitment from cytosol to the membrane where ARF-GAP has been described to participate in cargo sorting and formation of COPI-coated transport carriers (Yang et al., 2002). We asked whether the amino acid determinants involved in coatomer recruitment also affected the interaction of the KDEL receptor with cytosolic ARF-GAP (Figure 5C). In our assay, ARF-GAP did not bind to a peptide corresponding to the complete C-terminal domain of the KDEL receptor unless serine 209 was replaced by aspartic acid. Again, the internal dilysine motif had a dominant effect because no binding occurred when it was altered by alanine replacement either in the presence or in the absence of aspartic acid at a position equivalent to 209 in the native protein (Figure 5C). Taken together, these results suggest that phosphorylation of serine 209 promotes the interaction of the KDEL receptor with both coatomer proteins and ARF-GAP.
Role of Serine 209 in Golgi-ER Transport and Retrieval of KDEL Ligands
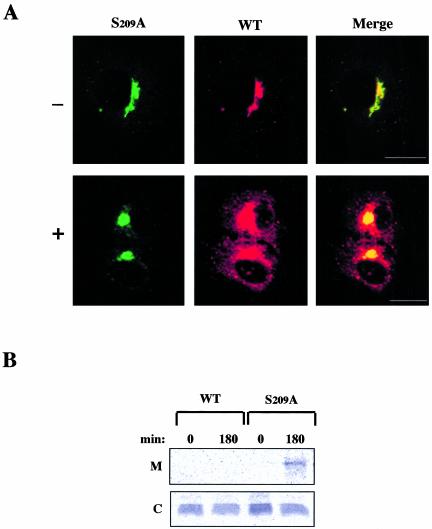
The above data suggested that within the C-terminal domain serine 209 could play a critical role in the interaction of the KDEL receptor with COPI coat proteins and hence in the Golgi-ER retrograde transport followed by the former. To investigate this further, we analyzed the redistribution of a mutated form of the receptor in which this particular amino acid was replaced by alanine. Cells expressing fluorescently tagged forms of both the wild-type and the S209A mutated receptor were permeabilized and used in the redistribution assay. During a 25–30-min incubation period a significant amount of the wild-type receptor redistributed to the ER in an ATP- and GTP-dependent manner. In contrast, the S209A mutated receptor lagged behind in the Golgi (Figure 6A) and only after prolonged (40–45 min) incubation periods started to appear in the ER network. This is consistent with the fact that most of the Golgi residents redistributed to the ER after long incubations (our unpublished results). These results revealed striking differences between both molecular forms in their ability to be transported from the Golgi complex to the ER within the same living cell. According to these data the S209A receptor does not travel from the Golgi to the ER at normal speed and most likely this could affect the retrieval of ligands containing the KDEL sequence. We therefore examined the effect of this amino acid replacement on the intracellular retention of lysozyme-KDEL (Figure 6B). Cells expressing simultaneously lysozyme-KDEL and either the wild-type or the S209A mutated receptor were pulse-labeled and chased for 3 h. During this time period 18.2% of the initially labeled lysozyme-KDEL was secreted to the culture medium by cells expressing the S209A mutant form. Because lysozyme-KDEL was not secreted to the medium by cells expressing the wild-type receptor (Figure 6B), this result indicated that expression of the mutated receptor decreased the efficiency of the retrieval system.
Figure 6.
Effects of S209A replacement on receptor redistribution and secretion of lysozyme-KDEL. (A) COS cells were cotransfected with two plasmids, one coding a YFP-tagged version of the wild-type KDEL receptor and the other coding a CFP-tagged version of a mutant form in which S209 was changed to A (S209A). Cells were permeabilized and incubated at 37°C for 25 min either in the presence (+) or in the absence (–) of both ATP and GTP before fixation. Bars, 16 μm. (B) COS cells were transfected with a plasmid coding both lysozyme-KDEL and the myc-tagged version of the KDEL receptor. Cells expressing either the wild-type receptor (WT) or the S209A mutant form were pulse-labeled for 10 min and chased for 3 h. Lysozyme-KDEL was immunoprecipitated from both medium (M) and cell pellet (C) and resolved by SDS-PAGE.
PKA Phosphorylation of the KDEL Receptor
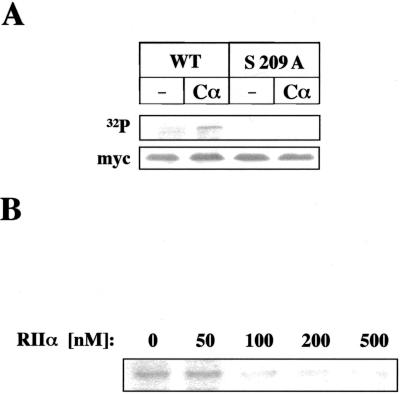
The above data indicated that serine 209 is a key residue for the normal functioning of the KDEL receptor. The sequence context in which Serine 209 is located (KKLSL) is a potential consensus site for PKA phosphorylation (K/R-K/R-X-S-X). We therefore examined the possibility that this kinase might be involved in phosphorylation of the C-terminal domain of the KDEL receptor (Figure 7). High-salt–washed microsomal membranes from insect cells expressing different versions of the myc-tagged KDEL receptor were incubated with both [γ-32P]ATP and pure PKA catalytic subunits, Cα. Membranes were then rinsed with buffer and lysed. The KDEL receptor constructs were immunoprecipitated with anti-myc antibody, subjected to SDS-PAGE, and transferred to Western blots. Protein bands labeled with 32P were identified by immunoblotting with anti-myc antibody. The wild-type KDEL receptor was efficiently phosphorylated in a Cα-dependent reaction. By contrast, the S209A mutant form was not phosphorylated either in the absence or in the presence of Cα in the incubation medium (Figure 7A). Phosphorylation of the wild-type KDEL receptor could also be achieved by incubation with crude cytosol (Figure 7B). An endogenous kinase activity catalyzed receptor phosphorylation in this case. We identified such kinase activity as PKA by using cytosol preincubated with pure RIIα regulatory subunits. These are expected to complex and inactivate endogenous Cα subunits present in the cytosol preparation. As shown in Figure 7B, addition of 50–100 nM RIIα abolished KDEL receptor phosphorylation induced by cytosol.
Figure 7.
PKA phosphorylation of the KDEL receptor. Microsomal membranes containing myc-tagged versions of the KDEL receptor were prepared from baculovirus-infected insect cells, washed with high-salt buffer, and subjected to PKA-mediated phosphorylation. (A) Membranes containing either the wild-type KDEL receptor (WT) or the S209A mutant form were incubated at 30°C for 10 min with [γ-32P]ATP either in the presence or in the absence (–) of 1 U Cα. (B) Membranes containing the wild-type KDEL receptor were similarly incubated with both [γ-32P]ATP and 100 μg rat liver cytosol. The latter was preincubated for 10 min with both phosphatase inhibitors and the indicated concentrations of recombinant RIIα. Membranes were rinsed with buffer and lysed with detergents before immunoprecipitation of the KDEL receptor with anti-myc antibody. Immunoprecipitates were resolved by SDS-PAGE and either directly visualized in PhosphorImager (B) or previously transferred to Western blot membranes (A). Radioactive (32P) bands in A were identified by immunoblotting with anti-myc antibody. In this case, protein G-Sepharose beads with anti-myc antibody covalently cross-linked were used during immunoprecipitation to avoid interference of immunoglobulin light chains during immunoblotting.
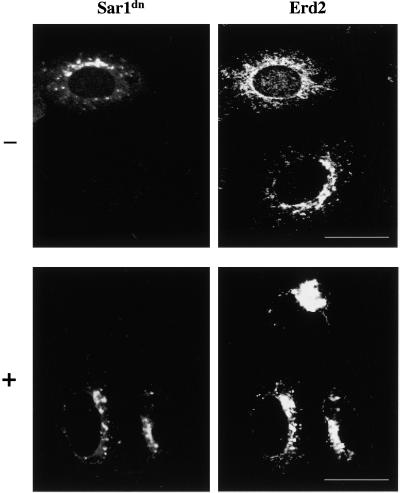
These data implied that PKA could potentially phosphorylate the C-terminal domain of the KDEL receptor at serine 209. The question that arose was the functional relevance of such modification. To address this, we performed an experiment aimed to evaluate the role of PKA phosphorylation in the dynamic cycling of the endogenous KDEL receptor. A GTP-restricted form of Sar1 (Sar1[H79G], Sar1dn) was used as a specific reagent to arrest the anterograde ER-Golgi transport while leaving the retrograde Golgi-ER pathway unaffected. Nontransfected cells were microinjected with recombinant Sar1dn-tagged with YFP. In the presence of this protein the KDEL receptor redistributed to the ER where it became trapped. This effect was observed in most (66%, n = 115) of the microinjected cells and seems to be the result of a situation of continuous retrograde transport in the absence of anterograde flow. By contrast, noninjected cells showed the typical Golgi/intermediate compartment localization pattern (Figure 8). We next used H89 as a selective inhibitor to reveal the involvement of PKA in the transport of the native receptor from the Golgi to the ER. Used at 5–10 μM, this reagent specifically inhibits PKA activity, whereas much higher concentrations (mM range) are required to inhibit other serine/threonine kinases. In cells incubated with 5 μM H89 the KDEL receptor was seen concentrated in the Golgi area either in the presence or in the absence of Sar1dn (Figure 8). Because redistribution did not occur in >70% of the cells microinjected with Sar1dn, this result indicated that the transport of the native receptor from the Golgi to the ER is indeed PKA modulated.
Figure 8.
Effect of H89 treatment on the Golgi-ER redistribution of the native KDEL receptor. Vero cells were preincubated (+) or not (–) for 10–15 min with 5 μM H89 in FCS-free medium supplemented with 25 mM HEPES. They were microinjected in the same medium with recombinant YFP-tagged Sar1dn and incubation continued at 37°C for 1 h. Cells were fixed and processed for indirect immunofluorescence with an antibody against the KDEL receptor. Bars, 16 μm
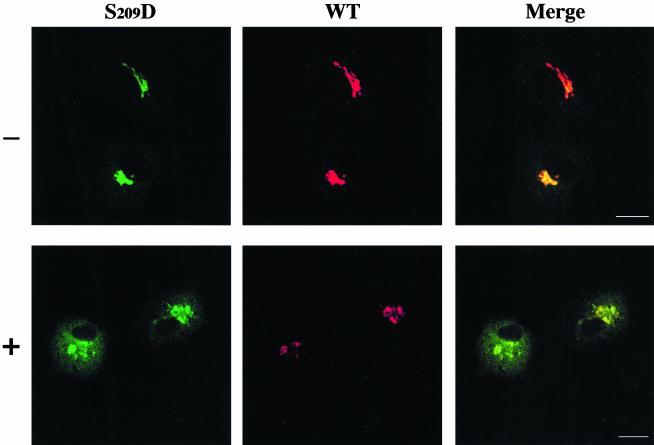
We took advantage of the inhibitory effect of H89 on ER-Golgi anterograde transport (Muñiz et al., 1996; Aridor and Balch, 2000; Lee and Linstedt, 2000) to demonstrate the redistribution from the Golgi to the ER of the S209D mutant receptor. We reasoned that if this form would mimic a phosphorylated receptor, it would travel from the Golgi to the ER in a H89 insensitive way. Then it would be retained in the ER because of blocking of the anterograde transport. Cells expressing fluorescently tagged versions of both the wild-type KDEL receptor and the S209D mutated form were therefore subjected to H89 treatment. Both molecular forms colocalized at the Golgi complex in control, untreated cells (Figure 9). As expected from the above data, H89 inhibited ER redistribution of the wild-type KDEL receptor that remained localized at the Golgi after 1-h incubation with a high dose, 20–30 μM, of this inhibitor. By contrast, under these conditions the mutant S209D receptor expressed by the same cell redistributed efficiently from the Golgi to the ER (Figure 9). These results emphasized the relevance of PKA phosphorylation of serine 209 to promote the retrograde traffic of the native KDEL receptor from the Golgi complex to the ER.
Figure 9.
Effect of H89 treatment on the Golgi-ER redistribution of the S209D mutant form of the KDEL receptor. Vero cells were cotransfected with two plasmids, one coding a YFP-tagged version of the wild-type KDEL receptor and the other coding a CFP-tagged version of a mutant form in which S209 was changed to D (S209D). Twenty-four hours after transfection they were incubated or not (–) at 37°C for 1 h with 20 μM H89 in FCS-free medium before fixation. Bars, 16 μm
DISCUSSION
Retrieval of endogenous ER proteins tagged with the KDEL-(HDEL) sequence at their C-terminus is an essential, conserved process in eukaryotic cells that contributes to the quality control in the secretory pathway. The wild-type KDEL receptor does not contain a classic dilysine ER retrieval motif. Despite of this, it is firmly established that, once loaded with cargo, the KDEL receptor travels from the Golgi complex to the ER in COPI-coated transport carriers (Orci et al., 1997; Girod et al., 1999). Therefore the mechanism that allows the receptor-ligand complexes to be sorted into these containers is a major unresolved problem.
In this study we have analyzed the functional role of the C-terminal cytoplasmic domain of the KDEL receptor. According to our results, this part of the protein does not contain dominant targeting information that could direct the KDEL receptor or any other membrane protein to the Golgi complex. By contrast, the data indicate that it is necessary for receptor sorting into COPI-coated transport intermediates and, therefore, is required for the retrograde transport of the KDEL receptor from the Golgi region to the ER as well as for the retrieval of KDEL ligands. Thus, in a Golgi-ER redistribution assay that makes use of SLO-permeabilized cells a truncated form of the receptor lacking the C-terminal domain remained retained in the Golgi complex, whereas the native receptor present in the same cell efficiently redistributed to the ER. Accordingly, a significant amount of lysozyme-KDEL was secreted to the extracellular medium by cells expressing this truncated form of the KDEL receptor. In contrast, those expressing the wild-type form retained lysozyme-KDEL intracellularly. Together, these results indicate that the short C-terminal domain plays an essential role for the functioning of the KDEL receptor. A relevant role of the C-terminal domain in retrograde transport is also supported by studies showing that the arrival of KDEL-bearing bacterial toxins to the ER is inhibited in cells microinjected with antibodies directed against this region of the receptor (Majoul et al., 1998; Jackson et al., 1999). Alternatively, it is possible that tail-less forms of the KDEL receptor would become aggregated in the Golgi complex where they would be unable to participate in retrograde transport.
Within the C-terminal domain serine 209 is an important residue for the function of the KDEL receptor. Replacement by alanine (S209A) affected the ability of the receptor to be transported from the Golgi complex to the ER as judged by results obtained with the redistribution assay. At least in this case formation of molecular aggregates that could not be transported along the retrograde pathway seems unlikely. The S209A mutant form remained arrested in the Golgi complex, whereas the wild-type receptor expressed by the same cell redistributed to ER at normal speed. Additionally, intact cells expressing the S209A mutant form did not retain lysozyme-KDEL properly. In contrast to these findings, Townsley et al. (1993) reported that point mutations at the different predicted cytoplasmic domains of the KDEL receptor including the C-terminal tail did not affect its retrograde traffic from the Golgi to the ER. This was evaluated by receptor redistribution to the ER during coexpression with lysozyme-KDEL. In particular, alanine replacement of serine 209 did not prevent receptor relocation to the ER induced by overexpression of lysozyme-KDEL. Instead, using the same criteria an aspartic acid residue located in the seventh transmembrane domain was judged to be critical for retrograde transport (Townsley et al., 1993). The molecular versions of the KDEL receptor here analyzed, including the S209A and S209D mutant forms and also a truncated form lacking the entire C-terminal domain, were all concentrated at the Golgi complex during transient expression. Still they exhibited different capabilities to be transported from the Golgi to the ER as shown by their relative contribution to the intracellular retention of lysozyme-KDEL. This indicates that receptor localization pattern is determined by several factors (i.e., ER-Golgi anterograde transport) and is not necessarily an indication of its functionality.
Phosphorylation of serine 209 seems be a key event in controlling the dynamic behavior of the KDEL receptor. The available evidences indicate that PKA is involved. Thus, the receptor can be phosphorylated in vitro by both pure, recombinant PKA catalytic subunit and by a cytosolic activity inhibited by PKA regulatory subunits. Involvement of PKA in Golgi-ER retrograde transport is supported by experiments with H89. Cells incubated with low concentrations of this inhibitor did not show redistribution of the native KDEL receptor from the Golgi region to the ER after a block in the ER-Golgi anterograde flow induced by Sar1dn. This suggests that in order to be transported from the Golgi complex to the ER the KDEL receptor must be first phosphorylated by PKA. Conversely, in the absence of PKA phosphorylation the native receptor would not be recruited into the Golgi-ER retrograde pathway and proteins bearing the KDEL signal would be secreted to the extracellular medium. In addition, because of its inhibitory effect on ER to Golgi anterograde transport (Muñiz et al., 1996; Aridor and Balch, 2000; Lee and Linstedt, 2000) H89 was used to evaluate the consequences of the S209D mutation. In this case, treatment with H89 does not interfere with the Golgi-ER retrograde traffic of the S209D mutant form but inhibits exit from the ER and this gives rise to retention at this location. Presence of an aspartic acid at position 209 would mimic a phosphorylated residue. This could have the effect of making the KDEL receptor to be permanently activated for retrograde transport. However, the Golgi localization pattern of this form in untreated cells suggests that it is also rapidly moving out of the ER to the Golgi.
Our observations indicate that the C-terminal domain is involved in the interaction of the KDEL receptor with components of the COPI coat such as coatomer proteins and also with the ARF1 regulator ARF-GAP. We have been able to study such interaction in assays using synthetic peptides that mimic the cytoplasmic tail of the KDEL receptor. From these experiments we conclude that binding of both coatomer proteins and ARF-GAP apparently depends on two factors. First, a hidden dilysine motif is required. This corroborates a recent study showing that mutation of these residues abolishes the interaction of the cytoplasmic domain with purified coatomer and recombinant ARF-GAP (Yang et al., 2002). On the other hand, serine 209 was shown to be critical for both coatomer and ARF-GAP recruitment. Binding in both cases was maximal after replacement of this residue by aspartic acid, whereas it was abolished after alanine replacement. This result places PKA phosphorylation in the center of the regulation of the interaction of the KDEL receptor with the retrograde transport machinery. A possibility would be that PKA phosphorylation of serine 209 could trigger a conformational change in the C-terminal domain of the KDEL receptor. This would result in the cryptic dilysine motif becoming exposed and available for interaction with ARF-GAP and coatomer. Although this mechanism might occur in mammalian cells, it certainly does not apply to other cell systems. Thus, neither the internal dilysine motif nor the critical serine residue are strictly conserved in evolution. For instance, the C-terminal domain of the yeast HDEL receptor have only one lysine residue, which is not sufficient to constitute a retrieval motif. Furthermore, no serine or other phospho-acceptor residue is present in such domain (Semenza et al., 1990). Interestingly, the two determinants here discussed are present in the C-terminal domain of yeast Rer1, which also functions in retrograde transport as a receptor for the COPI-dependent retrieval of certain type II transmembrane proteins (Nishikawa and Nakano, 1993; Boehm et al., 1997). Therefore, it is possible that PKA activity regulates the interaction with the COPI transport machinery of several membrane receptors in different organisms.
We postulate the existence of a signal-transduction pathway across the membrane of the Golgi complex. This could become activated once the KDEL receptor interacts with ligands. The predicted topology of the KDEL receptor, structurally similar to G-protein–coupled receptors, supports the existence of such a pathway (Townsley et al., 1993; Scheel and Pelham, 1998). PKA, on the other hand, is associated to the cytoplasmic side of the Golgi membranes (Martín et al., 1999), and it could also be activated upon ligand binding. Activated PKA phosphorylates serine 209 at the C-terminus of the KDEL receptor and this would trigger its recruitment into the Golgi-ER retrograde pathway by promoting ARF-GAP and coatomer association and sorting into COPI-coated carriers.
Acknowledgments
We thank Drs. A. Caruz, H. R. B. Pelham, R. Pepperkok, R. Rios, M. M. Riottot, and L. M. Roberts for providing reagents used in this study. This work was supported by Grants PB98–1119 and BMC2002–00295 from Ministerio de Ciencia y Tecnología (to A.V.) and Grant 00/1065 from Ministerio de Sanidad y Consumo (to J.H.). M.C. and L.V. were supported by predoctoral fellowships from Ministerio de Educación and Junta de Andalucía, respectively.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–04–0194. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-04-0194.
References
- Amara, A., Gall, S.L., Schwartz, O., Salamero, J., Montes, M., Loetscher, P., Baggiolini, M., Virelizier, J.L., and Arenzana-Seisdedos, F. (1997). HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 186, 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoe, T., Cukierman, E., Lee, A., Cassel, D., Peters, P.J., and Hsu, V.W. (1997). The KDEL receptor, ERD2, regulates intracellular traffic by recruiting a GTPase-activating protein for ARF1. EMBO J. 16, 7305–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoe, T., Lee, A.J., v. Donselaar, E., Peters, P.J., and Hsu, V.W. (1998). Modulation of intracellular transport by transported proteins: insight from regulation of COPI-mediated transport. Proc. Natl. Acad. Sci. USA 95, 1624–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor, M., and Balch, W.E. (2000). Kinase signaling initiates coat complex II (COPII) recruitment and export from mammalian endoplasmic reticulum. J. Biol. Chem. 275, 35673–35676. [DOI] [PubMed] [Google Scholar]
- Barlowe, C. (2000). Traffic COPs of the early secretory pathway. Traffic 1, 371–377. [DOI] [PubMed] [Google Scholar]
- Boehm, J., Letourneur, F., Ballensiefen, W., Ossipov, D., Démollière, C., and Schmitt, H.D. (1997). Sec12p requires Rer1p for sorting to coatomer (COPI)-coated vesicles and retrieval to the ER. J. Cell Sci. 110, 991–1003. [DOI] [PubMed] [Google Scholar]
- Bremser, M., Nickel, W., Schweikert, M., Ravazzola, M., Amherdt, M., Hughes, C.A., Sollner, T.H., Rothman, J.E., and Wieland, F.T. (1999). Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell 96, 495–506. [DOI] [PubMed] [Google Scholar]
- Cosson, P., and Letourneur, F. (1994). Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science 263, 1629–1631. [DOI] [PubMed] [Google Scholar]
- Cukierman, E., Huber, I., Rotman, M., and Cassel, D. (1995). The ARF1-GTPase-activating protein: zinc finger motif and Golgi complex localization. Science 270, 1999–2002. [DOI] [PubMed] [Google Scholar]
- Dean, N., and Pelham, H.R.B. (1990). Recycling of proteins from the Golgi compartments to the ER in yeast. J. Cell Biol. 111, 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson, J.G., and Lippincott-Schwartz, J. (2000). Sorting and signalling at the Golgi complex. Cell 101, 693–696. [DOI] [PubMed] [Google Scholar]
- Girod, A., Storrie, B., Simpson, J.C., Johannes, L., Goud, B., Roberts, L.M., Lord, J.M., Nilsson, T., and Pepperkok, R. (1999). Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat. Cell Biol. 1, 423–430. [DOI] [PubMed] [Google Scholar]
- Goldbaum, F.A., Cauerhff, A., Velikovsky, C.A., Llera, A.S., Riottot, M.M., and Poljak, R.J. (1999). Lack of signalling differences in association rates and affinities from short-term and long-term responses to hen egg lysozyme. J. Immunol. 162, 6040–6045. [PubMed] [Google Scholar]
- Griffiths, G., Ericsson, M., Krijnse-Locker, J., Nilsson, T., Goud, B., Söling, H.D., Tang, B.L., Wong, S.H., and Hong, W. (1994). Localization of the Lys, Asp, Glu, Leu tetrapeptide receptor to the Golgi complex and the intermediate compartment in mammalian cells. J. Cell Biol. 127, 1557–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, C., and Helenius, A. (1994). Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J. Cell Biol. 126, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo, J., Muñiz, M., and Velasco, A. (1995). Trimeric G proteins regulate the cytosol-induced redistribution of Golgi enzymes into the endoplasmic reticulum. J. Cell Sci. 108, 1805–1815. [DOI] [PubMed] [Google Scholar]
- Ho, S.N., Hunt, H.D., Horton, R.M., Pullen, J.K., and Pease, L.R. (1989). Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59. [DOI] [PubMed] [Google Scholar]
- Infante, C., Ramos-Morales, F., Fedriani, C., Bornens, M., and Rios, R.M. (1999). GMAP-210, a cis-Golgi network-associated protein, is a minus end microtubule-binding protein. J. Cell Biol. 145, 83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, C.L., and Casanova, J.E. (2000). Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 10, 60–67. [DOI] [PubMed] [Google Scholar]
- Jackson, M.E., Simpson, J.C., Girod, A., Pepperkok, R., Roberts, L.M., and Lord, J.M. (1999). The KDEL retrieval system is exploited by Pseudomonas exotoxin A, but not by Shiga-like toxin-1, during retrograde transport from the Golgi complex to the endoplasmic reticulum. J. Cell Sci. 112, 467–475. [DOI] [PubMed] [Google Scholar]
- Jackson, M.R., Nilsson, T., and Peterson, P.A. (1990). Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 9, 3153–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, M.R., Nilsson, T., and Peterson, P.A. (1993). Retrieval of transmembrane proteins to the endoplasmic reticulum. J. Cell Biol. 121, 317–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, C. (2000). Thinking about p24 proteins and how transport vesicles select their cargo. Proc. Natl. Acad. Sci. USA 97, 3783–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoix, J., Ouwendijk, J., Stark, A., Szafer, E., Cassel, D., Dejgaard, K., Weiss, M., and Nilsson, T. (2001). Sorting of Golgi resident proteins into different subpopulations of COPI vesicles: a role for ArfGAP1. J. Cell Biol. 155, 1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, T.H., and Linstedt, A.D. (2000). Potential role for protein kinases in regulation of bidirectional endoplasmic reticulum-to-Golgi transport revealed by protein kinase inhibitor H89. Mol. Biol. Cell 11, 2577–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur, F., Gaynor, E.C., Hennecke, S., Déollière, C., Duden, R., Emr, S.D., Riezman, H., and Cosson, P. (1994). Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell 79, 1199–1207. [DOI] [PubMed] [Google Scholar]
- Lewis, M.J., and Pelham, H.R.B. (1990). A human homologue of the yeast HDEL receptor. Nature 348, 162–163. [DOI] [PubMed] [Google Scholar]
- Lewis, M.J., and Pelham, H.R.B. (1992). Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell 68, 353–364. [DOI] [PubMed] [Google Scholar]
- Lord, J.M., and Roberts, L.M. (1998). Toxin entry: retrograde transport through the secretory pathway. J. Cell Biol. 140, 733–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoul, I., Sohn, K., Wieland, F.T., Pepperkok, R., Pizza, M., Hillemann, J., and Söling, H.D. (1998). KDEL receptor (Erd2p)-mediated retrograde transport of the cholera toxin A subunit from the Golgi involves COPI, p23, and the COOH terminus of Erd2p. J. Cell Biol. 143, 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoul, I., Straub, M., Hell, S.W., Duden, R., and Söling, H.D. (2001). KDEL-cargo regulates interactions between proteins involved in COPI vesicle traffic: measurements in living cells using FRET. Develop. Cell 1, 139–153. [DOI] [PubMed] [Google Scholar]
- Majoul, I.V., Bastiaens, P.I.H., and Söling, H.D. (1996). Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells. J. Cell Biol. 133, 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín, M.E., Hidalgo, J., Rosa, J.L., Crottet, P., and Velasco, A. (2000). Effect of protein kinase A activity on the association of ADP-ribosylation factor 1 to Golgi membranes. J. Biol. Chem. 275, 19050–19059. [DOI] [PubMed] [Google Scholar]
- Martín, M.E., Hidalgo, J., Vega, F.M., and Velasco, A. (1999). Trimeric G proteins modulate the dynamic interaction of PKAII with the Golgi complex. J. Cell Sci. 112, 3869–3878. [DOI] [PubMed] [Google Scholar]
- Munro, S., and Pelham, H.R.B. (1987). A C-terminal signal prevents secretion of luminal ER proteins. Cell 48, 899–907. [DOI] [PubMed] [Google Scholar]
- Muñiz, M., Alonso, M., Hidalgo, J., and Velasco, A. (1996). A regulatory role for cAMP-dependent protein kinase in protein traffic along the exocytic route. J. Biol. Chem. 271, 30935–30941. [DOI] [PubMed] [Google Scholar]
- Nilsson, T., Jackson, M.R., and Peterson, P.A. (1989). Short cytoplasmic sequences serve as retention signals for transmembrane proteins in the endoplasmic reticulum. Cell 58, 707–718. [DOI] [PubMed] [Google Scholar]
- Nishikawa, S.I., and Nakano, A. (1993). Identification of a novel gene required for membrane protein retention in the early secretory pathway. Proc. Natl. Acad. Sci. USA 90, 8179–8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci, L., Stamnes, M., Ravazzola, M., Amherdt, M., Perrelet, A., Söllner, T.H., and Rothman, J.E. (1997). Bidirectional transport by distinct populations of COP I-coated vesicles. Cell 90, 335–349. [DOI] [PubMed] [Google Scholar]
- Pelham, H.R.B. (1988). Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 1988, 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, H.R.B. (1991). Recycling of proteins between the endoplasmic reticulum and Golgi complex. Curr. Opin. Cell Biol. 3, 585–591. [DOI] [PubMed] [Google Scholar]
- Pelham, H.R.B., Hardwick, K.G., and Lewis, M.J. (1988). Sorting of soluble ER proteins in yeast. EMBO J. 7, 1757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley, J.F., Smith, C., Hirschberg, K., Miller, C., Cole, N.B., Zaal, K.J.M., and Lippincott-Schwartz, J. (1998). Golgi membrane dynamics. Mol. Biol. Cell 9, 1617–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel, A.A., and Pelham, H.R.B. (1998). Identification of amino acids in the binding pocket of the human KDEL receptor. J. Biol. Chem. 273, 2467–2472. [DOI] [PubMed] [Google Scholar]
- Semenza, J.C., Hardwick, K.G., Dean, N., and Pelham, H.R.B. (1990). ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell 61, 1349–1357. [DOI] [PubMed] [Google Scholar]
- Storrie, B., White, J., Röttger, S., Stelzer, E.H.K., Suganuma, T., and Nilsson, T. (1998). Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J. Cell Biol. 143, 1505–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley, F.M., Wilson, D.W., and Pelham, H.R.B. (1993). Mutational analysis of the human KDEL receptor: distinct structural requirements for Golgi retention, ligand binding and retrograde transport. EMBO J. 12, 2821–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist, S., Kim, W., Belden, W.J., Spear, E.D., Barlowe, C., and Ng, D.T.W. (2001). Distinct retrieval and retention mechanisms are required for the quality control of endoplasmic reticulum protein folding. J. Cell Biol. 155, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland, F., and Harter, C. (1999). Mechanisms of vesicle formation: insights from the COP system. Curr. Opin. Cell Biol. 11, 440–446. [DOI] [PubMed] [Google Scholar]
- Wilson, D.W., Lewis, M.J., and Pelham, H.R.B. (1993). pH-dependent binding of KDEL to its receptor in vitro. J. Biol. Chem. 268, 7465–7468. [PubMed] [Google Scholar]
- Yamamoto, K., Fujii, R., Toyofuku, Y., Saito, T., Koseki, H., Hsu, V.W., and Aoe, T. (2001). The KDEL receptor mediates a retrieval mechanism that contributes to quality control at the endoplasmic reticulum. EMBO J. 20, 3082–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J.-S., Lee, S.Y., Gao, M., Bourgoin, S., Randazzo, P.A., Premont, R.T., and Hsu, V.W. (2002). ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J. Cell Biol. 159, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaal, K.J.M. et al. (1999). Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell 99, 589–601. [DOI] [PubMed] [Google Scholar]