Abstract
Formation of filamentous F-actin drives many cellular processes, including phagocytosis and cell spreading. We have recently reported that mouse macrophage 12/15-lipoxygenase (12/15-LO) activity promotes F-actin formation in filopodia during phagocytosis of apoptotic cells. Oxidized low-density lipoprotein (OxLDL) also stimulates robust F-actin formation and spreading of macrophages. However, unlike apoptotic cells, OxLDL did not cause specific translocation of 12/15-LO to the cell membrane, neither in macrophages nor in GFP-15LO–transfected COS-7 cells. Moreover, inhibition of 12/15-LO activity in macrophages by a specific inhibitor or by 12/15-LO gene disruption did not affect OxLDL-induced actin polymerization. Among LDL modifications modeling OxLDL, LDL modified by incubation with 15LO-overexpressing fibroblasts was as active in eliciting F-actin response as was OxLDL. This LDL modification is well known to produce minimally modified LDL (mmLDL), which is bioactive and carries lipid oxidation products similar to those produced by 12/15-LO catalysis. MmLDL activated phosphoinositide 3-kinase (PI3K), and PI3K inhibitors abolished mmLDL-induced macrophage spreading. We hypothesize that OxLDL and mmLDL may contribute oxidized lipids to the macrophage cell membrane and thereby mimic intracellular 12/15-LO activity, which leads to uncontrolled actin polymerization and dramatic cytoskeletal changes in macrophages.
INTRODUCTION
Actin filaments form the cytoskeleton and determine cell morphology, motility, and endocytosis. During the process of phagocytosis the macrophage forms filopodia to surround an apoptotic cell, which leads to an actin-dependent internalization of the apoptotic cell. Filopodia are rich in filamentous F-actin and, as we have recently shown in mouse peritoneal macrophages, the filopodial F-actin colocalizes with 12/15-lipoxygenase (12/15-LO; Miller et al., 2001). Moreover, 12/15-LO activity was required for in vitro and in vivo F-actin formation and for phagocytosis (Miller et al., 2001). These findings might be of special interest for macrophage function in atherosclerotic tissue, because on the one hand, atherosclerotic tissue, unlike the normal vascular wall, is characterized by a large number of apoptotic cells and phagocytes (Kockx and Herman, 2000). Phagocytosis of dying cells is crucial for preventing the release of toxic cellular compounds and it regulates immune responses (Savill et al., 2002). Inhibition of efficient phagocytosis leads to the accumulation of proinflammatory necrotic debris, plaque instability, and thrombogenesis (Libby, 2001). Therefore, the prophagocytic activity of 12/15-LO should be considered anti-inflammatory. On the other hand, in the context of hypercholesterolemia, macrophage 15-LO in humans and 12/15-LO in mice is believed to be an important proatherogenic enzyme because of its ability to oxidize low-density lipoproteins (LDL; Ylä-Herttuala et al., 1991; Benz et al., 1995; Ezaki et al., 1995). Absence of 12/15-LO expression decreases lipid peroxidation and atherogenesis in both ApoE-deficient (Cyrus et al., 2001) and LDL receptor–deficient mice (George et al., 2001). Mouse 12/15-LO and human 15-LO both produce 12-hydroxyeicosatetraenoic acid (12-HETE) and 15-HETE from arachidonic acid (in various ratios) and predominantly 13-hydroxyoctadecadienoic acid (13-HODE) from linoleic acid. These particular enzymes are closely related, and subtle amino acid substitutions can account for the species differences (human and rabbit 15-LO vs. mouse, and rat 12/15-LO) by altering the ratio of 12-HETE to 15-HETE metabolites.
Oxidized LDL (OxLDL), and its many oxidized lipid products, is responsible for numerous proinflammatory and proatherogenic processes (Glass and Witztum, 2001). Intriguingly, OxLDL appears to be very similar to apoptotic cells in that both carry many of the same oxidation-specific epitopes recognized by monoclonal autoantibodies (Chang et al., 1999; Shaw et al., 2000) and by macrophage receptors (Bird et al., 1999; Hörkkö et al., 1999; Boullier et al., 2000). In addition, OxLDL and more so minimally modified LDL (mmLDL) carry hydroperoxides and hydroxides of fatty acids and phospholipids, similar to those produced by 12/ 15-LO catalysis (Ezaki et al., 1995). Because we previously showed that exposure of macrophages to apoptotic cells led to translocation of 12/15-LO from the cytosol to the filopodia surrounding the apoptotic cells and because of similar oxidation-specific epitopes on apoptotic cells and on OxLDL, we investigated actin polymerization in macrophages exposed to OxLDL.
We have recently demonstrated that mmLDL stimulates robust actin polymerization in macrophages via CD14/TLR4/MD2 receptors (Miller et al., 2003). Here we report that OxLDL-induced actin polymerization, unlike the stimulation induced by apoptotic cells, does not require 12/15-LO translocation and is independent of 12/15-LO activity. We hypothesize that oxidized lipids of OxLDL and mmLDL simulate 12/15-LO activity, leading to a generalized actin polymerization in macrophages.
MATERIALS AND METHODS
Cell Culture and Materials
Resident or elicited peritoneal macrophages were harvested from 8–10-week-old female mice, either wild-type C57BL/6 or 12/15-LO knockout mice (12/15-LO KO) (Cyrus et al., 2001), as described (Miller et al., 2001). The macrophages were plated in RPMI 1640 (BioWhittaker, Walkersville, MD) supplemented with 20% heat-inactivated fetal bovine serum (FBS, Omega Scientific, Tarzana, CA) and adhered to the plate for 5 h. For the actin polymerization experiments, macrophages were preconditioned for 16 h in medium containing 2% lipoprotein-deficient serum (LPDS) prepared from FBS by ultracentrifugation. Murine macrophage-like cell lines J774A.1 and RAW 264.7 were maintained in 10% FBS/RPMI 1640. Murine fibroblast cell lines stably overexpressing human 15-LO or β-galactosidase (Benz et al., 1995) were cultured in DMEM (BioWhittaker) with 10% FBS and 0.5 mg/ml G418 (Calbiochem, La Jolla, CA) to maintain selection. Thymocytes were harvested from 4–6-week-old C57BL/6 female mice, as described (Chang et al., 1999).
1-Palmitoyl-2-(5-oxovaleroyl) phosphatidylcholine (POVPC) was a gift from P. Friedman (University of California, San Diego; Friedman et al., 2002). A specific 15-LO inhibitor PD 146176 was a gift from J. Cornicelli of Pfizer. Mouse M-CSF was from R&D Systems (Minneapolis, MN). Escherichia coli LPS was purchased from List Biological Laboratory (Campbell, CA) and from Sigma (St. Louis, MO). Wortmannin and LY294,002 were from Alexis (San Diego, CA). All other chemicals, if not otherwise stated, were from Sigma.
LDL Isolation and Modification
LDL was isolated from plasma of normolipidemic donors by sequential ultracentrifugation (Havel et al., 1955). For oxidation, the LDL was diluted to 1 mg protein/ml with EDTA-free PBS and incubated with 10 μm CuSO4 for 18 h at 37°C. This procedure resulted in profound LDL oxidation and resulting preps are referred to in the text as OxLDL. Alternatively, 50 μg/ml LDL in serum-free DMEM was modified by an 18-h incubation with a murine fibroblast cell line overexpressing 15-LO (Benz et al., 1995). We have previously documented that this procedure generates minimally modified LDL (mmLDL; Benz et al., 1995; Ezaki et al., 1995; Sigari et al., 1997). In some experiments, the mmLDL was purified by removing from the media the components with molecular weight lower than 100,000 Daltons on a Biomax-100 spin filter device (Millipore, Bedford, MA). Incubation of LDL in the medium without cells produced a no cell control sample (nLDL). POVPC-LDL, acetylated LDL (Ac-LDL) and malondialdehyde LDL (MDA-LDL) were prepared as described (Hörkkö et al., 1999). Aggregated LDL was prepared by vortexing LDL for 1 min (Khoo et al., 1988) on a Fisher Vortex Genie 2 (Pittsburgh, PA).
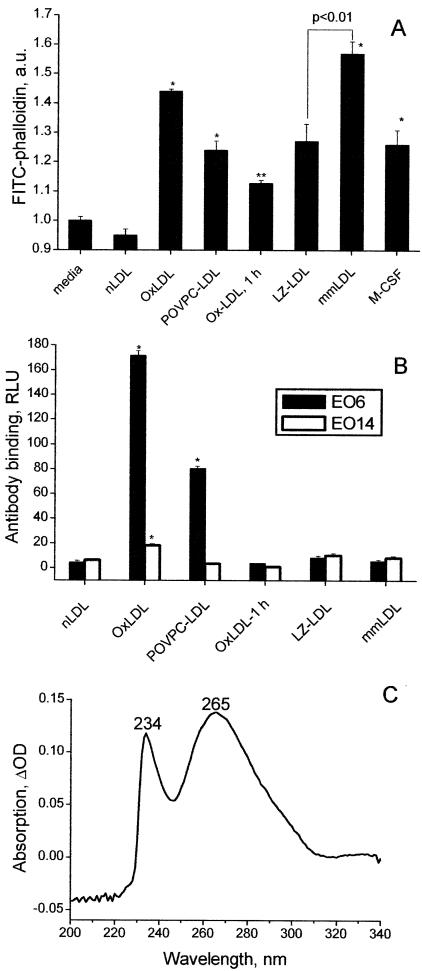
Contamination of native and modified LDL preparations by bacterial LPS was assessed with a LAL kit (BioWhittaker). Samples with LPS higher than 2 ng/mg protein were discarded. Because in most experiments LDL was used at a final concentration of 50 μg/ml, the LPS contamination in experimental samples was kept below 0.1 ng/ml. The extent of LDL oxidation was assessed by measuring its binding to monoclonal autoantibody EO6 (specific to oxidized PC-containing phospholipids or oxidized phospholipid-protein adducts), and EO14 (specific to MDA-lysine epitopes; Hörkkö et al., 1999). The number of modified lysine residues in the variously modified apoB was estimated by a TNBS assay (Steinbrecher et al., 1987).
Transfection
COS-7 cells grown on glass coverslips were transiently transfected with a vector containing a 15-LO fusion with green fluorescent protein, pEGFP-C2–15LO-1 (Chen and Funk, 2001) or with pEGFP-C2 empty vector (Clontech, Palo Alto, CA), using Lipofectamine Plus Transfection reagent (Invitrogen, San Diego, CA). Transfected cells (designated further in the text as GFP-15LO) were treated, fixed, and stained as outlined below.
Immunocytochemistry and Imaging
Macrophages or GFP-15LO–transfected COS-7 cells were exposed for 15 min to either apoptotic thymocytes or aggregated biotin-labeled OxLDL and fixed with 3.7% paraformaldehyde. To avoid staining of mitochondrial biotin, the intact cells exposed to biotinylated LDL were first stained with AMCA-streptavidin (Jackson ImmunoResearch, West Grove, PA). Then cells were permeabilized with 0.2% Triton X-100 for 5 min. The coverslips were blocked with 0.8 μg/ml Fc block (PharMingen, San Diego, CA), stained with a guinea pig anti-rabbit 15-LO antibody (Ylä-Herttuala et al., 1990), and with a rhodamine red-X–conjugated F(ab′)2 fragment donkey anti–guinea pig Ig G (H+L) antibody (Jackson ImmunoResearch). The specificity of this antibody to 15-LO was previously demonstrated because it failed to bind to mouse macrophages deficient of 12/15-LO (Miller et al., 2001). F-actin was stained by addition of 1.5 μm FITC-conjugated phalloidin (TRITC-phalloidin for GFP cells) to the solution of the secondary antibody as previously described (Miller et al., 2001). Cell nuclei were stained blue with 1 μg/ml Hoechst 33342 (Sigma). The coverslips were mounted on microscopic glass slides with ProLong antifade medium (Molecular Probes, Eugene, OR). Images were captured by deconvolution microscopy (Agard et al., 1989) using a DeltaVision deconvolution microscopic system operated by SoftWorx software (Applied Precision, Issaquah, WA) as described (Miller et al., 2001). Pixel intensities were kept in the linear response range of the digital camera. Optical sections through the samples were taken with increments of 0.2 μm. The images were deconvolved and examined either section by section or by volume views generated by combining areas of maximal intensity of each optical section with SoftWorx programs. DataInspector application (Applied Precision) was used to quantitatively analyze the images. The analysis of both raw and deconvolved images yielded similar results. Adobe Photoshop 7.0 software (San Jose, CA) was used to design figures.
FACS Assay for F-actin
Relative content of F-actin in cells activated by addition of LDL samples was assessed by flow cytometry as described in Howard and Meyer (1984) with previously described modifications (Miller et al., 2001, 2003). In brief, at the end of incubation of the LDL samples with adherent macrophages, one volume of a solution containing 0.4 μm FITC-phalloidin, 1.2 μm unlabeled phalloidin, 15% formaldehyde, and 0.8% saponin (all from Sigma) was added to three volumes of the culture medium and incubated for additional 10 min at 37°C. Cells were then washed, scraped from the plate, and analyzed on a FACScan (Becton Dickinson, Mountain View, CA). Data were collected as histograms of distribution of cell fluorescence intensities, and geometrical means of each histogram were used for further analysis. Experiments were performed in duplicates and repeated at least three times.
Western Blot
Cells were lysed on the plate with 5% SDS in PBS. Protein content was determined with a BCA kit (Pierce, Rockford, IL), and 15–100 μg of the cell lysates were run on a 4–12% SDS-PAGE (Invitrogen) and then transferred to a nitrocellulose membrane (Millipore). The membrane was incubated with a protein A–purified polyclonal guinea pig anti-rabbit 15-LO antibody (Ylä-Herttuala et al., 1990), which cross-reacts with mouse 12/15-LO (Miller et al., 2001), and the bands were visualized with an alkaline phosphatase–conjugated goat anti–guinea pig IgG antibody (Sigma) and Western Blue stabilized alkaline phosphatase substrate (Promega, Madison, WI).
PI3K Assay
Macrophage cell lysates were immunoprecipitated with PY20 antiphosphotyrosine antibody (Transduction Laboratories, Lexington, KY) and protein G agarose (Upstate Biotechnology, Lake Placid, NY) overnight at 4°C. PI3K activity was measured as described (Kruszynska et al., 2002) in a reaction with phosphatidylinositol (Sigma) in the presence of [γ-32P]ATP. After TLC separation, phosphatidylinositol-3-phosphate was visualized by autoradiography.
RESULTS
12/15-LO Localization in the Cells Exposed to Apoptotic Cells and OxLDL
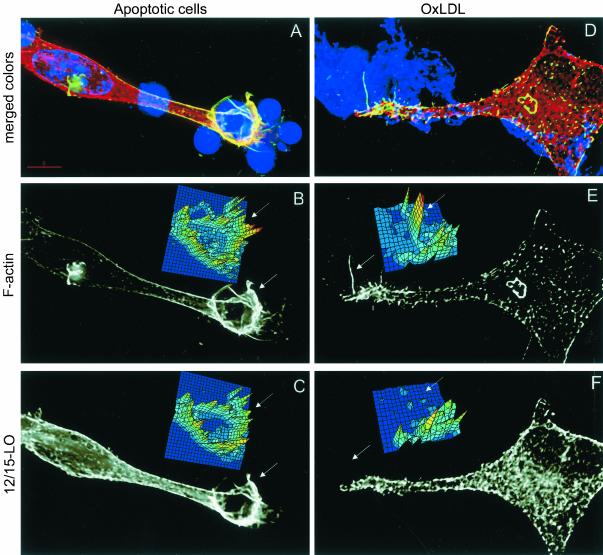
We have previously reported that 12/15-LO concentrates in macrophage filopodia during phagocytosis of apoptotic cells (Miller et al., 2001). Because both apoptotic cells and OxLDL carry similar surface oxidation-specific epitopes (Chang et al., 1999), we expected to see similarities in 12/15-LO localization in macrophages exposed to either apoptotic cells or OxLDL. Because monomeric OxLDL cannot be seen by light microscopy, it was aggregated by vigorous vortexing to visualize the OxLDL aggregates binding to the cells. As previously described (Miller et al., 2001), in macrophages phagocytosing apoptotic cells, 12/15-LO (red) translocated to the membrane to sites where apoptotic cells were bound (blue condensed nuclei) and colocalized with the areas enriched for F-actin (green). The colocalization results in appearance of yellow color on the image (Figure 1A; Miller et al., 2001). Filopodia (arrowheads) can be seen that contain both F-actin (Figure 1B) and 12/15-LO (Figure 1C) staining. Digital intensity maps (insets to B and C) also show colocalization of F-actin and 12/15-LO. (A 3D movie derived from these data that shows the relevant morphology of this cell is available as an online supplement to this article.) In contrast, binding of aggregated OxLDL (blue) to macrophages caused formation of what appeared to be similar filopodia, which contained F-actin (green) but not 12/15-LO (red) (Figure 1D). This is better seen in the grayscale Figure 1, E and F (F-actin and 12/15-LO individual stainings, respectively). Note especially the difference in the topography of digital intensity maps of 1E and 1F. These results suggest that in contrast to 12/15-LO translocation toward bound apoptotic cells, 12/15-LO does not translocate toward bound OxLDL.
Figure 1.
Intracellular localization of 12/15-LO in macrophages stimulated by OxLDL and apoptotic cells. Resident macrophages were incubated for 15 min with apoptotic thymocytes (A–C) or aggregated biotinylated OxLDL (D–F) and then stained for 12/15-LO (red), F-actin (green), and either nucleic acid (blue, A) or with AMCA-streptavidin to visualize aggregated OxLDL (blue, D). A and D, Merged color images. (B and E) Black and white images of F-actin staining. (C and F) Black and white images of 12/15-LO staining. Insets to B, C, E, and F: digital maps for respective colors in the selected areas (arrowheads) showing fluorescence intensities from low (depicted blue) to high (depicted red). A 3D movie reconstructing morphology of the cell from panel A is available as an online supplement to this article.
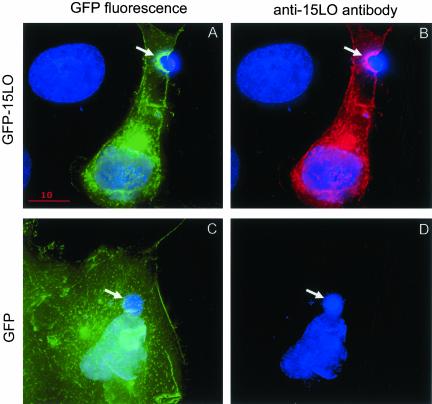
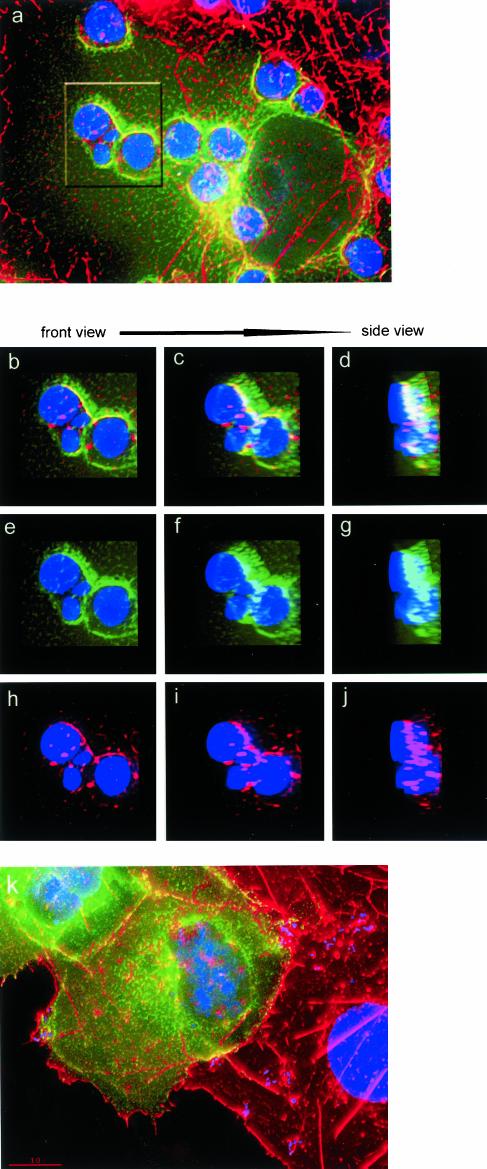
To confirm the specificity of 12/15-LO localization in macrophages, we transfected COS-7 cells with GFP-15LO or with the empty GFP vector and exposed these cells to apoptotic thymocytes. Consistent with the data on the endogenous 12/15-LO, GFP-15LO concentrated in vicinity of bound apoptotic cells (Figure 2A, green GFP fluorescence). The same cell stained with the anti-15LO antibody showed exactly the same pattern of 15-LO staining (red) as the GFP-15LO fluorescence (green; compare Figure 2, A and B). In contrast, GFP, which was not fused to 15LO, did not translocate to the bound apoptotic cells (Figure 2C), and the COS-7 cells transfected with GFP alone exhibited no specific staining with the anti-15LO antibody (Figure 2D). In another set of experiments, we stained the GFP-15LO–transfected COS-7 cells, which were exposed to apoptotic thymocytes, with TRITC-phalloidin (red) to visualize F-actin. Similarly to peritoneal macrophages, both GFP-15LO and F-actin concentrated and colocalized in vicinity of bound apoptotic cells (Figure 3, A–J). Volume view projections, which were rotated around the Y axis, in both separated and merged colors, clearly show GFP-15LO and F-actin colocalization (Figure 3, B–J). COS-7 cells do not bind OxLDL (Boullier et al., 2000), and consistent with that, we were unable to detect any specific binding of aggregated OxLDL to GFP-15LO–transfected COS-7 cells. GFP-15LO (green) and F-actin (red) were clearly separated in COS-7 exposed to aggregated OxLDL (Figure 3K), consistent with our findings with peritoneal macrophages (Figure 1).
Figure 2.
GFP-15LO localization in transfected COS-7 cells stimulated by apoptotic cells. COS-7 cells transfected with GFP-15LO (A and B) or GFP (C and D) were exposed for 15 min to apoptotic thymocytes, fixed, and stained with Hoechst to visualize cell nuclei (blue) and with anti-15LO antibody (red). (A and C) Green GFP fluorescence; (B) red 15-LO staining. Absence of 15LO protein in GFP-transfected cells is evident from the absence of red staining in D. There are two COS-7 cells in A and B: one nontransfected in the top-left corner (nucleus only is stained) and one transfected (nucleus plus cytosol stained green in A or red in B). There is one large COS-7 cell transfected with GFP in C and D. Arrows point to apoptotic thymocytes (small blue nuclei) bound to transfected COS-7 cells; they also show areas of GFP-15LO concentration in A and B.
Figure 3.
F-actin and GFP-15LO in transfected COS-7 cells stimulated by apoptotic cells and OxLDL. (A–J) GFP-15LO–transfected COS-7 cells were exposed for 15 min to apoptotic thymocytes, fixed, and stained with Hoechst to visualize cell nuclei (blue; large of COS-7 and small of apoptotic thymocytes) and with TRITC-phalloidin for F-actin (red). (Note that colors for 15LO and F-actin are switched in this figure compared with Figure 1.) An area high-lighted in A is analyzed by rotating the volume views in merged colors (B–D), green for GFP-15LO (E–G), and red for F-actin (H–J), from frontal to side orientation. The images show colocalization of GFP-15LO and F-actin. (K) GFP-15LO–transfected COS-7 cells were exposed for 15 min to aggregated biotinylated OxLDL, fixed, and stained with Hoechst for nuclei (blue), TRITC-phalloidin for F-actin (red), and AMCA-streptavidin for OxLDL. Absence of any blue color outside the cell indicates no binding of aggregated OxLDL. No specific colocalization of GFP-15LO and F-actin is observed in this image.
F-actin Response to OxLDL Is Independent of 12/15-LO Activity
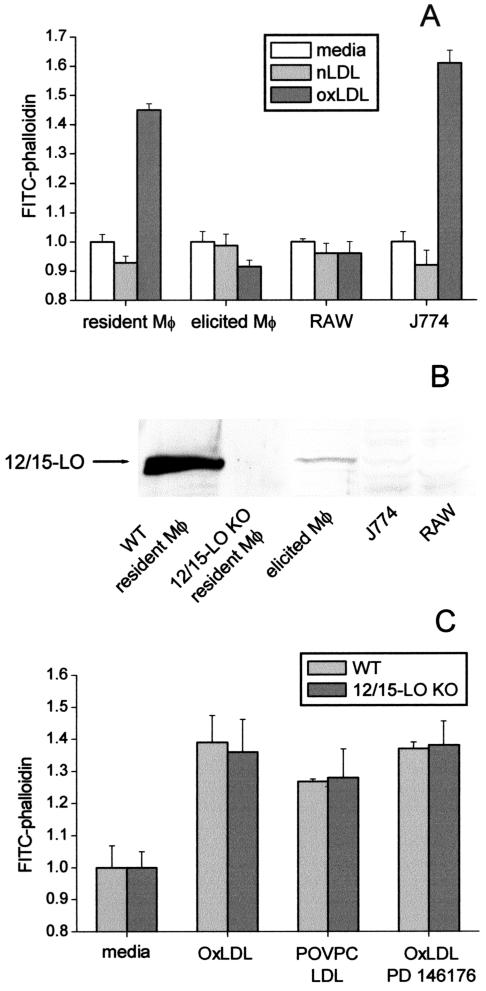
We previously reported that 12/15-LO activity was essential for the F-actin response to apoptotic cells (Miller et al., 2001). The data above strongly suggest that in sharp contrast, the F-actin response to OxLDL was independent of 12/15-LO activity. Indeed, although there was no colocalization of 12/15-LO with F-actin in the cells exposed to OxLDL (Figures 1 and 3), robust actin polymerization in response to OxLDL, but not to native LDL, was observed (Miller et al., 2003; Figure 4A). Interestingly, mouse peritoneal resident macrophages and the macrophage-like J774 cell line responded to OxLDL, whereas thioglycollate-elicited macrophages and another macrophage cell line RAW did not. Thus, we tested whether the ability to respond to OxLDL correlated with 12/15-LO expression. The enzyme was highly expressed only in resident macrophages, was much lower in elicited macrophages, and was not detected in either J774 or RAW cells (Figure 4B). Macrophage lysates from 12/15-LO–/– mice served as a negative control. These data show that there is no correlation between 12/15-LO expression and F-actin response to OxLDL.
Figure 4.
Lack of the role of 12/15-LO activity in the OxLDL-induced actin polymerization. (A) Resident and elicited peritoneal macrophages, J774A.1 and RAW 264.7 cells were exposed to 50 μg/ml indicated LDL samples and then subjected to the FACS analysis for F-actin. (B) Western Blot of cell lysates with an anti–15-LO antibody. Because of different cell density one can achieve with these cells, gel was loaded with maximal possible amounts of total lysate protein: wild-type (WT) resident macrophages, 14 μg; 12/15-LO KO resident macrophages, 10 μg; WT elicited macrophages, 75 μg; J774 and RAW, 55 μg each. (C) Resident macrophages harvested either from wild-type (WT) or 12/15-LO KO mice were incubated for 1 h with 50 μg/ml OxLDL or POVPC-LDL, and F-actin content was measured. The specific 12/15-LO inhibitor PD 146176 at 5 μM was added to some cells 1 h before OxLDL addition.
Direct evidence that 12/15-LO activity was not involved in OxLDL-induced F-actin response was obtained in experiments with macrophages from wild-type and 12/15-LO–/– macrophages. Both macrophage populations showed the same degree of F-actin formation when stimulated with either OxLDL or its model modification, POVPC-LDL (see next section for detailed POVPC definition; Figure 4C). Moreover, the specific 12/15-LO inhibitor PD 146176, which inhibited F-actin response to apoptotic cells (Miller et al., 2001), did not affect F-actin formation in macrophages exposed to OxLDL (Figure 4C). These results suggest no role for macrophage 12/15-LO activity in OxLDL-induced F-actin formation.
F-actin Response to Various LDL Modifications
We made an initial attempt to find a specific oxidative modification(s) of LDL capable of causing an increase in actin polymerization in macrophages. POVPC has been identified as a biologically active phospholipid oxidation product in OxLDL (Berliner et al., 2001), which is also recognized by a naturally occurring autoantibody EO6 (Chang et al., 1999; Hörkkö et al., 1999). POVPC-modified LDL (POVPC-LDL) caused a significant increase in macrophage F-actin (Figure 5A). Comparing the F-actin responses induced by OxLDL and POVPC-LDL with their concentrations of the EO6 epitopes showed a direct correlation between these two parameters (compare 2nd and 3rd columns in Figure 5, A and B). There was no correlation between the F-actin response and the level of apoB lysine modification because MDA-LDL and Ac-LDL with up to 85% of modified lysine residues caused the least pronounced actin polymerization. Surprisingly, an F-actin response to mmLDL surmounted that of OxLDL (Figure 5A). This LDL, modified by incubation with fibroblasts overexpressing 15-LO, has been previously shown by our laboratory to possess the properties of mmLDL (Sigari et al., 1997). Under the conditions used in the current experiments, in which the incubations were done in the presence of DMEM, mmLDL exhibited very low levels of EO6 and EO14 epitopes, making it indistinguishable from that of native LDL incubated in the absence of cells (Figure 5B). However, the difference UV spectrum of mmLDL measured against native LDL as a reference revealed the presence of fatty acid hydroperoxides and/or hydroxides (a 234-nm peak) and of dihydroxides and/or dienals (a 265-nm peak; Figure 5C). The mmLDL purified on a filter with a 100-kDa cutoff had the same activity as the nonpurified mmLDL, whereas the eluent fraction of molecular weight below 100 kDa had significantly lower activity. LDL incubated with control β-galactosidase overexpressing fibroblasts (LZ) produced “LZ-LDL,” which elicited a weaker F-actin response than mmLDL (Figure 5A). We have previously shown that the transformed LZ cells have some LDL-oxidizing activity (Benz et al., 1995). As an alternative, mmLDL was produced by a different method, using a 1-h incubation of nLDL with copper (Ox-LDL, 1h). Although OxLDL-1h induced F-actin above that of native LDL, this preparation also showed no EO6 or EO14 binding and was less effective than mmLDL produced by incubation with 15-LO fibroblasts. As an LDL-independent positive control for these experiments, we used M-CSF, which is known to stimulate osteoclast and human monocyte spreading (Diederich et al., 2001; Nakamura et al., 2001) and would thus be expected to induce mouse peritoneal macrophage spreading and actin polymerization as well. Indeed, a significant increase in macrophage F-actin in response to M-CSF was observed (Figure 5A).
Figure 5.
F-actin response to various LDL modifications. (A) Resident macrophages were incubated for 1 h with 50 μg/ml indicated LDL samples or 5 nM M-CSF and then subjected to the FACS analysis for F-actin. LZ designates LDL modified with LacZ-fibroblasts, a control for mmLDL produced with 15LO-fibroblasts. *p < 0.01; **p < 0.05 vs. media. (B) The LDL samples used in A were characterized for the presence of oxidation-specific epitopes recognized by monoclonal antibodies EO6 and EO14. *p < 0.01 vs. nLDL. (C) Difference absorption spectrum of 50 μg/ml LDL incubated in DMEM with 15LO-fibroblasts for 4 h taken against a reference cuvette with native LDL of the same concentration in DMEM.
MmLDL Activates PI3K
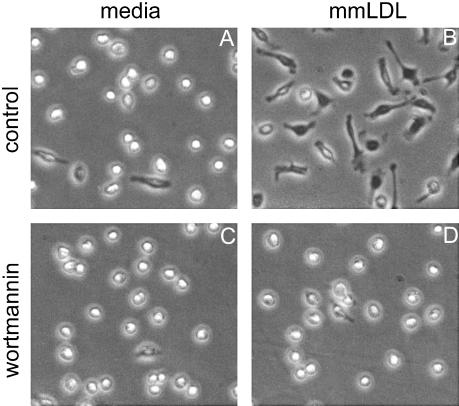
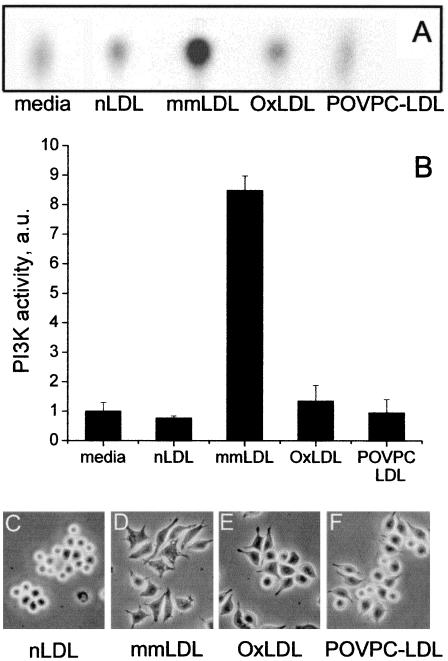
Phosphoinositide 3-kinase (PI3K) regulates cell morphology, motility, and phagocytosis (Stephens et al., 2002). Thus, we asked whether PI3K was involved in F-actin response to mmLDL. Mm-LDL-induced spreading of mouse peritoneal macrophages was effectively inhibited by the specific PI3K inhibitor wortmannin (Figure 6, A–D). This agrees with our previous data demonstrating wortmannin and LY294002 inhibition of mmLDL-induced F-actin formation in J774 cells (Miller et al., 2003). In addition, phosphotyrosine-associated PI3K activity in J774 cells increased as much as eightfold 10 min after addition of mmLDL but not of native LDL (Figures 7, A and B). Interestingly, profoundly oxidized OxLDL caused only minimal increase (not statistically significant) in the PI3K activity, and POVPC-LDL did not activate PI3K in J774 cells (Figures 7, A and B). OxLDL and POVPC-LDL were strong stimuli for F-actin formation in J774 cells (Figure 4A), but they did not cause as robust cell spreading as mmLDL (Figure 7, C–F). Thus, these data suggest that PI3K participates in the mmLDL-induced spreading, but F-actin formation initiated by either mmLDL or OxLDL may employ some additional mechanisms.
Figure 6.
Effect of wortmannin on macrophage spreading. Peritoneal macrophages were preincubated for 30 min with or without 50 nM wortmannin and then exposed for 1 h to 50 μg/ml mmLDL. Phase-contrast microphotographs were taken at the end of the incubation.
Figure 7.
PI3K activity and J774 cell spreading. (A and B) J774 cells were exposed to media alone, 50 μg/ml either native LDL, mmLDL, OxLDL, or POVPC-LDL for 10 min and then lysed on ice. PI3K activity was measured in phosphotyrosine-immunoprecipitates and normalized to a “media alone” sample. A representative TCL auto-radiogram (A) and quantified relative PI3K activities (expressed in arbitrary units; B) are shown. (C–F) J774 cells were incubated with 50 μg/ml either native LDL, mmLDL, OxLDL, or POVPC-LDL for 1 h. Phase-contrast microphotographs were taken at the end of the incubation.
Inhibition of PI3K Does Not Affect 12/15-LO Translocation
We previously showed that in contrast to the situation with mmLDL, incubation of macrophages with apoptotic cells caused translocation of 12/15-LO toward the apoptotic cells (Figure 1, A and C). Because PI3K is known to regulate phagocytosis (Stephens et al., 2002), we next tested whether this 12/15-LO translocation depended on PI3K activity. Macrophages were treated with wortmannin and then exposed to apoptotic thymocytes. Wortmannin caused most of the macrophages to retract to a round shape, but did not prevent apoptotic cell binding. In the round-shaped macrophages, 12/15-LO was visibly concentrated in the vicinity of bound apoptotic cells (Figure 8, A–C) as was the case in control nontreated macrophages. However, it was challenging to quantify the protein distribution in the retracted, rounded cells, which had a low cytosol volume and in which the plasma membrane was close to the nucleus. To compare the intensity of 12/15-LO staining in areas of the cell membrane adjacent to apoptotic cells with areas not adjacent to apoptotic cells, we selected polygonal areas (0.2–0.3-μm thick) around the macrophage perimeter, which were ∼1 μm away from the macrophage's nucleus. Then, we quantified the 12/15-LO intensity in the polygons adjacent to bound apoptotic cells vs. the polygons encircling other parts of the macrophage not adjacent to apoptotic cells. The measurements were averaged for 3–4 optical sections in four individual cells. In the wortmannin-treated macrophages, the relative intensity of 12/15-LO in the vicinity of bound apoptotic cells was 1.69 ± 0.24 vs. 1.0 ± 0.13 in other areas of the cell membrane not adjacent to apoptotic cells (p < 0.005). In the control untreated cells, the respective 12/15-LO intensities were 1.92 ± 0.31 vs. 1.0 ± 0.27 (p < 0.001). Thus, wortmannin did not appear to affect 12/15-LO distribution.
Figure 8.
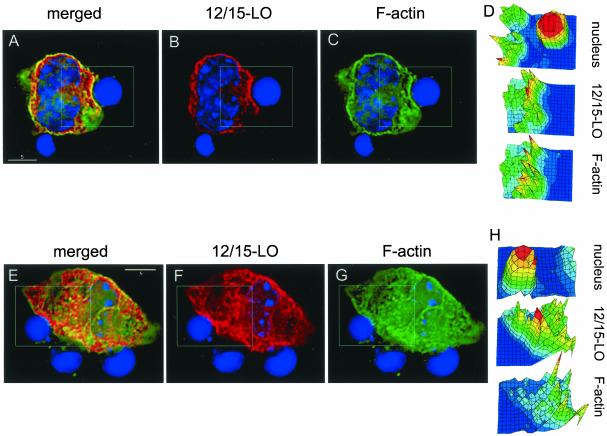
12/15-LO and F-actin localization in the phagocytosing macrophages treated with wortmannin. Two representative macrophages (A–D and E–H), treated for 30 min with 50 nM wortmannin and then exposed for 15 min to apoptotic thymocytes, are shown. Staining: red, 12/15-LO; green, F-actin; and blue, nuclei—as described in MATERIALS AND METHODS. Highlighted areas of the images were analyzed with DataInspector software to produce intensity maps for individual colors (D and H). (A--D) Both 12/15-LO and F-actin concentrate and colocalize at the site of an apoptotic cell binding. (E–H) In the highlighted area, 12/15-LO concentrates at the site of an apoptotic cell binding, but there is no enrichment with F-actin and no colocalization.
Formation of an F-actin–rich cup at the site of contact with apoptotic cells was not prevented in the majority of wortmannin-treated macrophages examined, although such F-actin enrichment was not seen in all macrophage-apoptotic cell contacts. In the wortmannin-treated macrophage shown in Figure 8, A–C, 12/15-LO concentration at the site of an apoptotic cell binding was strongly colocalized with the F-actin formation; this is also shown in the intensity maps for each individual color (Figure 7D). In contrast, in another wortmannin-treated macrophage, the apoptotic cell binding in the selected area in Figure 8, E–G, caused 12/15-LO translocation but not actin polymerization; this is quantified in Figure 8H. These findings appear to be similar to the situation observed in Fcγ-mediated phagocytosis, where PI3K does not regulate initial F-actin cup formation, but rather controls filopodia extension and the closure of phagosomes (Cox et al., 1999; Araki et al., 2003). The process of the apoptotic cell engulfment includes many other pathways in addition to the Fcγ, and the interplay of 12/15-LO and PI3K with other cellular components remains to be elucidated.
DISCUSSION
This work contrasts acute macrophage responses to apoptotic cells and OxLDL. A striking difference between these two stimuli was found in the mode of 12/15-LO cellular localization. In response to contact with apoptotic cells, 12/15-LO specifically translocated from the cytosol and concentrated in association with F-actin in the vicinity of bound apoptotic cells. In contrast, 12/15-LO failed to do so when the cells were exposed to OxLDL. This differing behavior was observed in peritoneal macrophages (Figure 1) and confirmed in GFP-15LO transfected COS-7 cells (Figures 2 and 3). Moreover, although 12/15-LO activity was required for the efficient F-actin formation during phagocytosis of apoptotic cells (Miller et al., 2001), this was not observed for F-actin response to OxLDL (Figure 4). Among various LDL modifications, mmLDL, which possessed lipid peroxidation products similar to those produced by 12/15-LO catalysis, but does not bind to scavenger receptors, was most active in eliciting F-actin formation (Figure 5). This mmLDL induced dramatic PI3K activation, and specific PI3K inhibitors abolished mmLDL-induced macrophage spreading (Figures 6 and 7).
To further characterize 12/15-LO translocation from the cytosol to the sites of apoptotic cell binding (Miller et al., 2001), we produced a 3D reconstruction of the filopodia surrounding an apoptotic cell, which confirmed 12/15-LO and F-actin colocalization (see online supplement). To confirm 12/15-LO translocation by an alternative method different from immunocytochemistry, we utilized GFP-15LO–transfected COS-7 cells. GFP-15LO, but not GFP alone, specifically translocated toward bound apoptotic cells where it colocalized with F-actin (Figures 2 and 3). Previously, we have shown that inhibiting actin polymerization by cytochalasin D or latrunculin A does not prevent 12/15-LO translocation. In the current study, we also demonstrated that inhibiting PI3K by wortmannin did not prevent 12/15-LO translocation toward bound apoptotic cells (Figure 8). This implies that 12/15-LO translocation is not downstream of PI3K activation. The mechanism(s) responsible for targeting 12/15-LO to the sites of apoptotic cell binding still remain elusive and are under study.
Here, we focused on testing whether OxLDL elicited a response in macrophages similar to the response to apoptotic cells. Interestingly, stimulation with OxLDL did not produce the same pattern of 12/15-LO or GFP-15LO distribution as observed with apoptotic cells; 12/15-LO remained mostly cytosolic (Figure 3). This implies no role for 12/15-LO in the robust F-actin formation in macrophages in response to OxLDL (Figure 4A). Indeed, there was no correlation between the levels of 12/15-LO expression in different macrophage cell types and the amplitude of their F-actin response to OxLDL (Figure 4, A and B). Moreover, the F-actin response was not abolished by a specific 12/15-LO inhibitor or by 12/15-LO gene disruption (Figure 4C).
Although endogenous 12/15-LO enzymatic activity was not required in the response to OxLDL, we hypothesize that OxLDL may still induce a similar “12/15-LO-like” mechanism by supplying the cell with certain products of lipid peroxidation that are similar to those produced by the 12/15-LO catalysis, thereby mimicking the intracellular 12/15-LO activity. This hypothesis was supported by our finding of a strong F-actin response to mmLDL, which was enriched by 15-LO products during LDL incubation with 15LO-fibroblasts (Figure 5A). This particular mmLDL modification, developed in our laboratory, is characterized by a high yield of phospholipid-, cholesteryl ester- and triglyceride-hydroperoxides as shown by HPLC with chemiluminescent detection (Benz et al., 1995; Ezaki et al., 1995). Evidence exists that fatty acid hydroxides and/or hydroperoxides stimulate actin polymerization and cell spreading. We have recently reported that there was a higher rate of spreading of 15LO-fibroblasts than of control LZ-fibroblasts and that adding the 15-LO inhibitor PD 146176 inhibited this effect (Miller et al., 2001). OxLDL and mmLDL, but not native LDL, also induced extensive macrophage spreading (Miller et al., 2003). Furthermore, we have shown that lysates of wild-type macrophages can promote in vitro actin polymerization, demonstrating a direct role for 12/15-LO products in promoting F-actin formation. This ability was significantly reduced in lysates of 12/15-LO deficient macrophages, but could be rescued by addition of the 12/15-LO product, 13(S)-HODE (Miller et al., 2001). Another 12/15-LO product, 12(S)-HETE, has been shown to induce spreading of B16a melanoma and HeLa cells, also accompanied by increased levels of F-actin (Chun and Jacobson, 1992; Timar et al., 1992; Rice et al., 1998). In smooth muscle cells, 12(S)-HETE induces p38MAPK and CREB activation, which result in cell hypertrophy (Reddy et al., 2002). Lipoxygenase products seem to be also involved in neurite outgrowth and retraction (Smalheiser et al., 1996). Thus, it seems likely that OxLDL and mmLDL stimulate actin polymerization by contributing oxidized lipids to the cell, thereby augmenting or bypassing the need for intracellular 12/15-LO activity. Defining the specific oxidized lipids that stimulate F-actin formation in macrophages remains to be accomplished.
Another question is whether the oxidized LDL stimulation first requires binding to cell surface receptors or can proceed through nonreceptor mediated uptake or even by direct transfer of oxidized lipids (or both). Recently, we proposed that mmLDL binds to CD14 and stimulates cell spreading via TLR4/MD-2 (Miller et al., 2003). This novel pathway is consistent with our recent demonstration that oxidation-specific epitopes are recognized by numerous components of the innate immune system (Binder et al., 2002), including pattern recognition receptors TLR4 (Miller et al., 2003) and CD36 (Boullier et al., 2000), C-reactive protein (Chang et al., 2002), and natural autoantibodies (Shaw et al., 2000; Shaw et al., 2001). However, our data do not rule out other potential pathways of oxidized lipid stimulation of actin polymerization in macrophages.
A recent report showing that M-CSF-induced spreading of human monocytes is inhibited by apoAI and HDL (Diederich et al., 2001) may also be in agreement with our hypothesis of oxidized lipid-induced cell spreading. Indeed, ApoAI is capable of directly reducing lipid peroxides (Garner et al., 1998), and ApoAI and HDL can remove phospholipid peroxidation products from lipoproteins or cell membranes (Navab et al., 2000; Klimov et al., 2001). Removal of oxidized moieties could be a part of the mechanism by which HDL inhibited monocyte spreading.
It appears that there may be more than one oxidized moiety that may stimulate F-actin formation. Among other LDL modifications used to model different epitopes on OxLDL, POVPC-LDL was found also capable of eliciting the F-actin response in macrophages (Figure 5A). The amplitude of this response correlated with the concentration of POVPC epitopes recognized by autoantibody EO6. The importance of POVPC arises from the findings that it constitutes a high-affinity ligand for macrophage scavenger receptors (Bird et al., 1999), among them are CD36 (Boullier et al., 2000) and SR-B1 (Gillotte-Taylor et al., 2001). POVPC is specifically recognized by the EO6 autoantibody (Hörkkö et al., 1999), which is structurally and functionally identical to the classic “natural” T15 antibody, which provides optimal protection to mice from virulent pneumococcal infection (Shaw et al., 2000). Additionally, EO6 recognizes apoptotic but not normal cells (Chang et al., 1999). Taken together with our finding that POVPC-LDL stimulated actin polymerization in macrophages (Figures 4 and 5), it is tempting to hypothesize that the F-actin response to OxLDL (which carries POVPC epitopes) mimics a macrophage response to infectious microorganisms, such as pneumococci, and/or to apoptotic cells.
Probing downstream signaling molecules, we found that PI3K activation was involved in the mmLDL-induced macrophage spreading and F-actin formation (Figures 6 and 7; Miller et al., 2003). This finding agrees with the known function of the downstream PI3K targets, which include guanine nucleotide exchange factors specific for virtually all small GTPases of Rho family (Leevers et al., 1999; Marignani and Carpenter, 2001), as well as PKC and PLCγ (Leevers et al., 1999; Arbuzova et al., 2002). All these molecules regulate actin cytoskeleton and accordingly, PI3K activity is required for phagocytosis (Stephens et al., 2002). Our data suggest that PI3K is also involved in the mmLDL-induced F-actin response in macrophages. Szekeres et al. (2000) have reported that the 12-LO product 12(S)-HETE activates PI3K in A431 epidermoid carcinoma. In neutrophils, the PI3K pathway is activated by arachidonic acid and 5(S)-HETE (Chang and Wang, 2001; Hii et al., 2001). These findings also support our hypothesis that mmLDL, by mimicking 12/15-LO activity, promotes cytoskeletal rearrangements via a PI3K-dependent mechanism. Interestingly, in the case of OxLDL stimulation, the F-actin response was not associated with as significant PI3K activation as with mmLDL (Figure 7). Biwa et al. (2000) have also reported rather modest activation of PI3K by OxLDL in macrophages. In endothelial cells, OxLDL inhibits Akt phosphorylation, which is downstream of PI3K (Breitschopf et al., 2001). Thus, it seems likely that different lipid peroxidation products, found in mmLDL and OxLDL, participate in a complex regulation of cell spreading and actin polymerization via different signaling pathways.
Supplementary Material
Acknowledgments
We thank the UCSD Cancer Center Digital Imaging Shared Resource, funded in part by NCI; Philip Fisher-Ogden at the San Diego Supercomputer Center for rendering perspective views using the NPACI Scalable Visualization Tools; Susan Butler for technical assistance; and Dennis J. Young in the UCSD Cancer Center Flow Cytometry Shared Resource for flow cytometer expertise. This study was supported by American Heart Association Grant AHA WSA 0160111Y (Y.I.M.), a start-up grant from the Sam and Rose Stein Institute for Research on Aging (Y.I.M.), a Scholars grant from Medicine Education and Research Foundation (Y.I.M.), National Institutes of Health Grants HL56989 to La Jolla SCOR in Molecular Medicine and Atherosclerosis (J.L.W. and Y.I.M), HL66941 (J.R.F.), HL53558 (C.D.F.), and DK62025 (D.S.W.), and the Charles H. Stern and Anna S. Stern Foundation (D.S.W.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–02–0063. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-02-0063.
Online version of this article contains video material. Online version is available at www.molbiolcell.org.
References
- Agard, D.A., Hiraoka, Y., Shaw, P., and Sedat, J.W. (1989). Fluorescence microscopy in three dimensions. Methods Cell Biol. 30, 353–377. [DOI] [PubMed] [Google Scholar]
- Araki, N., Hatae, T., Furukawa, A., and Swanson, J.A. (2003). Phosphoinositide-3-kinase-independent contractile activities associated with Fc{gamma}-receptor-mediated phagocytosis and macropinocytosis in macrophages. J. Cell Sci. 116, 247–257. [DOI] [PubMed] [Google Scholar]
- Arbuzova, A., Schmitz, A.A., and Vergeres, G. (2002). Cross-talk unfolded: MARCKS proteins. Biochem. J. 362, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz, D.J. et al. (1995). Enhanced levels of lipoperoxides in low density lipoprotein incubated with murine fibroblast expressing high levels of human 15-lipoxygenase. J. Biol. Chem. 270, 5191–5197. [DOI] [PubMed] [Google Scholar]
- Berliner, J.A., Subbanagounder, G., Leitinger, N., Watson, A.D., and Vora, D. (2001). Evidence for a role of phospholipid oxidation products in atherogenesis. Trends Cardiovasc. Med. 11, 142–147. [DOI] [PubMed] [Google Scholar]
- Binder, C.J., Chang, M.K., Shaw, P.X., Miller, Y.I., Hartvigsen, K., Dewan, A., and Witztum, J.L. (2002). Innate and acquired immunity in atherogenesis. Nat. Med. 8, 1218–1226. [DOI] [PubMed] [Google Scholar]
- Bird, D.A., Gillotte, K.L., Hörkkö, S., Friedman, P., Dennis, E.A., Witztum, J.L., Steinberg, D. (1999). Receptors for oxidized low-density lipoprotein on elicited mouse peritoneal macrophages can recognize both the modified lipid moieties and the modified protein moieties: implications with respect to macrophage recognition of apoptotic cells. Proc. Natl. Acad. Sci. USA 96, 6347–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biwa, T., Sakai, M., Matsumura, T., Kobori, S., Kaneko, K., Miyazaki, A., Hakamata, H., Horiuchi, S., and Shichiri, M. (2000). Sites of action of protein kinase C and phosphatidylinositol 3-kinase are distinct in oxidized low density lipoprotein-induced macrophage proliferation. J. Biol. Chem. 275, 5810–5816. [DOI] [PubMed] [Google Scholar]
- Boullier, A., Gillotte, K.L., Hörkkö, S., Green, S.R., Friedman, P., Dennis, E.A., Witztum, J.L., Steinberg, D., and Quehenberger, O. (2000). The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J. Biol. Chem. 275, 9163–9169. [DOI] [PubMed] [Google Scholar]
- Breitschopf, K., Zeiher, A.M., and Dimmeler, S. (2001). Pro-atherogenic factors induce telomerase inactivation in endothelial cells through an Akt-dependent mechanism. FEBS Lett. 493, 21–25. [DOI] [PubMed] [Google Scholar]
- Chang, L.C., and Wang, J.P. (2001). Signal transduction pathways for activation of extracellular signal-regulated kinase by arachidonic acid in rat neutrophils. Leukoc. Biol. 69, 659–665. [PubMed] [Google Scholar]
- Chang, M.K., Bergmark, C., Laurila, A., Hörkkö, S., Han, K.H., Friedman, P., Dennis, E.A., Witztum, J.L. (1999). Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. USA 96, 6353–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, M.K., Binder, C.J., Torzewski, M., and Witztum, J.L. (2002). From the cover: C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: phosphorylcholine of oxidized phospholipids. Proc. Natl. Acad. Sci. USA 99, 13043–13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X.S., and Funk, C.D. (2001). The N-terminal “β-barrel” domain of 5-lipoxygenase is essential for nuclear membrane translocation. J. Biol. Chem. 276, 811–818. [DOI] [PubMed] [Google Scholar]
- Chun, J.S., and Jacobson, B.S. (1992). Spreading of HeLa cells on a collagen substratum requires a second messenger formed by the lipoxygenase metabolism of arachidonic acid released by collagen receptor clustering. Mol. Biol. Cell 3, 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, D., Tseng, C.C., Bjekic, G., and Greenberg, S. (1999). A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 274, 1240. [DOI] [PubMed] [Google Scholar]
- Cyrus, T., Pratico, D., Zhao, L., Witztum, J.L., Rader, D.J., Rokach, J., FitzGerald, G.A., and Funk, C.D. (2001). Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein e-deficient mice. Circulation 103, 2277–2282. [DOI] [PubMed] [Google Scholar]
- Diederich, W., Orso, E., Drobnik, G., and Schmitz, G. (2001). Apolipoprotein AI and HDL3 inhibit spreading of primary human monocytes through a mechanism that involves cholesterol depletion and regulation of CDC42. Atherosclerosis 159, 313–324. [DOI] [PubMed] [Google Scholar]
- Ezaki, M., Witztum, J.L., and Steinberg, D. (1995). Lipoperoxides in LDL incubated with fibroblasts that overexpress 15-lipoxygenase. J. Lipid Res. 36, 1996–2004. [PubMed] [Google Scholar]
- Friedman, P., Hörkkö, S., Steinberg, D., Witztum, J.L., and Dennis, E.A. (2002). Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids: importance of Schiff base formation and Aldol condensation. J. Biol. Chem. 277, 7010–7020. [DOI] [PubMed] [Google Scholar]
- Garner, B., Waldeck, A.R., Witting, P.K., Rye, K.A., and Stocker, R. (1998). Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J. Biol. Chem. 273, 6088–6095. [DOI] [PubMed] [Google Scholar]
- George, J. et al. (2001). 12/15-Lipoxygenase gene disruption attenuates atherogenesis in LDL receptor-deficient mice. Circulation 104, 1646–1650. [DOI] [PubMed] [Google Scholar]
- Gillotte-Taylor, K., Boullier, A., Witztum, J.L., Steinberg, D., and Quehenberger, O. (2001). Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J. Lipid Res. 42, 1474–1482. [PubMed] [Google Scholar]
- Glass, C.K., and Witztum, J.L. (2001). Atherosclerosis. The road ahead. Cell 104, 503–516. [DOI] [PubMed] [Google Scholar]
- Havel, R.J., Bragdon, J.H., and Eder, H.A. (1955). The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34, 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hii, C.S., Moghadammi, N., Dunbar, A., and Ferrante, A. (2001). Activation of the phosphatidylinositol 3-kinase-Akt/protein kinase B signaling pathway in arachidonic acid-stimulated human myeloid and endothelial cells: involvement of the ErbB receptor family. J. Biol. Chem. 276, 27246–27255. [DOI] [PubMed] [Google Scholar]
- Hörkkö, S. et al. (1999). Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Invest. 103, 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, T.H., and Meyer, W.H. (1984). Chemotactic peptide modulation of actin assembly and locomotion in neutrophils. J. Cell Biol. 98, 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo, J.C., Miller, E., McLoughlin, P., and Steinberg, D. (1988). Enhanced macrophage uptake of low density lipoprotein after self-aggregation. Arteriosclerosis 8, 348–358. [DOI] [PubMed] [Google Scholar]
- Klimov, A.N., Kozhevnikova, K.A., Kuzmin, A.A., Kuznetsov, A.S., and Belova, E.V. (2001). On the ability of high density lipoproteins to remove phospholipid peroxidation products from erythrocyte membranes. Biochemistry (Moscow) 66, 300–304. [DOI] [PubMed] [Google Scholar]
- Kockx, M.M., and Herman, A.G. (2000). Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovasc. Res. 45, 736–746. [DOI] [PubMed] [Google Scholar]
- Kruszynska, Y.T., Worrall, D.S., Ofrecio, J., Frias, J.P., Macaraeg, G., and Olefsky, J.M. (2002). Fatty acid-induced insulin resistance: decreased muscle PI3K activation but unchanged Akt phosphorylation. J. Clin. Endocrinol. Metab. 87, 226–234. [DOI] [PubMed] [Google Scholar]
- Leevers, S.J., Vanhaesebroeck, B., and Waterfield, M.D. (1999). Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr. Opin. Cell Biol. 11, 219–225. [DOI] [PubMed] [Google Scholar]
- Libby, P. (2001). What have we learned about the biology of atherosclerosis? The role of inflammation. Am. J. Cardiol. 88, 3J–6J. [DOI] [PubMed] [Google Scholar]
- Marignani, P.A., and Carpenter, C.L. (2001). Vav2 is required for cell spreading. J. Cell Biol. 154, 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, Y.I., Chang, M.K., Funk, C.D., Feramisco, J.R., and Witztum, J.L. (2001). 12/15-Lipoxygenase translocation enhances site-specific actin polymerization in macrophages phagocytosing apoptotic cells. J. Biol. Chem. 276, 19431–19439. [DOI] [PubMed] [Google Scholar]
- Miller, Y.I., Viriyakosol, S., Binder, C.J., Feramisco, J.R., Kirkland, T.N., and Witztum, J.L. (2003). Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J. Biol. Chem. 278, 1561–1568. [DOI] [PubMed] [Google Scholar]
- Nakamura, I., Lipfert, L., Rodan, G.A., and Le, T.D. (2001). Convergence of alpha(v)beta(3) integrin- and macrophage colony stimulating factor-mediated signals on phospholipase Cgamma in prefusion osteoclasts. J. Cell Biol. 152, 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab, M. et al. (2000). Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J. Lipid Res. 41, 1481–1494. [PubMed] [Google Scholar]
- Reddy, M.A., Thimmalapura, P.R., Lanting, L., Nadler, J.L., Fatima, S., Natarajan, R. (2002). The oxidized lipid and lipoxygenase product 12(S)-hydroxyeicosatetraenoic acid induces hypertrophy and fibronectin transcription in vascular smooth muscle cells via p38 MAPK and cAMP response element-binding protein activation. Mediation of angiotensin II effects. J. Biol. Chem. 277, 9920–9928. [DOI] [PubMed] [Google Scholar]
- Rice, R.L., Tang, D.G., Haddad, M., Honn, K.V., and Taylor, J.D. (1998). 12(S)-hydroxyeicosatetraenoic acid increases the actin microfilament content in B16a melanoma cells: a protein kinase-dependent process. Int. J. Cancer 77, 271–278. [DOI] [PubMed] [Google Scholar]
- Savill, J., Dransfield, I., Gregory, C., and Haslett, C. (2002). A blast from the past: clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2, 965–975. [DOI] [PubMed] [Google Scholar]
- Shaw, P.X., Hörkkö, S., Chang, M.K., Curtiss, L.K., Palinski, W., Silverman, G.J., and Witztum, J.L. (2000). Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 105, 1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, P.X., Hörkkö, S., Tsimikas, S., Chang, M.K., Palinski, W., Silverman, G.J., Chen, P.P., and Witztum, J.L. (2001). Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler. Thromb. Vasc. Biol. 21, 1333–1339. [DOI] [PubMed] [Google Scholar]
- Sigari, F., Lee, C., Witztum, J.L., and Reaven, P.D. (1997). Fibroblasts that overexpress 15-lipoxygenase generate bioactive and minimally modified LDL. Arterioscler. Thromb. Vasc. Biol. 17, 3639–3645. [DOI] [PubMed] [Google Scholar]
- Smalheiser, N.R., Dissanayake, S., and Kapil, A. (1996). Rapid regulation of neurite outgrowth and retraction by phospholipase A2-derived arachidonic acid and its metabolites. Brain Res. 721, 39–48. [DOI] [PubMed] [Google Scholar]
- Steinbrecher, U.P., Witztum, J.L., Parthasarathy, S., and Steinberg, D. (1987). Decrease in reactive amino groups during oxidation or endothelial cell modification of LDL. Correlation with changes in receptor-mediated catabolism. Arteriosclerosis 7, 135–143. [DOI] [PubMed] [Google Scholar]
- Stephens, L., Ellson, C., and Hawkins, P. (2002). Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr. Opin. Cell Biol. 14, 203–213. [DOI] [PubMed] [Google Scholar]
- Szekeres, C.K., Trikha, M., Nie, D., and Honn, K.V. (2000) Eicosanoid 12(S)-HETE activates phosphatidylinositol 3-kinase. Biochem. Biophys. Res. Commun. 275, 690–695. [DOI] [PubMed] [Google Scholar]
- Timar, J., Chen, Y.Q., Liu, B., Bazaz, R., Taylor, J.D., and Honn, K.V. (1992). The lipoxygenase metabolite 12(S)-HETE promotes alpha IIb beta 3 integrin-mediated tumor-cell spreading on fibronectin. Int. J. Cancer 52, 594–603. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala, S., Rosenfeld, M.E., Parthasarathy, S., Glass, C.K., Sigal, E., Witztum, J.L., and Steinberg, D. (1990). Colocalization of 15-lipoxygenase mRNA and protein with epitopes of oxidized low density lipoprotein in macrophage-rich areas of atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 87, 6959–6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala, S., Rosenfeld, M.E., Parthasarathy, S., Sigal, E., Sarkioja, T., Witztum, J.L., Steinberg, D. (1991). Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J. Clin. Invest. 87, 1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.