Abstract
Members of the California serogroup of bunyaviruses (family Bunyaviridae) are the leading cause of pediatric viral encephalitis in North America. Significant cell death is observed as part of the infection pathology. We now report that a Bunyaviral nonstructural protein termed NSs shows sequence similarity to Reaper, a proapoptotic protein from Drosophila. Although NSs proteins lack the Reaper N-terminal motif critical for IAP inhibition, they do retain other functions of Reaper that map to conserved C-terminal regions. Like Reaper, NSs proteins induce mitochondrial cytochrome c release and caspase activation in cell-free extracts and promote neuronal apoptosis and mortality in a mouse model. Independent of caspase activation, Bunyavirus NSs proteins also share with Reaper the ability to directly inhibit cellular protein translation. We have found that the shared capacity to inhibit translation and induce apoptosis resides in common sequence motifs present in both Reaper and NSs proteins. Data presented here suggest that NSs induce apoptosis through a mechanism similar to that used by Reaper, as both proteins bind to an apoptotic regulator called Scythe and can relieve Scythe inhibition of Hsp70. Thus, bunyavirus NSs proteins have multiple Reaper-like functions that likely contribute to viral pathogenesis by promoting cell death and/or inhibiting cellular translation.
INTRODUCTION
Programmed cell death, or apoptosis, is a physiological process required in metazoans for normal development and tissue homeostasis (reviewed by Bergmann et al., 1998; Vaux and Korsmeyer, 1999; Bangs and White, 2000; Meier et al., 2000). In response to diverse apoptotic signals, both internally and externally generated, most cells activate a specific set of proteases, the caspases, to cleave intracellular substrates essential for the orderly dismantling of the dying cell (reviewed by Zimmermann and Green, 2001; Shi, 2002).
Caspases are ubiquitously expressed, but their enzymatic activity can be regulated by a family of proteins known as Inhibitors of Apoptosis, or IAPs (reviewed by Richter and Duckett, 2000; Shi, 2002). Although IAPs can inhibit caspase activity through direct binding, it has also been reported that RING domain-containing IAP proteins can act as E3 ligases to catalyze caspase ubiquitination and degradation (Yang et al., 2000; Suzuki et al., 2001). Acting in opposition to IAPs is the RHG protein family, which can bind to IAPs and disrupt the IAP-caspase interaction. These IAP inhibitors, which include the Drosophila proteins Reaper, Grim, Hid, Jafrac2, and Sickle and the mammalian proteins Smac/Diablo and Omi/HtrA2, share a short N-terminal motif that can physically contact IAPs and inhibit their function (Wang et al., 1999; Goyal et al., 2000; Christich et al., 2002; Holley et al., 2002; Martins et al., 2002; Srinivasula et al., 2002; Tenev et al., 2002; Verhagen et al., 2002; Verhagen and Vaux, 2002; Wing et al., 2002; Yoo et al., 2002).
Although the Reaper, Grim, and Hid proteins inhibit IAPs through their similar N-termini, they do not share marked homology outside of the N-terminus. In the case of Reaper, a truncated protein lacking the N-terminus is unable to bind IAPs but can still induce apoptosis, suggesting that the C-terminal 2/3 of the protein mediates IAP-independent functions (Evans et al., 1997a; Vucic et al., 1997; McCarthy and Dixit, 1998). One suggested function of the C-terminal 2/3 of Reaper is induction of cytochrome c-release from mitochondria. Although the involvement of mitochondria in Drosophila apoptosis is controversial, ectopic expression of Reaper clearly induces release of mitochondrial cytochrome c in cells of vertebrate origin, leading to Apaf-1–mediated caspase 9 activation (Evans et al., 1997a). Furthermore, the Trp/GH3 motif of Grim, a block of sequence conserved in Sickle and Reaper, is critical for Grim's localization to mitochondria and its capacity to induce programmed cell death in flies (Claveria et al., 2002).
The ability of Reaper to induce apoptosis in intact cells of both fly and vertebrate origin has been well documented (Evans et al., 1997a; Vucic et al., 1997; McCarthy and Dixit, 1998). We have shown in particular that recombinant Reaper can induce apoptotic changes in a cell-free extract derived from Xenopus eggs. Specifically, addition of either GST-Reaper or a peptide encoding the entire 65 amino acids of Reaper can trigger both mitochondrial cytochrome c release and caspase activation (Evans et al., 1997a; Holley et al., 2002). Although the IAP inhibitory properties of the full-length untagged Reaper most likely contribute to apoptotic induction, in the egg extract system mitochondria are absolutely required for measurable caspase activation in response to Reaper (Evans et al., 1997a). While searching for Reaper-interacting proteins that mediate the observed mitochondrial effects, we identified Scythe, a member of the Bag family of Hsc 70/Hsp 70 regulators (Thress et al., 1998, 1999). As shown for Bag 1, the founding member of the family, Scythe can inhibit Hsc/Hsp 70–mediated protein folding (Thress et al., 2001). However, Scythe is unique in that this inhibition can be reversed by Reaper binding. Because removal of Scythe from egg extracts or addition of the recombinant Bag domain from Scythe prevented Reaper-induced caspase activation, we speculated that regulation of protein folding by Reaper might underlie its ability to control mitochondrial cytochrome c release (Thress et al., 2001).
In addition to Reaper's ability to promote mitochondrial cytochrome c release and inhibit IAP function, we and others have recently reported that Reaper can destabilize IAPs (most likely through promotion of auto-ubiquitination) and perhaps more surprisingly, can suppress general protein translation (Thress et al., 2001; Hays et al., 2002; Holley et al., 2002; Ryoo et al., 2002; Yoo et al., 2002). Regions outside of the Reaper N-terminus are necessary for these effects and the C-terminal 2/3 of the protein appears to be sufficient for the translational inhibition. Although the mechanism of translational inhibition by Reaper is not yet understood, it is attractive to speculate that translational inhibition, coupled to IAP destabilization, leads to effective IAP elimination.
Given that multiple activities of Reaper reside in a region of the protein with no known homologues, we decided to search sequence databases for proteins with similarity to the C-terminal 2/3 of Reaper. In this report we show that the nonstructural (NSs) protein encoded by some members of the Bunyaviridae family is similar at the sequence level to Drosophila Reaper. The Bunyaviridae are a large family of mainly arthropod-transmitted viruses characterized by a tripartite, negative-sense RNA genome. Several members of the family cause human disease including febrile illness, encephalitis, and hemorrhagic fevers (Elliott, 1997). Viruses in the California serogroup of the Orthobunyavirus genus, the bunyaviruses in which these Reaper-homologous NSs proteins are found, are the major cause of viral pediatric encephalitis in North America (Gonzalez-Scarano et al., 1992). Although the precise function of the bunyavirus NSs protein is not known, studies on the prototype Bunyamwera virus in which NSs was deleted indicate that it is important for both viral pathogenesis and suppression of host cell protein translation (Bridgen et al., 2001).
We demonstrate here that sequence similarities between Reaper and the NSs proteins extend to functional similarities. Specifically, we show that bunyaviral NSs protein can act in the absence of other viral proteins to inhibit protein synthesis. Moreover, we find that NSs, like Reaper, can induce mitochondrial cytochrome c release and caspase activation. The NSs protein, like Reaper, can reverse Scythe-mediated inhibition of Hsc 70, suggesting that Reaper and NSs share a common mechanism of apoptotic induction. Interestingly, the shared capacity of NSs proteins and Reaper to inhibit translation and induce apoptosis appear to reside in common motifs present in both proteins. These results raise the intriguing possibility that induction of apoptosis by the NSs protein contributes to the pathological cell death observed during bunyaviral encephalitis.
MATERIALS AND METHODS
Secondary Structure Prediction
Secondary structure predictions for NSs and Reaper proteins were made using the PredictProtein server (http://www.embl-heidelberg.de/predict-protein/submit_def.html), specifically, the SSpro program (Baldi et al., 1999). The DAS program was used to generate the hydrophobicity profiles (Cserzo et al., 1997; Server address: http://www.sbc.su.se/~miklos/DAS/maindas.html).
Extract Preparation
Preparation of crude interphase Xenopus egg extracts (CS) was performed as previously described (Evans et al., 1997a). Crude interphase translation extracts (CSt) were prepared the same way as CS, but no cycloheximide was added.
Production of GST Recombinant Proteins
GST and GST-Reaper were prepared as previously described (Evans et al., 1997b). GST-NSsCe, GST-NSsLac, and GST-NSsSa were prepared the same as GST-Reaper.
In Vitro Translation Assays
In vitro translation assays using Xenopus extracts were conducted by adding 1 μCi μl–1 of Trans label (ICN, Costa Mesa, CA), 100 μm zVAD-fmk (BioMol, Plymouth Meeting, PA) and 100 ng μl–1 of recombinant GST, GST-Reaper, GST-NSsCe, or GST-NSsSa to CSt extracts. Protein translation was assayed by SDS-PAGE analysis, or by TCA precipitation (80 μg of extract in 20% TCA). In vitro assays using RRL were conducted as previously described (Holley et al., 2002).
Immunodepletion Assays
Affi-Prep protein A-beads (40 μl; Bio-Rad, Hercules, CA) were washed in PBS and incubated with 135 μl of preimmune or anti-scythe antisera overnight. The beads were washed twice in ELB and incubated 150 μl of CSt for 1 h at 4°C. Beads were then allowed to pellet by gravity, the supernatant was transferred to a fresh Microfuge tube, and the depletion process was repeated, using fresh beads, twice more. Depleted extracts were then assayed for their ability to translate in the presence of recombinant GST, GST-Reaper, or GST-NSsCe.
Cell Culture, Transfections, and Pulse Analysis
All cell culture reagents were obtained from Invitrogen (Carlsbad, CA) unless otherwise specified. BHR cells were maintained in G-MEM supplemented with 10% FBS, 10% tryptose phosphate broth, and 1 mg/ml geneticin. For pulse chase analysis, 200,000 cells were plated per well in six-well plates in medium lacking geneticin and transfected 24 h later using LipofectAMINE 2000 (Invitrogen), OptiMEM, and 2 μg of DNA, according to the manufacturer's instructions. Sixteen to 20 h after transfections, cells were washed once with prewarmed pulse medium (DMEM minus l-Met and l-Cys supplemented with 10% dialyzed fetal bovine serum and 1 mM sodium pyruvate) and then incubated for 15 min in pulse medium to deplete Met and Cys levels. Cells were then incubated for the times indicated in pulse medium containing 200 μCi ml–1 of 35S-trans label. Radiolabeled proteins were harvested and separated by SDS-PAGE as previously described (Holley et al., 2002).
DEVD and Nuclear Fragmentation Assay
Recombinant GST, GST-Reaper, GST-NSsCe, GST-NSsLac, and GST-NSsSa were added in 1:10 dilution to crude egg extracts. At the indicated times, 3 μl aliquots were withdrawn and incubated with 90 μl of assay buffer and 200 μm Ac-DEVD pNA colorimetric substrate (BioMol) as previously described (Holley et al., 2002). Caspase-3 activity was monitored by the measurement of absorbance at 405 nm using a LabSystems MultiSkan MS microtiter 96-well plate reader (Helsinki, Finland). Nuclear fragmentation assay was performed as previously described (Evans et al., 1997a).
Cytochrome c Release Assays
Recombinant GST, GST-Reaper, GST-NSsCe, or GST-NSsLac was added 1:10 dilution to CS extracts supplemented with 100 μM zVAD-fmk. At the indicated times, 20-μl aliquots were withdrawn and filtered through a 0.1-μm ultrafree-MC filter (Millipore, Bedford, MA). Aliquots were then analyzed for cytochrome c content by SDS-PAGE and immunoblotting as previously described (Evans et al., 1997a).
Protein Refolding Assays
Protein refolding assays were performed as previously described (Freeman and Morimoto, 1996).
Scythe Binding and Release Assays
Scythe binding and release assays were performed as previously described (Thress et al., 1999).
Viruses
Recombinant Sindbis virus either empty (dsTE12Q) or carrying the NSsSA or NSsCE genes under the regulation of an extra copy of the subgenomic promoter were generated as described (Levine et al., 1996). Infectious viruses recovered from the supernatants of transfected BHK (baby hamster kidney) cells (American Type Culture Collection, Manassas, VA) were titered by standard plaque assay.
Mice Mortality Assays
Twelve-day-old CD-1 mice (Charles River Laboratories, Wilmington, MA) were inoculated by intracerebral injection of 5 × 103 plaque-forming units of each recombinant virus diluted in 30 μl HBSS.
TUNNEL Assays
Mice were inoculated as described above. At 2–3 d after infection brains were collected and fixed in 4% paraformaldehyde. The fixed tissue was processed and paraffin-embedded. Sections 5 μm in thickness were deparaffinized, rehydrated, and permeabilized using 0.1% Triton X-100 in 0.1% sodium citrate to perform TUNNEL assays as described by the manufacturer (Roche Diagnostics, Indianapolis, IN).
RESULTS
Sequence and Structural Similarities between California Serogroup Nonstructural NSs Protein and Drosophila Reaper
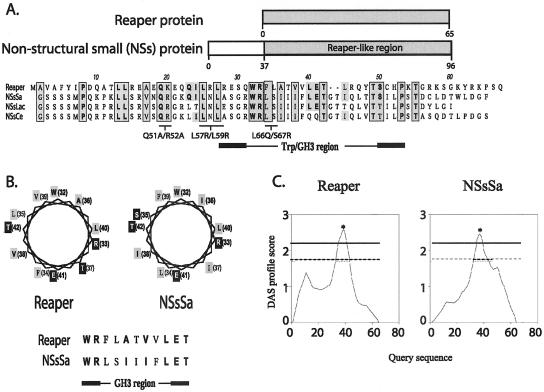
Although the extreme N-terminus of Reaper is required for IAP binding and stimulated degradation, this region of the protein is not critical for Reaper-induced mitochondrial cytochrome c release or translational inhibition (Evans et al., 1997a; Holley et al., 2002). Given that these activities of Reaper mapped to a region of the protein with no known orthologues, we wished to search known protein databases for proteins with sequence similarity to Reaper. We were particularly interested in identifying related proteins known to regulate protein synthesis. Accordingly, we queried public databases using a tBLASTn algorithm for proteins similar to Reaper amino acids 16–65. This search yielded a group of Non-Structural small-segment–encoded proteins (NSs) from some members of the Orthobunyavirus genus in the Bunyaviridae family of viruses. The Orthobunyavirus genus comprises 172 named viruses that are subdivided into 18 serogroups. The smallest of the three orthobunyavirus genomic RNA segments encodes two proteins, the nucleocapsid (N) protein and, in an overlapping distinct reading frame, a nonstructural protein termed NSs. We found that in the California group, the major cause of viral pediatric encephalitis in North America (Gonzalez-Scarano et al., 1992), NSs proteins are similar to Reaper at the C-terminal region in a 59-amino acid area we termed the Reaper-like region (RLR). The most similar of these to Reaper, the NSs from San Angelo virus (SA), has an RLR that is 50% similar to Reaper with 12 exact amino acid identities (25%; Figure 1A; also shown are NSs from slightly more distantly related viruses, La Crosse and California encephalitis).
Figure 1.
Sequence alignment of NSs and Reaper. (a) Public databases queried for Reaper homologues using a tBLASTn algorithm yielded a group of nonstructural proteins encoded by the small genome segment (NSs) expressed in the Bunyaviridae viral family. The Reaper-like region in the NSs protein is highlighted in the schematic diagram of the NSs. Areas of similarity between the NSs and Reaper are shaded. Amino acid identities are represented in bold. Areas of similarity and identity between the three NSs proteins and Reaper are boxed. Also indicated are areas of sequence similarity mutated and assayed for function (see Figure 3). (b) Helical wheel projection diagram of the Reaper and NSsSa GH3 region, which is predicted to conform to an amphipathic α-helix. Similar and identical residues are represented in bold. Hydrophobic residues are shaded in gray, and hydrophilic residues in black. (c) Hydrophobicity analysis of Reaper and the NSsSa RLR by DAS TM-segment prediction. Dashed horizontal line correspond to loose cutoff DAS score of 1.7, and the solid gray line corresponds to the strict cutoff DAS score of 2.2. Starred area of high hydrophobicity corresponds to amino acids 33–42 (in both Reaper and NSs RLR).
The RLR overlaps the Trp/GH3 motif, a small homologous block shared by Reaper, Grim, and Sickle (Wing et al., 1998, 2001, 2002; Christich et al., 2002; Claveria et al., 2002). This motif has been predicted to form an amphipathic α-helix (Claveria et al., 2002; Wing et al., 2002). Secondary structure analysis of the NSs proteins suggested that the RLR's Trp/GH3 motif also conforms to an amphipathic α-helix, with four hydrophilic amino acids in this highly hydrophobic region positioned at the same face of the helix (Figure 1B). Hydrophobicity analysis of the Trp/GH3 region corroborated that both proteins had similar hydrophobicity profiles and conserved amino acids responsible for the observed hydrophobicity patterns (Figure 1C). Collectively, these observations suggest that sequence similarities between NSs proteins and Reaper might extend to structural similarities of functional significance.
NSs Proteins Inhibit General Protein Translation
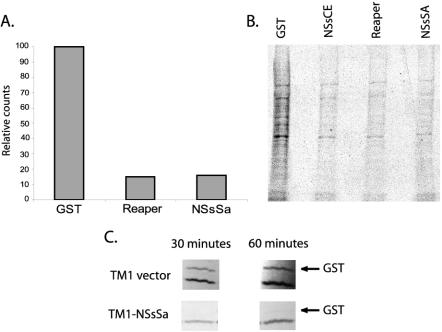
Little is known about NSs biological function, but these proteins are demonstrably important for viral pathogenesis. Moreover, a mutant Bunyamwera virus (Bunyamwera serogroup, Orthobunyavirus genus) deleted for its NSs (BUN-delNSs) lacks the characteristic ability to inhibit host cell protein synthesis during viral infection (Bridgen et al., 2001). Although the failure of BUNdelNSs to impair translation implied that the NSs protein might be a translational regulator, the ability of NSs to inhibit translation directly has not been reported. Hence, we wished to determine whether the NSs proteins and Drosophila Reaper shared the ability to downregulate general protein synthesis. To test this, NSs protein from San Angelo virus (NSsSa) was produced in bacteria as a GST fusion protein. When equivalent levels of NSsSa, Reaper, or GST were added to translationally competent but transcriptionally inactive Xenopus eggs extracts, a profound inhibition of translation was observed in the presence of the NSs protein and Drosophila Reaper, but not GST alone (Figure 2, A and B). Interestingly, the dose-response curve for protein translation was very similar for Drosophila Reaper and viral NSs proteins (unpublished data). Additionally, to assess the translational effects of the NSs proteins in mammalian cells, we cloned the gene of NSsSa downstream of an EMCV IRES sequence, which allows protein expression even when bulk cap-dependent protein translation is attenuated (Moss et al., 1990). This construct, along with a plasmid encoding GST, was transfected into BHR cells treated with the general caspase inhibitor zVAD-fmk to circumvent any translational inhibition that might result from NSs-mediated apoptotic induction (see further below). To assess de novo protein synthesis in these cells, we pulsed them briefly with [35S]methionine, prepared lysates, and isolated the newly synthesized GST protein on glutathione Sepharose beads. As shown in Figure 2C, the NSs protein was able to dampen GST synthesis markedly (note that synthesis of the endogenous protein migrating just below GST appears unaffected because most of the cells in the culture do not express the NSs/GST because of low transfection efficiency). Collectively, these observations extend those made using BUNdelNSs virus and argue strongly that NSs proteins can suppress general protein translation.
Figure 2.
Repression of translation by NSs proteins. (a) Translationally competent, but transcriptionally inactive, Xenopus egg extracts were supplemented with 35S-Met/Cys and zVAD-fmk plus GST, GST-Reaper, or GST-NSsSa. Translation was allowed to proceed for 45 min, and products were subjected to TCA precipitations and quantified by scintillation counting. (b) Samples prepared as in panel a were resolved by SDS-PAGE. (c) BHR cells were transfected with GST and either NSs or vector control. Cells were radiolabeled in a pulse experiment, and the resulting radiolabeled GST protein was precipitated from cell lysates using GSH beads. GST expression was then analyzed by autoradiography. The synthesis of the endogenous protein that interacts with GSH beads (lower band) was also observed in mock-transfected cells (unpublished data). Its synthesis appears unaffected because most cells do not express NSs/GST because of low transfection efficiencies.
Residues Conserved between Reaper and NSs Are Critical for Translational Inhibition
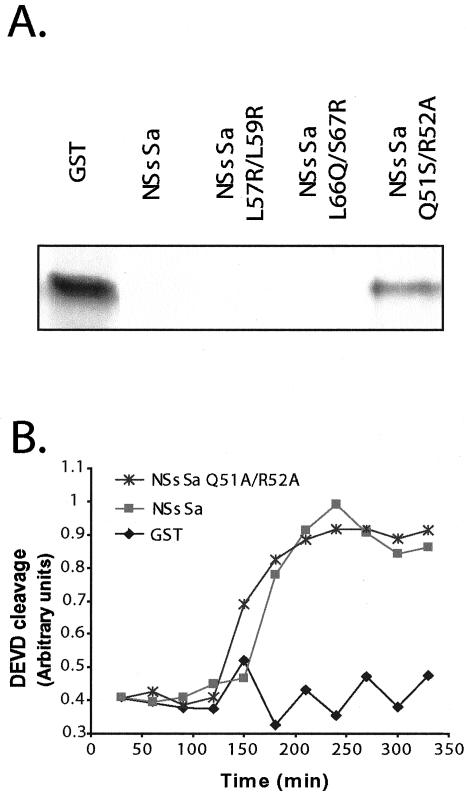
The shared ability of the NSs proteins and Reaper to inhibit protein synthesis suggested that the sequence similarities described above were functionally significant. Therefore, we speculated that a shared sequence motif might be responsible for the common ability of these proteins to inhibit translation. To address this possibility, we performed site directed mutagenesis of motifs shared by Reaper and NSs proteins. As shown in Figure 3A, NSsSa proteins altered at residues L57R/L59R or residues L66Q/S67R were still capable of inhibiting protein synthesis, suggesting that these conserved residues (equivalent to residues 25/27 and 34/35, respectively, in Reaper) were not required for the inhibitory activity. However an NSsSa mutant altered at residues 51 and 52 (Q51A/R52A) was incapable of inhibiting translation. Mutations of the corresponding residues in Reaper (residues 19–20) also abrogated its capacity to inhibit translation (unpublished data). These data suggest that there are areas of sequence similarity shared by these proteins that are critical for their capacity to inhibit protein synthesis, perhaps responsible for mediating a common interaction with some component of the translation machinery.
Figure 3.
Sequence similarities are functionally significant. (a) GST, GST-NSsSa, GST-NSsSa L57R/L59R, GST-NSsSa L66Q/S67R, or GST-NSsSa Q51A/R52A was added to rabbit reticulocyte lysates programmed with nonspecific Cdc25 cDNA. The reticulocyte lysates were allowed to transcribe and translate for 45 min, and the product was resolved by SDS-PAGE as previously described (Holley et al., 2002). Note that the GST-NSsSa Q51A/R52A mutant was still markedly defective in translational inhibition even when added at concentrations four-fold higher than the GST-NSsSa L57R/L59R or GST-NSsSa L66Q/S67R proteins. (b) GST, GST-NSsSa, or GST-NSsSa Q51A/R52A was added to crude Xenopus egg extracts and caspase activity was monitored by cleavage of the colorimetric peptide substrate DEVD-pNA.
NSs Proteins Can Trigger Caspase Activation in Cell-free Xenopus Egg Extracts
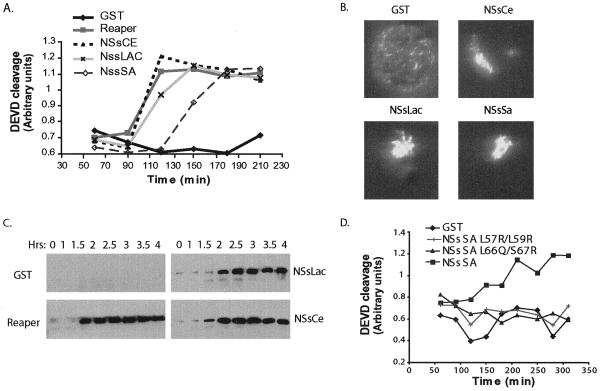
The ability of Reaper to promote caspase activation and cell death stems in part from its ability to inhibit IAP function. However, the Reaper 16–65 protein, lacking IAP inhibitory functions, is also able to induce apoptosis, suggesting that distinct determinants of cell killing lie within the C-terminal 2/3 of the protein (Chen et al., 1996; Evans et al., 1997a; Vucic et al., 1997; McCarthy and Dixit, 1998; Wing et al., 2001). Given the sequence homology between NSs proteins and Reaper in this region, we speculated that the viral NSs proteins could also affect apoptotic pathways. Xenopus egg extracts allow us to characterize the pro- or anti-apoptotic properties of proteins by assaying their ability to inhibit or accelerate caspase activation (Kluck et al., 1997). As described above, we have previously reported that addition of Reaper to cell-free Xenopus egg extracts triggers several hallmarks of apoptosis, including caspase activation and nuclear fragmentation. To determine if the NSs proteins could modulate apoptotic caspase activation, we supplemented interphase extracts with GST fused NSsSa, NSsCe, NSsLac, and Reaper proteins. As with all of our previously reported apoptosis assays, these were performed in the presence of cycloheximide, so that the ability of these proteins to inhibit translation would not impact the assay. Surprisingly, we found that GST-Reaper and the NSs proteins (but not GST alone) were equivalently active in inducing caspase activation, as measured by cleavage of the artificial caspase 3 substrate, DEVD-pNA, and nuclear fragmentation (Figure 4, A and B). These data suggest that NSs proteins, like fly Reaper, have proapoptotic properties.
Figure 4.
Induction of apoptosis by NSs proteins. (a) GST, GST-Reaper, GST-NSsCe, GST-NSsLac, and GST-NSsSa were added to crude Xenopus egg extracts and caspase activity was monitored by cleavage of the colorimetric peptide substrate DEVD-pNA. (b) Photomicrographs of representative nuclei formed in vitro by incubation of sperm chromatin in egg extracts after supplementation of extracts with either GST alone, GST-NSsCe, GST-NSsLac, or GST-NSsSa protein. Nuclear morphology was normal in the presence of GST, but the addition of GST-NSsCe, GST-NSsLac, or GST-NSsSa resulted in apoptosis and nuclear fragmentation. (c) Recombinant GST, GST-Reaper, GST-NSsLac, or GST-NSsCe proteins were added to crude extract in the presence of the caspase inhibitor zVAD-fmk. Samples were collected at the indicated times, and mitochondria were removed by passage of the extract through a 0.1-μm filter. The filtered extract was examined for cytochrome c release by anticytochrome c immunoblotting. (d) GST, GST-NSsSa, GST-NSsSa L57R/L59R, or GST-NSsSa L66Q/S67R was added to crude Xenopus egg extracts and caspase activity was monitored by cleavage of the colorimetric peptide substrate DEVD-pNA. Note that although the kinetics of caspase activation varies slightly from extract to extract, the relative activities of the wild-type and mutant proteins are constant.
NSs Proteins Induce Mitochondrial Cytochrome c Release
Using Xenopus egg extracts, we have previously demonstrated that Drosophila Reaper can trigger mitochondrial cytochrome c release and that this release is absolutely required in this system for Reaper-induced caspase activation (Evans et al., 1997a; Kluck et al., 1997). To determine if the NSs proteins could also induce cytochrome c release, GST-NSsSa, GST-NSsCe, or GST-NSsLac proteins were incubated in Xenopus egg extracts supplemented with the caspase inhibitor zVAD-fmk. Caspase inhibitor was included in these incubations to preclude mitochondrial cytochrome c release induced after the activation of caspases. At various times after protein addition, aliquots of extract were withdrawn and filtered to remove mitochondria. The resulting supernatants were then immunoblotted for released cytochrome c. Like Drosophila Reaper, the NSs proteins accelerated cytochrome c release in a caspase-independent manner (Figure 4C). These data suggest that the observed caspase activation by the NSs proteins in egg extracts results from an acceleration of mitochondrial cytochrome c release.
Residues Conserved between Reaper and NSs Are Critical for Induction of Apoptosis
Given that residues shared between Reaper and NSs proteins were central for their ability to inhibit translation, we speculated that a shared sequence motif might also be responsible for the common ability of these proteins to induce apoptosis. To address this possibility, we tested the mutants described above for their capacity to activate caspases in Xenopus egg extracts. As shown in Figure 4D, NSsSa mutants L57R/L59R and L66Q/S67R, both which disrupt hydrophobic residues in or near the Trp/GH3 region, were unable to induce apoptosis (as opposed to the Q51A/R52A mutants shown in Figure 3). These data suggest that there are regions of sequence similarity between Reaper and NSsSa proteins, which are critical for apoptotic induction and for translation inhibition. It also strongly suggests that the observed sequence similarities are functionally significant.
NSs Proteins Bind to Scythe and Modulate Its Function
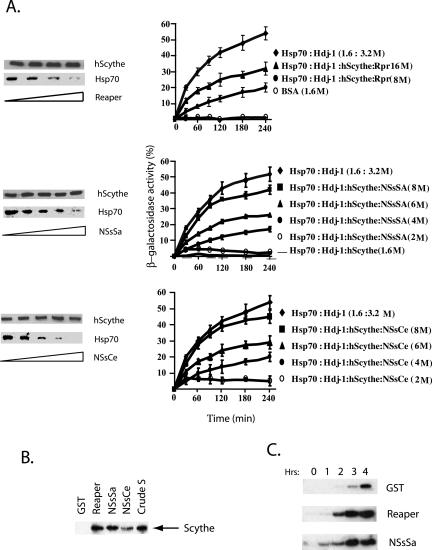
We have previously demonstrated that Reaper can bind to and regulate the action of the Hsc/Hsp 70 modulator, Scythe, and that this activity of Reaper is central to its mitochondrial cytochrome c-releasing activity (Thress et al., 2001). To determine if NSs and Reaper might promote cytochrome c release by a similar mechanism, we first wished to determine if the NSs proteins could, like Reaper, alleviate Scythe-mediated Hsp 70 suppression. To test this we followed the in vitro protein-folding assay for Hsp 70 chaperones described by Freeman and Morimoto (1996). Briefly, β-galactosidase was denatured in 6 M guanidine HCl and then incubated in refolding buffer with purified recombinant Hsp 70, ATP, and the cochaperone Hdj-1 in the presence or absence of Scythe, Reaper, NSsSa, or NSsCe proteins. After various times of incubation, samples were assayed for β-galactosidase activity using the colorimetric substrate o-nitrophenyl-β-d-galactopyranoside. As shown in Figure 5A and reported previously, Scythe very effectively suppressed Hsp 70–mediated protein folding. Strikingly, the NSs proteins were as effective as Reaper at reversing this inhibition of protein folding (Figure 5A). Consistent with these data, we found that the NSs proteins bound well to Scythe (both Xenopus, Figure 5B, and Human Scythe/BAT3, unpublished data) and could, upon binding, displace Scythe-bound Hsp 70 (Figure 5A). Collectively, these observations strongly suggest that Reaper and NSs proteins can modulate Scythe in a similar manner.
Figure 5.
NSs bind to Scythe and modulate its function. (a) His-hScythe (2 μm) was incubated with Hsp70 (2 μm) and the complex was allowed to form. After complex formation, increasing concentrations of recombinant Reaper (2, 5, 8, and 10 μM in lanes 1–4, respectively), NSsCe or NSsSa (2, 4, 6, 8, and 10 μM in lanes 1–5, respectively) were added. Bound proteins were precipitated with Ni+-agarose beads, washed, resolved by SDS-PAGE, and analyzed by immunoblotting using an Hsp70 mAb 5a5 or an anti-His antibody for hScythe detection (left panel). Hsp 70–dependent refolding of β-galactosidase was measured in the presence of full-length human Scythe and increasing concentrations of recombinant Reaper, NSsSa, or NSsCe (right-panel). (b) Recombinant GST, GST-Reaper, GST-NSsSA, or GST-NSsCe proteins attached to glutathione Sepharose beads were incubated in Xenopus egg extracts, retrieved by centrifugation, washed, and analyzed for the presence of bound Scythe by immunoblotting. (c) Anti-Scythe immunoprecipitates formed by incubation of protein A-linked anti-Scythe antibodies with Xenopus egg extracts were washed and then incubated with recombinant GST, Reaper, or NSsSa protein. The released activity was then incubated with purified isolated mitochondria. After removal of the mitochondria by filtration, released cytochrome c was detected by immunoblotting with anticytochrome c antibody.
Although the precise substrate of the Reaper/Scythe/Hsp 70 machine responsible for mitochondrial cytochrome c release has been elusive, on an empirical level we know that Scythe immunoprecipitates contain a Reaper-releasable factor that can trigger cytochrome c release from isolated mitochondria (Thress et al., 1999). Therefore, we assayed the NSs proteins for the Reaper-like ability to induce the dissociation of a cytochrome c-releasing activity from Scythe precipitates. Immunoprecipitates from Xenopus egg extracts formed using either preimmune or Scythe antisera linked to protein A Sepharose were washed extensively and then incubated with either recombinant GST protein, GST-Reaper, or GST-NSsSa to initiate release of presumptive cytochrome c-releasing factors. Bead-bound proteins (including Scythe, Reaper, and NSsSa) were removed by centrifugation and the collected supernatants were concentrated and added to purified mitochondria. As shown in Figure 5C and previously reported (Thress et al., 1999), Reaper induced the release of proapoptotic activity from the immune resin and GST did not induce release of such factors. Interestingly, the NSsSa behaved like Reaper, promoting dissociation of a cytochrome c–releasing activity from anti-Scythe immunoprecipitates. These results point to a common mechanism whereby Reaper and NSs act in conjunction with Scythe to trigger mitochondrial cytochrome c release and consequent caspase activation.
Despite the apparent involvement of Scythe in both Reaper- and NSs-mediated apoptosis, it should be noted that their shared ability to regulate Scythe does not appear to account for their effects on protein translation. Specifically, removal of >95% of Scythe from Xenopus egg extracts by immunodepletion did not interfere with the ability of either Reaper or the NSs proteins to dampen translation (unpublished data). These data suggest that the 16–65 region of Reaper and the NSs proteins must interact with factors other than Scythe to mediate translational repression.
NSs Proteins Can Induce Apoptosis in the Mouse Brain
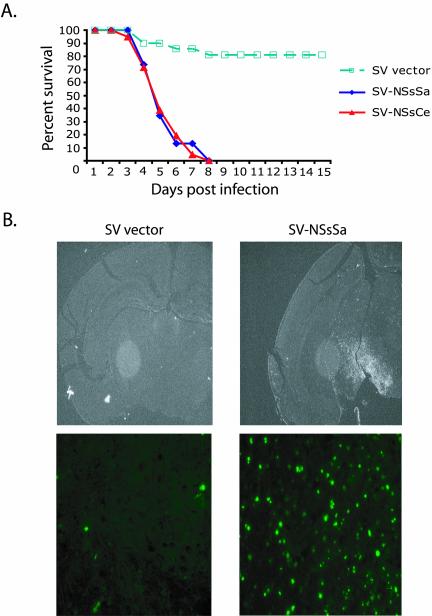
Although the data above strongly suggest that NSs proteins can have proapoptotic activity, these experiments were all performed in vitro in the heterologous Xenopus system. We wished to extend these in vitro observations further by determining whether the NSs proteins could also induce apoptosis in vivo. Bunyaviruses are normally neuronotropic viruses; peripheral infection of young mammals with bunyaviruses leads to invasion of the CNS and increased cytopathy (Gonzalez-Scarano et al., 1992; Nathanson, 1997). To induce expression of the NSs proteins in neuronal mouse tissue, we subcloned NSsSa and NSsCe genes into a Sindbis virus-based expression vector. Sindbis virus infection causes age-dependent encephalitis in mice, and its severity correlates with the degree of virus-induced apoptosis in neuronal cells (Griffin and Hardwick, 1997). Recombinant Sindbis virus (dsTE12Q) carrying genes coding for the NSs proteins were introduced by intracerebral injection into the brain of twelve-day-old mice, and animals were monitored for 10 d. As shown in Figure 6A and previously reported (Levine et al., 1996), mice this age are resistant to Sindbis-induced death, with >80% of animals surviving the infection. Remarkably, all of the animals inoculated with the recombinant virus expressing the NSs proteins died at approximately 6 days postinjection (Figure 6A).
Figure 6.
NSs induce programmed cell death in vivo. (a) Percent survival of 12-d-old CD-1 mice inoculated with recombinant Sindbis virus carrying no exogenous gene (SV vector) or carrying the NSsSa or NSsCe genes (SV-NSsSa or SV-NSsCe, respectively). A total of 50–60 mice were analyzed per treatment group. (b) TUNNEL staining of tissue sections from brains of 12-d-old CD-1 mice inoculated with SV vector or SV-NSsSa (top panel). Close-up of TUNNEL staining of brain tissue sections (bottom panel).
To determine if the observed increase in mortality following NSs expression was due to the induction of neuronal programmed cell death, sagittal cross sections of brains from mice infected with the various Sindbis virus constructs were analyzed by TUNNEL staining. As shown in Figure 6B, brains from mice inoculated with control SV vector displayed low levels of TUNNEL-positive cells. In contrast, brain sections from SV-NSs–infected animals showed highly elevated levels of TUNNEL-positive cells, indicating a significant increase in virus-induced apoptosis. Taken together, these results indicate that expression of NSs proteins during SV infection resulted in exacerbated programmed cell death of infected neurons and efficient induction of fatal encephalitis in infected animals, demonstrating the ability of NSs proteins to function as prodeath factors in vivo.
DISCUSSION
Drosophila Reaper is a potent apoptotic inducer in cells of both vertebrate and invertebrate origin. Although Reaper is only 65 amino acids long, it can inhibit IAPs, promote IAP autoubiquitination, trigger mitochondrial cytochrome c release, and inhibit general protein translation (Evans et al., 1997a; Goyal et al., 2000; Holley et al., 2002). A bona fide vertebrate Reaper homolog able to perform all of these functions has not yet been reported, though the extreme Reaper N-terminus shares limited similarity at the sequence level with Smac/Diablo and Omi/HtrA2, which translates into the functional ability to bind IAPs and displace them from caspases (Martins et al., 2002; Verhagen et al., 2002; Verhagen and Vaux, 2002). We report here that a group of bunyaviral nonstructural NSs proteins share limited homology with the C-terminal region of fly Reaper. Although these sequence similarities do not necessarily indicate common evolutionary origins, the shared ability of the California NSs proteins and Reaper to inhibit generalized protein translation, induce mitochondrial cytochrome c release/caspase activation and modulate Scythe/Hsp 70 activities strongly suggest that the shared sequence motifs are functionally significant.
Homology and Functional Similarities
Structural predictions for the NSs RLR and Reaper suggest that the sequence similarities between these proteins might translate to structural similarities. For instance, both NSs proteins and Reaper share a similar pKi (NSsSa pKi is 10.10; Reaper pKi is 10.13), and their conserved GH3 motif is predicted to form an amphipathic α-helix. Interestingly, this region is also present in the Drosophila proteins Sickle and Grim (Claveria et al., 2002; Wing et al., 2002). Although the function of this region is not fully understood, it is important for Grim's apoptotic activity, and mutations in this region affect its capacity to localize to the mitochondria (Claveria et al., 2002). Consistent with these observations, hydrophobic residues altered in or near this conserved α-helical area also affected NSsSa capacity to induce apoptosis. Moreover, recent data in our lab indicates that similar mutations in Reaper also disrupt its capacity to induce apoptosis (M. Olson and S. Kornbluth, unpublished observations). Interestingly, these mutations did not affect the capacity of the protein to inhibit translation. Instead, a conserved motif present outside of the Trp/GH3 area is apparently responsible for the inhibitory activity of these proteins. These data collectively show that sequence similarities in the RLR area are likely to account for the similar activities observed for Reaper and NSs proteins.
One interesting conclusion to be drawn from these studies concerns the evolutionary separation of Reaper's functions. One hallmark function of Reaper is the ability to inhibit IAP-caspase interactions. This activity of Reaper resides in its extreme N-terminus in a motif shared by the proteins Grim and Hid, as well as the mammalian proteins SMAC and Omi (Wang et al., 1999; Goyal et al., 2000; Christich et al., 2002; Holley et al., 2002; Martins et al., 2002; Srinivasula et al., 2002; Tenev et al., 2002; Verhagen et al., 2002; Verhagen and Vaux, 2002; Wing et al., 2002; Yoo et al., 2002). As far as we know, there is no evidence to suggest that either SMAC or Omi, which are unrelated to the C-terminal 2/3 of Reaper, can either induce mitochondrial cytochrome c release or inhibit protein synthesis. Based on the identification of regions of significant sequence homology shared between the C-terminal 2/3 of Reaper and NSs proteins and the absence of IAP inhibitory motifs in the NSs proteins, we would suggest that the N- and C-terminal functions of Reaper have evolved to be present in separate proteins. Moreover, this suggests that there may be as yet unreported mammalian proteins that share sequence similarity/function with the C-terminal 2/3 of Reaper. In this regard, it is interesting to note that we have found that coexpression of SMAC and amino acids 16–65 of Reaper in human cells (which are both very weak apoptotic inducers on their own) can reconstitute full Reaper-like cell killing activity (C. Holley and S. Kornbluth, unpublished observations).
Translational Inhibition by NSs Proteins and Reaper
In this report we show that NSs proteins, like Reaper, can act in isolation to inhibit protein synthesis. These observations support previous studies demonstrating that a mutant virus lacking NSs (BUNdelNSs) is impaired in its ability to inhibit host cell translation (Bridgen et al., 2001). The ability of these Reaper-related proteins to inhibit protein translation also helps to validate this rather unexpected function of Reaper itself. As the nearly complete immunodepletion of Scythe did not compromise the translational inhibition, we suspect that there are other ligands for the NSs proteins/Reaper that mediate effects on the translational machinery. Further work will be required to elucidate the mechanism of this inhibition, but the availability of several proteins that act in a similar manner, and the identification of a common motif important for translation inhibition should facilitate isolation of the relevant targets.
The capacity of the viruses to inhibit translation raises the intriguing question as to how they can avoid inhibiting synthesis of their own RNA while affecting the host protein synthesis program. Interestingly, NSs proteins are encoded by the smallest of the three orthobunyavirus genomic RNA segments and are in an overlapping, but distinct reading frame with the nucleocapsid (N) protein (Bridgen et al., 2001). This suggests that the synthesis of NSs proteins might be controlled by a cap-independent translational pathway such as that driven by internal ribosome entry sites. This is the case for other known classes of viral proteins able to inhibit translation (reviewed by Vagner et al., 2001). If this is the case for the NSs proteins, they may inhibit cap-dependent host translation initiation while preserving their own expression (and presumably that of other bunyaviral proteins).
Apoptotic Induction by NSs Proteins
Viruses frequently inhibit cap-dependent translation of host proteins. For viruses whose own reading frames are translated from IRES sequences, this can serve as a means to enhance translation of their own IRES-driven viral proteins. We speculated that Reaper-mediated translational inhibition could contribute to apoptotic induction by preventing synthesis of short-lived apoptotic inhibitors, such as the IAPs (Holley et al., 2002). It is not yet clear whether the translational inhibitory properties of the NSs proteins also contribute to their apoptotic activity. However, NSs proteins must possess apoptotic regulatory activity distinct from their ability to inhibit translation as they accelerated mitochondrial cytochrome c release and caspase activation in cycloheximide-treated Xenopus egg extracts.
Although the IAP inhibitory functions of fly Reaper are clearly significant for its full apoptotic potential, several reports have attributed distinct apoptotic activities to the C-terminal 2/3 of Reaper where the similarities to the RLR NSs lay (Evans et al., 1997a; Vucic et al., 1997; McCarthy and Dixit, 1998). Indeed, the 16–65 Reaper fragment, which lacks the RHG domain, can still induce apoptosis (Chen et al., 1996; McCarthy and Dixit, 1998; Thress et al., 1999). We have shown that NSs proteins, which lack an RHG domain, can induce apoptosis in both cell-free extracts and in vivo when introduced into mice. Furthermore, NSs proteins appear to act similarly to Reaper in modulating the BAG domain-containing protein Scythe. Reaper was the first protein documented to relieve inhibition of Hsc 70/Hsp 70 by BAG family members (Thress et al., 2001). The NSs proteins constitute a second group of these ligands, potentially able to modulate a number of cellular functions by controlling protein chaperones. Like Reaper, the NSs proteins appear to induce mitochondrial alterations (e.g., cytochrome c release) through dissociation of a Scythe-sequestered cytochrome c–releasing activity. Although the nature of this activity is not yet clear, the common ability of NSs and Reaper to invoke these activities further underscores their likely similar mechanism of action.
Viruses and Apoptosis
Under most circumstances, known viral regulators of cell death act to inhibit, rather than promote, apoptosis to preserve the host cells in which they replicate (reviewed by Hardwick, 2001; Benedict et al., 2002). However, viruses have also been shown to elicit and use apoptosis as part of their infection strategy. For example, the Aleutian mink disease parvovirus (ADV) induces caspase activation and apoptosis. Direct inhibition of caspase 3 not only prevented viral-induced apoptosis, but also compromised ADV replication (Best et al., 2002). Studies on coxsackie virus B2, hepatitis C virus, herpes simplex virus, influenza A virus, and HIV-1 have also found that particular viral proteins critical for the viral life cycle also directly induce apoptosis (Henke et al., 2000; Anderson, 2001; Goh et al., 2001; Henke et al., 2001; Schultz-Cherry et al., 2001; Muthumani et al., 2002a, 2002b). It has been hypothesized that induction of apoptosis could facilitate the dissemination of viral particles. Furthermore, programmed cell death may also play an important role in the viral strategy to avoid the host immune response (Fazakerley, 2001; Fazakerley and Allsopp, 2001).
Bunyaviruses are cytopathic, causing hemorrhagic fevers and encephalitis in humans (Gonzalez-Scarano et al., 1992; Elliott, 1997). Apoptotic cell death by members of the Bunyaviridae has been described for hantaviruses (Kang et al., 1999) and of direct relevance for our study, for La Crosse virus (Pekosz et al., 1996). La Crosse virus induces apoptosis in the brains of newborn mice and in some, but not all, neuronal cell lines. Moreover, BUN NSs protein has been shown to be critical for viral pathogenesis (Bridgen et al., 2001; Weber et al., 2002). Given our findings on NSs apoptotic activity, it is attractive to speculate that the NSs proteins are directly responsible, through the induction of apoptosis, for the observed bunyavirus-induced cytopathy. This makes them interesting targets of regulation in controlling human arbovirus encephalitis.
Neuronal Apoptosis and Reaper
Although Reaper was originally thought to be a universal regulator of apoptosis, analysis of flies defective only at the Reaper locus revealed that Reaper is required solely for developmental apoptosis of the central fly ganglia (Peterson et al., 2002). Bunyaviruses are neurotropic viruses and the NSs proteins appear to be potent inducers of neuronal apoptosis (Gonzalez-Scarano et al., 1992; Pekosz et al., 1996; Figure 6). In several cytopathic neurotropic viruses, neurovirulence can be dependent upon the age of the host, with younger hosts being more susceptible to neurotropic virus infections (reviewed by Anderson, 2001; Fazakerley, 2001; Fazakerley and Allsopp, 2001; Gosztonyi and Koprowski, 2001; Liebert, 2001). This may be because these viruses can hijack the endogenous cell death pathways that are being actively used in wiring the developing nervous system. It is attractive to speculate that Reaper and bunyaviral NSs proteins share underlying properties that make them particularly suited to induce apoptosis in the neuronal milieu during development of the young nervous system. Factors associating with both Reaper and the NSs proteins will be particularly attractive candidates for mediators of these biological functions.
Acknowledgments
We thank Kenneth Thress, Seth Margolis, Douglas Weitzel, Ashley Richardson, and Christopher Holley for helpful comments and discussion. This work was supported by a National Institutes of Health grant to S.K. (RO1 GM61919), March of Dimes Research Grant to S.K. (1-FY02–186), NIH grant to M.H. (NS 34175), and a Wellcome Trust grant to R.E. (065121). S.K. is a Scholar of the Leukemia and Lymphoma Society. P.I. is a D'Arbeloff Fellow in Biological Sciences. D.C.-R. is supported by an NIH Minority Supplement to RO1 GM61919.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–03–0139. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-03-0139.
References
- Anderson, J.R. (2001). The mechanisms of direct, virus-induced destruction of neurons. Curr. Top. Microbiol. Immunol. 253, 15–33. [DOI] [PubMed] [Google Scholar]
- Baldi, P., Brunak, S., Fransconi, P., Pollastri, G., and Soda, G. (1999). Exploiting the past and the future in protein secondary structure prediction. Bioinformatics 15, 937–946. [DOI] [PubMed] [Google Scholar]
- Bangs, P., and White, K. (2000). Regulation and execution of apoptosis during Drosophila development. Dev. Dyn. 218, 68–79. [DOI] [PubMed] [Google Scholar]
- Benedict, C.A., Norris, P.S., and Ware, C.F. (2002). To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3, 1013–1018. [DOI] [PubMed] [Google Scholar]
- Bergmann, A., Agapite, J., and Steller, H. (1998). Mechanisms and control of programmed cell death in invertebrates. Oncogene 17, 3215–3223. [DOI] [PubMed] [Google Scholar]
- Best, S.M., Wolfinbarger, J.B., and Bloom, M.E. (2002). Caspase activation is required for permissive replication of Aleutian mink disease parvovirus in vitro. Virology 292, 224–234. [DOI] [PubMed] [Google Scholar]
- Bridgen, A., Weber, F., Fazakerley, J.K., and Elliott, R.M. (2001). Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis. Proc. Natl. Acad. Sci. USA 98, 664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P., Lee, P., Otto, L., and Abrams, J. (1996). Apoptotic activity of REAPER is distinct from signaling by the tumor necrosis factor receptor 1 death domain. J. Biol. Chem. 271, 25735–25737. [DOI] [PubMed] [Google Scholar]
- Christich, A., Kauppila, S., Chen, P., Sogame, N., Ho, S.I., and Abrams, J.M. (2002). The damage-responsive Drosophila gene sickle encodes a novel IAP binding protein similar to but distinct from reaper, grim, and hid. Curr. Biol. 12, 137–140. [DOI] [PubMed] [Google Scholar]
- Claveria, C., Caminero, E., Martinez, A.C., Campuzano, S., and Torres, M. (2002). GH3, a novel proapoptotic domain in Drosophila Grim, promotes a mitochondrial death pathway. EMBO J. 21, 3327–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserzo, M., Wallin, E., Simon, I., von Heijne, G., and Elofsson, A. (1997). Prediction of transmembrane alpha-helices in procariotic membrane proteins: the Dense Alignment Surface method. Prot. Eng. 10, 673–676. [DOI] [PubMed] [Google Scholar]
- Elliott, R.M. (1997). Emerging viruses: the Bunyaviridae. Mol. Med. 3, 572–577. [PMC free article] [PubMed] [Google Scholar]
- Evans, E.K., Kuwana, T., Strum, S.L., Smith, J.J., Newmeyer, D.D., and Kornbluth, S. (1997a). Reaper-induced apoptosis in a vertebrate system. EMBO J. 16, 7372–7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, E.K., Lu, W., Strum, S.L., Mayer, B.J., and Kornbluth, S. (1997b). Crk is required for apoptosis in Xenopus egg extracts. EMBO J. 16, 230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazakerley, J.K. (2001). Neurovirology and developmental neurobiology. Adv. Virus Res. 56, 73–124. [DOI] [PubMed] [Google Scholar]
- Fazakerley, J.K., and Allsopp, T.E. (2001). Programmed cell death in virus infections of the nervous system. Curr. Top. Microbiol. Immunol. 253, 95–119. [DOI] [PubMed] [Google Scholar]
- Freeman, B.C., and Morimoto, R.I. (1996). The human cytosolic molecular chaperones hsp90, hsp70 (hsc70), and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO J. 15, 2969–2979. [PMC free article] [PubMed] [Google Scholar]
- Goh, P.Y., Tan, Y.J., Lim, S.P., Lim, S.G., Tan, Y.H., and Hong, W.J. (2001). The hepatitis C virus core protein interacts with NS5A and activates its caspase-mediated proteolytic cleavage. Virology 290, 224–236. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano, F., Jacoby, D., Griot, C., and Nathanson, N. (1992). Genetics, infectivity and virulence of California serogroup viruses. Virus Res. 24, 123–135. [DOI] [PubMed] [Google Scholar]
- Gosztonyi, G., and Koprowski, H. (2001). The concept of neurotropism and selective vulnerability (“pathoclisis”) in virus infections of the nervous system—a historical overview. Curr. Top. Microbiol. Immunol. 253, 1–13. [DOI] [PubMed] [Google Scholar]
- Goyal, L., McCall, K., Agapite, J., Hartwieg, E., and Steller, H. (2000). Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. EMBO J. 19, 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, D.E., and Hardwick, J.M. (1997). Regulators of apoptosis on the road to persistent alphavirus infection. Annu. Rev. Microbiol. 51, 565–592. [DOI] [PubMed] [Google Scholar]
- Hardwick, J.M. (2001). Apoptosis in viral pathogenesis. Cell Death Differ. 8, 109–110. [DOI] [PubMed] [Google Scholar]
- Hays, R., Wickline, L., and Cagan, R. (2002). Morgue mediates apoptosis in the Drosophila melanogaster retina by promoting degradation of DIAP1. Nat. Cell Biol. 4, 425–431. [DOI] [PubMed] [Google Scholar]
- Henke, A., Launhardt, H., Klement, K., Stelzner, A., Zell, R., and Munder, T. (2000). Apoptosis in coxsackievirus B3-caused diseases: interaction between the capsid protein VP2 and the proapoptotic protein siva. J. Virol. 74, 4284–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke, A. et al. (2001). The apoptotic capability of coxsackievirus B3 is influenced by the efficient interaction between the capsid protein VP2 and the proapoptotic host protein Siva. Virology 289, 15–22. [DOI] [PubMed] [Google Scholar]
- Holley, C.L., Olson, M.R., Colon-Ramos, D.A., and Kornbluth, S. (2002). Reaper eliminates IAP proteins through stimulated IAP degradation and generalized translational inhibition. Nat. Cell Biol. 4, 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J.I., Park, S.H., Lee, P.W., and Ahn, B.Y. (1999). Apoptosis is induced by hantaviruses in cultured cells. Virology 264, 99–105. [DOI] [PubMed] [Google Scholar]
- Kluck, R.M., Bossy-Wetzel, E., Green, D.R., and Newmeyer, D.D. (1997). The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275, 1132–1136. [DOI] [PubMed] [Google Scholar]
- Levine, B., Goldman, J.E., Jiang, H.H., Griffin, D.E., and Hardwick, J.M. (1996). Bcl-2 protects mice against fatal alphavirus encephalitis. Proc. Natl. Acad. Sci. USA 93, 4810–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebert, U.G. (2001). Slow and persistent virus infections of neurones—a compromise for neuronal survival. Curr. Top. Microbiol. Immunol. 253, 35–60. [DOI] [PubMed] [Google Scholar]
- Martins, L.M. et al. (2002). The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a reaper-like motif. J. Biol. Chem. 277, 439–444. [DOI] [PubMed] [Google Scholar]
- McCarthy, J.V., and Dixit, V.M. (1998). Apoptosis induced by Drosophila reaper and grim in a human system. Attenuation by inhibitor of apoptosis proteins (cIAPs). J. Biol. Chem. 273, 24009–24015. [DOI] [PubMed] [Google Scholar]
- Meier, P., Finch, A., and Evan, G. (2000). Apoptosis in development. Nature 407, 796–801. [DOI] [PubMed] [Google Scholar]
- Moss, B., Elroy-Stein, O., Mizukami, T., Alexander, W.A., and Fuerst, T.R. (1990). Product review. New mammalian expression vectors. Nature 348, 91–92. [DOI] [PubMed] [Google Scholar]
- Muthumani, K., Hwang, D.S., Desai, B.M., Zhang, D., Dayes, N., Green, D.R., and Weiner, D.B. (2002a). HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J. Biol. Chem. 277, 37820–37831. [DOI] [PubMed] [Google Scholar]
- Muthumani, K. et al. (2002b). Adenovirus encoding HIV-1 Vpr activates caspase 9 and induces apoptotic cell death in both p53 positive and negative human tumor cell lines. Oncogene 21, 4613–4625. [DOI] [PubMed] [Google Scholar]
- Nathanson, N. (1997). Viral Pathogenesis. Philadelphia: Lippincott-Raven.
- Pekosz, A., Phillips, J., Pleasure, D., Merry, D., and Gonzalez-Scarano, F. (1996). Induction of apoptosis by La Crosse virus infection and role of neuronal differentiation and human bcl-2 expression in its prevention. J. Virol. 70, 5329–5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, C., Carney, G.E., Taylor, B.J., and White, K. (2002). reaper is required for neuroblast apoptosis during Drosophila development. Development 129, 1467–1476. [DOI] [PubMed] [Google Scholar]
- Richter, B.W., and Duckett, C.S. (2000). The IAP proteins: caspase inhibitors and beyond. Sci. STKE 2000, PE1. [DOI] [PubMed] [Google Scholar]
- Ryoo, H.D., Bergmann, A., Gonen, H., Ciechanover, A., and Steller, H. (2002). Regulation of Drosophila IAP1 degradation and apoptosis by reaper and ubcD1. Nat. Cell Biol. 4, 432–438. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry, S., Dybdahl-Sissoko, N., Neumann, G., Kawaoka, Y., and Hinshaw, V.S. (2001). Influenza virus ns1 protein induces apoptosis in cultured cells. J. Virol. 75, 7875–7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. (2002). Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 9, 459–470. [DOI] [PubMed] [Google Scholar]
- Srinivasula, S.M. et al. (2002). sickle, a novel Drosophila death gene in the reaper/hid/grim region, encodes an IAP-inhibitory protein. Curr. Biol. 12, 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, Y., Nakabayashi, Y., and Takahashi, R. (2001). Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc. Natl. Acad. Sci. USA 98, 8662–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenev, T., Zachariou, A., Wilson, R., Paul, A., and Meier, P. (2002). Jafrac2 is an IAP antagonist that promotes cell death by liberating Dronc from DIAP1. EMBO J. 21, 5118–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thress, K., Evans, E.K., and Kornbluth, S. (1999). Reaper-induced dissociation of a Scythe-sequestered cytochrome c-releasing activity. EMBO J. 18, 5486–5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thress, K., Henzel, W., Shillinglaw, W., and Kornbluth, S. (1998). Scythe: a novel reaper-binding apoptotic regulator. EMBO J. 17, 6135–6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thress, K., Song, J., Morimoto, R.I., and Kornbluth, S. (2001). Reversible inhibition of Hsp70 chaperone function by Scythe and Reaper. EMBO J. 20, 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagner, S., Galy, B., and Pyronnet, S. (2001). Irresistible IRES. Attracting the translation machinery to internal ribosome entry sites. EMBO Rep. 2, 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux, D.L., and Korsmeyer, S.J. (1999). Cell death in development. Cell 96, 245–254. [DOI] [PubMed] [Google Scholar]
- Verhagen, A.M. et al. (2002). HtrA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteins. J. Biol. Chem. 277, 445–454. [DOI] [PubMed] [Google Scholar]
- Verhagen, A.M., and Vaux, D.L. (2002). Cell death regulation by the mammalian IAP antagonist Diablo/Smac. Apoptosis 7, 163–166. [DOI] [PubMed] [Google Scholar]
- Vucic, D., Seshagiri, S., and Miller, L.K. (1997). Characterization of reaper- and FADD-induced apoptosis in a lepidopteran cell line. Mol. Cell. Biol. 17, 667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S.L., Hawkins, C.J., Yoo, S.J., Muller, H.A., and Hay, B.A. (1999). The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell 98, 453–463. [DOI] [PubMed] [Google Scholar]
- Weber, F., Bridgen, A., Fazakerley, J.K., Streitenfeld, H., Kessler, N., Randall, R.E., and Elliott, R.M. (2002). Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon. J. Virol. 76, 7949–7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing, J.P., Karres, J.S., Ogdahl, J.L., Zhou, L., Schwartz, L.M., and Nambu, J.R. (2002). Drosophila sickle is a novel grim-reaper cell death activator. Curr. Biol. 12, 131–135. [DOI] [PubMed] [Google Scholar]
- Wing, J.P., Schwartz, L.M., and Nambu, J.R. (2001). The RHG motifs of Drosophila Reaper and Grim are important for their distinct cell death-inducing abilities. Mech. Dev. 102, 193–203. [DOI] [PubMed] [Google Scholar]
- Wing, J.P., Zhou, L., Schwartz, L.M., and Nambu, J.R. (1998). Distinct cell killing properties of the Drosophila reaper, head involution defective, and grim genes. Cell Death Differ. 5, 930–939. [DOI] [PubMed] [Google Scholar]
- Yang, Y., Fang, S., Jensen, J.P., Weissman, A.M., and Ashwell, J.D. (2000). Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 288, 874–877. [DOI] [PubMed] [Google Scholar]
- Yoo, S.J. et al. (2002). Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat. Cell Biol. 4, 416–424. [DOI] [PubMed] [Google Scholar]
- Zimmermann, K.C., and Green, D.R. (2001). How cells die: apoptosis pathways. J. Allergy Clin. Immunol. 108, 99S–103S. [DOI] [PubMed] [Google Scholar]