Abstract
We report a novel nucleolar interaction between the AAA ATPase p97/VCP and the Werner protein (WRNp), a member of the RecQ helicase family. p97/VCP mediates several important cellular functions in eucaryotic cells, including membrane fusion of the endoplasmic reticulum and Golgi and ubiquitin-dependent protein degradation. Mutations in the WRN gene cause Werner syndrome, a genetic disorder characterized by premature onset of aging symptoms, a higher incidence of cancer, and a high susceptibility to DNA damage caused by topoisomerase inhibitors. We observed that both WRNp and valosin-containing protein (VCP) were present in the nucleoplasm and in nucleolar foci in mammalian cells and that WRNp and p97/VCP physically interacted in the nucleoli. Importantly, the nucleolar WRNp/VCP complex was dissociated by treatment with camptothecin, an inhibitor of topoisomerase I, whereas other WRNp-associated protein complexes, such as WRNp/Ku 80, were not dissociated by this drug. Because WRN syndrome cells are sensitive to topoisomerase inhibitors, these observations suggest that the VCP/WRNp interaction plays an important role in WRN biology. We propose a novel role for VCP in the DNA damage response pathway through modulation of WRNp availability.
INTRODUCTION
The ATPases associated with diverse cellular activities (AAA) proteins are a common family of Mg2+-dependent ATPases that contain one or two conserved ATP-binding domains (Ogura and Wilkinson, 2001). These domains, or AAA cassettes (Patel and Latterich, 1998), consist of a conserved sequence of 230-amino acid residues that include the Walker A and B motifs. Another sequence-conserved domain, the second region of homology, is found in part of the AAA cassette (Confalonieri and Duguet, 1995) but is not present in the closely related AAA+ family (Neuwald et al., 1999) of proteins. Cdc48p, p97, and valosin-containing protein (VCP) are 92- to 97-kDa orthologous members of a subfamily of AAA ATPases originally defined in yeast, Xenopus, and mammals, respectively (Moir et al., 1982; Peters et al., 1990; Frohlich et al., 1991). This subfamily has a known propensity to oligomerize and form homohexamers (Peters et al., 1992) that are a part of multiprotein complexes (Ogura and Wilkinson, 2001).
Cdc48p/p97/VCP is involved in two major and distinct cellular pathways: homotypic membrane fusion of endoplasmic reticulum (ER) and Golgi fragments and ubiquitin-dependent protein degradation. This versatility is achieved through different sets of adaptor proteins (Patel and Latterich, 1998). In ER membrane fusion, Cdc48p/p97/VCP is complexed with Ufe1p and Shp1p (Latterich et al., 1995; Patel et al., 1998; Lin et al., 2001) or with their vertebrate orthologs p47 and syntaxin 5 in Golgi membrane fusion (Acharya et al., 1995; Rabouille et al., 1995). It is likely that the role of Cdc48p in ER membrane fusion is to remove a fusion inhibitor, thus permitting membrane fusion to occur (Lin et al., 2001). Cdc48p/p97/VCP also participates in the degradation of ubiquitinated proteins in yeast via Ufd1p and Npl4p and in mammals (Ghislain et al., 1996; Dai et al., 1998; Meyer et al., 2000). Overall, the emerging hypothesis is that Cdc48p and orthologs such as VCP may serve as an “unwindase” or molecular motor to unfold proteins and extract them from protein complexes or membranes, with pathway specificity being conferred by adapter proteins (Patel and Latterich, 1998). It is known that Cdc48p is required for nuclear division, because cdc48 mutants arrest in the cell cycle with an undivided nucleus (Latterich and Schekman, 1994; Latterich et al., 1995). Cdc48p has a nuclear localization signal (NLS) located in the N-terminal domain that is essential for its nuclear localization (Madeo et al., 1998). It is unclear whether VCP is also nuclear and whether it has a functional NLS (Madeo et al., 1997; Muller et al., 1999). Herein, we demonstrate that VCP has both nuclear and nucleolar localization and embark on a study to determine the role of nucleolar VCP.
Werner syndrome (WS) is a rare autosomal recessive genetic disorder characterized by premature onset of aging symptoms and higher incidence of cancer (Shen and Loeb, 2001). The WRN gene product is a 160-kDa protein homologous to the Escherichia coli RecQ DNA helicase (Yu et al., 1996). Recombinant human Werner helicase (WRNp) exhibits RNA and DNA unwinding activities (Gray et al., 1997; Suzuki et al., 1997) as well as exonuclease activity (Huang et al., 1998; Suzuki et al., 1999). WRNp has an NLS near the C terminus of the protein and has been described in both the nucleoplasm and nucleolus. Recently, a nucleolar localization signal has been determined for WRNp (von Kobbe and Bohr, 2002). The regulation of WRNp nuclear dynamics is unclear and differs between growing and resting cells and also between mouse and human cells (Suzuki et al., 2001, and references therein). The WRN protein forms functional complexes with several cellular proteins, some of which facilitate its helicase activity, such as replication protein A (RPA) (Shen et al., 1998; Brosh et al., 1999) and telomere-repeat binding factor 2 (TRF2) (Opresko et al., 2002). However, none of these interacting proteins explain the regulation of WRNp nuclear localization.
In this study, we demonstrate that VCP is found in mammalian nuclei and report a novel nucleolar protein complex between members of two protein families, the Werner protein of the RecQ Helicase family, and the AAA ATPase p97/VCP. We further show that this complex is disrupted by a drug to which WS cells show high sensitivity, the topoisomerase I inhibitor camptothecin (CPT). These observations propose a novel role for members of the AAA protein family in regulating the availability of enzymes involved in nucleic acid metabolism through physical interaction.
MATERIALS AND METHODS
Proteins, Antibodies, and Cells
Rabbit anti-VCP antibody 5860 and chicken anti-VCP antibody 1469 (Aves, Tigard, OR) were raised against purified bovine liver VCP (Indig and Latterich, unpublished data). Briefly, liver homogenate was prepared in the presence of ATP and clarified by freezing –80°C overnight, followed by pelleting the precipitate by centrifugation. The clarified supernatant was loaded on DEAE-Sepharose FF (Amersham Biosciences, Piscataway, NJ) and eluted with a 60–500 mM KCl gradient. VCP-containing fractions were detected by Coomassie-stained gels, pooled, and precipitated with ammonium sulfate at 40% saturation. After dialysis, VCP was purified using a 5–30% sucrose gradient and fractions collected were snap frozen and stored at –80°C.
The antibodies 5860 and 1469 were compared with known mouse anti-p97 antibodies (see below) and found to be highly specific and selective, reacting only with VCP from human, monkey, and hamster cells. Preimmune sera did not detect VCP (Figure 4) and was negative in immunofluorescence (our unpublished data). Antibodies 5860 and 1469 did not cross-react with other AAA ATPases, including nuclear VCP-Like protein, a 110-kDa AAA protein with unknown function (Germain-Lee et al., 1997).
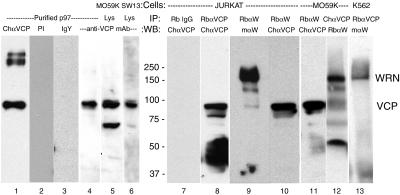
Figure 4.
VCP and WRNp reciprocally coimmunoprecipitate. Nuclear extracts were immunoprecipitated and immunoblotted as described in MATERIALS AND METHODS. Equal amounts of total protein were immunoprecipitated with 20 μl of rabbit anti-Werner helicase (RbαW) and either the chicken (1469) or rabbit (5860) anti-VCP polyclonal antibodies. Rabbit IgG was used as a negative control. Immunoprecipitated proteins (40 μl/lane) were electrophoresed on a 7.5% polyacrylamide gel and then immunoblotted to polyvinylidene difluoride membranes. After a 1 h incubation with primary antibodies and appropriate horseradish peroxidase-conjugated secondary antibodies, proteins were visualized by enhanced chemiluminescence. The results are presented as a composite image of Jurkat (lanes 7–10), MO59K (lanes 11 and 12) or K562 (lane 13) precipitates, or total cell lysates of MO56K (lane 5) or SW13 (lane 6) cells. Lanes 1–4 contain purified bovine liver VCP, 0.5 μg, immunoblotted with chicken anti-VCP 1469 (1:2000, lane 1), or anti-VCP mAb (lane 4). As a control, 2.0 μg of purified VCP was immunoblotted with 10 μg/ml preimmune chicken serum (preimmune 1:200, lane 2) or 10 μg/ml purified chicken IgY (IgY, lane 3). The expected position of VCP and WRNp are indicated on the right and molecular masses in kilodaltons are in the middle. In some cell lysates (example, lane 5), an ∼60-kDa protein, apparently a VCP fragment, is detected by anti-VCP antibody. VCP multimers are occasionally detected, for example, in purified VCP (lane 1).
The anti-VCP monoclonal antibody (mAb) MARA-1 (Schulte et al., 1994) was a kind gift of Robbie Schulte and Bart Sefton (The Salk Institute, La Jolla, CA). Anti-p97 mAb 58.13.3 was purchased from Research Diagnostics (Flanders, NJ). Rabbit anti-WRN1 was purchased from Novus (Littleton, CO) and mouse anti-WRNp mAb and SW13 cell lysate were from BD Transduction Laboratories (San Diego, CA). Rabbit anti-WRN H-300 and rabbit anti-MPP2 polyclonals were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-Ku 80 was purchased from Chemicon International (Temecula, CA) and mouse anti-Ku 80 from NeoMarkers (Fremont, CA). Rabbit anti-BRCA1 was purchased from Lab Vision (Fremont, CA) and mouse anti-BRCA1 clone antibody-1 was from Oncogene Science (Cambridge, MA). Horseradish peroxidase- and Cy3-conjugated secondary mAbs were purchased from Jackson Immunoresearch Laboratories (West Grove, PA). Alexa 488-conjugated secondary mAbs and the DNA stain 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) were purchased from Molecular Probes (Eugene, OR).
The monkey kidney cell line CV-1, the human glioma cell line MO59-K, the human mesothelioma cell line NCI-H226, and the human lymphoblastoid lines K562 and Jurkat were obtained from the American Type Culture Collection (Manassas, VA).
Protease inhibitors leupeptin, phenylmethylsulfonyl fluoride, E64, chymostatin, and pepstatin A were purchased from Calbiochem (San Diego, CA). RNase A was from Worthington Biochemicals (Lakewood, NJ).
Plasmid Construction
The murine VCP cDNA clone was a kind gift from Dr. Ron Trible (National Institutes of Health, Bethesda, MD). The following restriction fragments were cloned into the pEGFP-c1 vector (BD Biosciences Clontech, Palo Alto, CA), by using standard molecular biology techniques (Sambrook et al., 1989): the whole VCP gene (Swissprot Q01853), VCPΔC (VCP without C-terminal residues 639–806), and VCPΔN (VCP without N-terminal residues 1–141).
Cell Culture, Transfection, and Camptothecin Treatment
Cells were maintained in DMEM (Quality Biological, Gaithersburg, MD) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), l-glutamine, and antibiotics (Quality Biological), by using standard cell culture technique (Indig et al., 1997). Cells were plated onto wells of a six-well plate, ∼100,000 cells/well. After overnight growth, subconfluent cells were transfected with 0.5 μg of DNA by using LipofectAMINE Plus (Invitrogen, Carlsbad, CA) according to manufacturer's instructions.
Subconfluent cells propagated for nuclear extraction were treated with camptothecin (CPT; Calbiochem) at 10 μM for 1 or 4 h. Control cells were left untreated. The cells were trypsinized and harvested, washed once with phosphate-buffered saline (PBS), and the cell pellet was either snap frozen and stored at –80°C, or immediately extracted as below.
Nuclear Extraction
The nuclear extraction procedure was adapted from (Abmayr and Workman, 2001). Hypotonic (10 mM KCl, 10 mM HEPES, pH 7.9, at 4°C, 1.5 mM MgCl2), low salt (0.2 M KCl, 20 mM HEPES, pH 7.9, at 4°C, 25% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA), and high salt (similar to low salt buffer except with 1.2 M KCl) buffers were prepared. The following protease inhibitor cocktail was added to hypotonic and low salt buffers immediately before use (final concentration): 2 mM phenylmethylsulfonyl fluoride, 0.5 mM leupeptin, 0.2 mM chymostatin, 20 μM pepstatin A, 0.2 mM E64. All procedures were at 4°C.
Cell pellets were washed in 5 packed cell volumes of hypotonic buffer plus inhibitors, centrifuged at ∼1800 × g for 5 min, and then resuspended in 3 packed cell volumes of hypotonic buffer, and cells were allowed to swell on ice for 10 min. More hypotonic buffer was added until cell density was 2 × 107 cells/ml. The cells were then lysed on ice by using 10–20 strokes of a glass Dounce homogenizer (type B pestle). Lysis (>90% of cells) was verified by staining a sample with trypan blue and observing under a light microscope. The lysate was centrifuged at 3300 × g, and this cytoplasmic extract was transferred to new tubes and saved for later immunoprecipitation. The remaining nuclear pellet was resuspended in 2 packed nuclear volumes of low salt buffer plus inhibitors. High salt buffer, half the new volume of nuclei in low salt buffer, was added dropwise while gently vortexing. The suspension was incubated at 4°C with continuous gentle mixing for 2 h and then centrifuged at ∼18,000 × g for 40 min. The nuclear extract was transferred to fresh tubes for immunoprecipitation or storage at –80°C.
SDS-PAGE, Immunoblotting, and Immunoprecipitation
Nuclear or cytoplasmic extracts of equal protein content (determined by BCA protein assay; Pierce Chemical, Rockford, IL) were immunoprecipitated as described by Indig et al., 1997. Precipitated proteins were separated on polyacrylamide gels, immunoblotted, and chemiluminescence detected as described previously (Indig et al., 1997).
Microscopy and Immunofluorescence
Cells were grown on coverslips overnight and fixed in 3.7% formaldehyde followed by permeabilization with 0.2% Triton X-100 and then incubated with appropriate antibodies and mounted on slides as described previously (Indig et al., 1997). The following changes and additions were made to the procedure. After incubation with secondary antibodies, coverslips were washed five times in PBS, pH 8.5. When coverslips were stained with DAPI, they were washed three times and then stained with 1 μM DAPI (in PBS), and coverslips were incubated for 20 min at 37°C. RNase A (20 μg/ml) was also added to this step. Coverslips were washed three more times in PBS, pH 8.5, before mounting.
Fixed cells were studied with an Axioplan 2 microscope (Carl Zeiss, Thornwood, NY) and images were captured with a Orca 2 charged-coupled device camera (Hamamatsu, Bridgewater, NJ). Images were processed and deconvolved using Openlab software (Improvision, Lexington, MA). Confocal microscopy was done with a TCS SP system (Leica Microsystems, Deerfield, IL), and images were transferred into Photoshop (Adobe Systems, Palo Alto, CA). Live cells were studied with an IX70 inverted microscope (Olympus, Melville, NY), and images were captured through a Cool Snap Fx charge-coupled device and processed with IPLab (BioVision, Exton, PA) software.
RESULTS
VCP Is Localized to the Nucleus and Nucleolus of Mammalian Cells
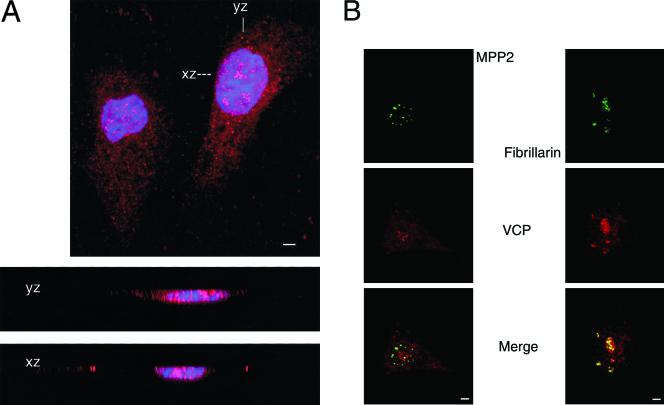
We examined the subcellular distribution of VCP in mammalian cells by indirect immunofluorescence. As shown in Figure 1, antibodies against VCP stained both the cytoplasm and the nucleus with a punctate pattern in simian and human cells (antibody Rb5860 in simian CV1 cells, Figure 1A; antibody chicken 1469 in human NCI-H226, Figure 1B). The cytoplasmic signal was primarily from the ER, where VCP colocalized with the ER-specific stain 3,3′-dihexyl-oxacarbocyanine iodide (our unpublished data). Two patterns of VCP nuclear fluorescence emerged: a punctate pattern throughout the nucleus and several foci of intense fluorescent signal. These intensely staining foci were found to correspond to nucleoli, as demonstrated by colocalization of VCP to nucleolar markers such as fibrillarin (Figure 1B) and nucleolin (our unpublished data). In contrast, antibodies against VCP did not colocalize with antibodies against other abundant nuclear proteins such as the M-phase phosphoprotein 2 (MPP2; Figure 1B). We used confocal microscopy to verify that the VCP signal was not merely superimposed on the nuclear area but intercalated within the nuclear DNA-staining regions (Figure 1A, xz and xy sections). Similar immunofluorescent patterns were observed with other human cell lines, including M059K (Figures 3 and 6), M059J, K562, U87MG, and Jurkat and in Chinese hamster ovary cells (our unpublished data). Identical patterns were observed with several anti-VCP antibodies: two murine monoclonals (anti-p97, MARA-1; our unpublished data) and with two polyclonal anti-VCP antibodies (rabbit 5860, Figure 1A; chicken 1469, Figure 1B).
Figure 1.
VCP is present in the nucleus and nucleolus of mammalian cells. Cells were grown on glass coverslips overnight and then fixed in 3.7% formaldehyde and permeabilized with Triton X-100 as described in MATERIALS AND METHODS. (A) Confocal section of CV-1 cells stained with Rb 5860 anti-VCP (1:500) and visualized with Cy3-conjugated secondary antibody (red), and with the DNA stain DAPI (blue). xz and yz are confocal cross sections through the right-hand cell as marked. 630×; bar, 10 μm. (B) NCI-H226 cells were stained with chicken 1469 anti-VCP (1:200, red) and mouse anti-MPP2 (1:500, green) or mouse anti-fibrillarin (1:200, green). Images were merged to view colocalization (yellow). 400×; bar, 10 μm.
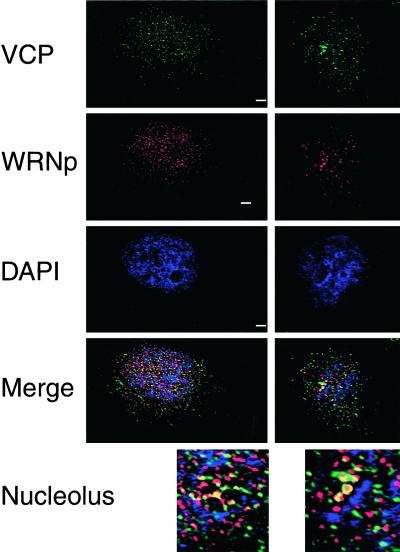
Figure 3.
VCP and WRNp colocalize in the nucleolus. MO59K cells were processed for indirect immunofluorescence as described above and stained with anti-WRNp mAb (1:100, red), chicken 1469 anti-VCP (1:250, green), and the DNA stain DAPI (blue). By using Openlab deconvolution software, 0.1-μm Z-sections were obtained on an Axioplan 2 microscope (Carl Zeiss) and deconvolved. Merge is the merged image of the three fluorescence channels examined of the same deconvolved Z-section. Two representative cells from different experiments are shown; a nucleolar area of each cell was enlarged (3×, bottom). 630×; bar, 5 μM.
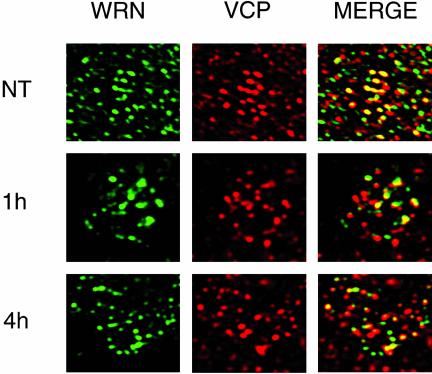
Figure 6.
CPT treatment dissociates VCP and WRNp in the nucleolus. MO59K cells were treated with 10 μM CPT for 1 or 4 h or were NT and then processed for indirect immunofluorescence as described in Figure 3. WRN (green) and VCP (red) are the different fluorescent channels of the same 0.1-μm section examined. Merged is the result of coloring both channels and presenting them in the same image, with yellow indicating colocalization. NT, 630×; 1 and 4 h, 1000×.
VCP Has an N-Terminal Domain Nuclear Localization Signal
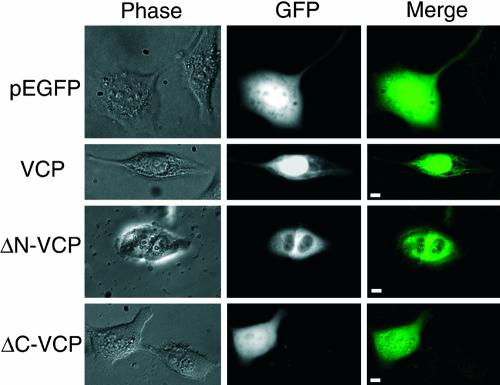
In contrast to its yeast ortholog Cdc48p, VCP did not possess a known NLS and was thought not to be a nuclear protein (Madeo et al., 1997, 1998). Because VCP clearly localized to the nucleus (Figure 1), it was important to determine the location of a putative NLS within the VCP sequence. Toward this goal, a series of VCP mutants fused to the green fluorescent protein (GFP) gene were cloned (Figure 2). The full-length VCP-GFP expressed throughout the cell, in an ER-like network in the cytoplasm and fluoresced intensely in the nuclear region (Figure 2, second row). Deletion of the C-terminal domain (ΔC-VCP-GFP) resulted in a diffuse nuclear and cytoplasmic signal (Figure 2, forth row). Deletion of the N-terminal domain (ΔN-VCP-GFP) prevented nuclear expression of the fusion protein and only an extranuclear cytoplasmic localization was visible (Figure 2, third row). Propidium iodide staining of nuclei verified that there was no nuclear GFP expression when cells were transfected with ΔN-VCP-GFP (our unpublished data).
Figure 2.
The N-terminal domain of VCP is crucial for nuclear localization. The murine VCP gene was fused to the pEGFP vector as described in MATERIALS AND METHODS, and several domain-deleted fusion proteins were constructed as described in text. Human glioma MO59K cells were transfected using the cationic lipid method. After 48 h, live cells were examined under an Olympus IX70 and images captured and merged with IPLab. Right, merged image of GFP fluorescence (middle) with phase contrast (left). 400×, bar, 5 μm.
VCP and WRNp Colocalize in the Nucleolus of Human Glioma Cells and Coimmunoprecipitate in Nuclear Extracts
To further characterize VCP function in the nucleus, we initiated a search for possible VCP interacting proteins, focusing on proteins that showed similar punctated nuclear and possible nucleolar distribution. VCP was not found in promyelocytic leukemia (PML) nuclear bodies and did not colocalize with proteins associated with DNA replication, such as proliferating cell nuclear antigen, or with cell cycle-specific proteins such as MPP2 (for example, Figure 1B). In contrast, WRNp showed a staining pattern similar to VCP throughout the nucleus with intense nucleolar signal that colocalized with the VCP signal (Figure 3). To verify that the pattern similarity was derived from colocalization and not superimposition of different nucleolar layers, we analyzed deconvolved, high-resolution nuclear images from 0.1-μm optical sections. As shown in Figure 3, the deconvolution data indicated that VCP (green) and WRNp (red) colocalized primarily in nucleolar foci (yellow, Figure 3, merge; also see Figure 6, nontreated [NT]). This colocalization extended to ∼1.0 μm in depth and was prominent in the DAPI-negative areas (i.e., that contained RNA before RNase A treatment) of the nucleolus (Figure 3).
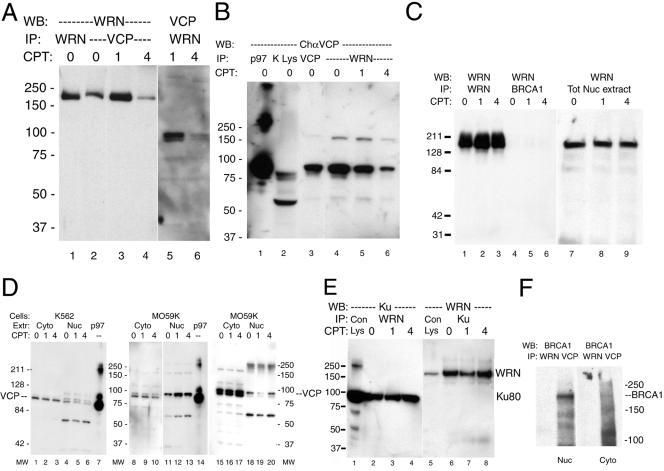
We next examined whether VCP and WRNp formed a stable complex in mammalian cells, as suggested by the colocalization data. To that end, we used immunoblotting to detect VCP in anti-WRNp immunoprecipitates of nuclear extracts. As shown in Figure 4, the chicken anti-VCP antibody (ChαVCP) detected an ∼100-kDa protein in purified p97 (lane 1). In control experiments, VCP was not detected in nuclear extracts immunoprecipitated by purified rabbit IgG (lane 7) or chicken IgY (not shown), nor did VCP react with preimmune chicken serum or purified IgY (lanes 2 and 3). Similarly, the monoclonal anti-VCP antibody (Figure 4, lanes 4–6) identified the same 100-kDa protein in purified p97 and in lysates from two cell lines, MO59K and SW13. An identically migrating protein was detected by the anti-VCP chicken antibody in immunoprecipitates with anti-WRNp from Jurkat and MO59K cells (Figure 4, lanes 10 and 11). Reciprocally, WRNp was detected in anti-VCP immunoprecipitates from both rabbit and chicken anti-VCP (Figure 4, lanes 12 and 13). Coimmunoprecipitation was apparent in several cell lines as well, such as MO59K, K562, and Jurkat, by using various antibodies against VCP and WRNp (Figure 4, lanes 8–13). These observations confirmed that VCP and WRNp are present in the same protein complex, as indicated by the indirect immunofluorescence studies.
The Topoisomerase I Inhibitor CPT modulates the VCP–WRNp Interaction
Camptothecin is known to have a specific effect on cells with mutated WRN function, causing chromosomal damage, cell-cycle arrest and rapid cell death (Poot et al., 1992, Pichierri et al., 2000). To examine whether the WRNp/VCP interaction was affected by DNA repair pathways, MO59K cells were treated with 10 μM CPT for 1 or 4 h before immunoprecipitation analysis or visualization of the WRN and VCP proteins by indirect immunofluorescence. As shown in Figure 5, a 4-h treatment with CPT led to a marked reduction in WRNp detected in anti-VCP immunoprecipitates (Figure 5A). We also observed a reciprocal reduction in VCP detected in anti-WRNp immonoprecipitates in nuclear extracts from cells treated for 4 h with CPT (Figure 5, A and B, compare 4 h to untreated cells or cells treated for only 1 h).
Figure 5.
CPT treatment effects VCP and WRNp coimmunoprecipitates. MO59K cells were treated with 10 μM CPT for 1 or 4 h or were untreated controls (0 h). In all panels, the molecular mass in kilodaltons is indicated to the right or left. (A) Rabbit anti-WRNp and rabbit anti-VCP precipitates were treated as described in Figure 4 and Western blotted with mouse anti-WRNp (lanes 1–4) or chicken anti-VCP (lanes 5 and 6) as described. (B) A single immunoblot from the same experiment of VCP (lane 3) and WRNp (lanes 4–6) IPs probed with chicken anti-VCP. Lane 1, 0.5 μg of purified bovine liver VCP; lane 2, MO59K total cell lysate (40 μg). (C) Mouse anti-WRNp detects WRNp in rabbit anti-WRNp precipitates (lanes 1–3) and in total nuclear extract (lanes 7–9), but not in rabbit anti-BRCA1 IPs (lanes 4–6). Cells were treated with CPT and Western blotted as described in text. (D) Cells were treated as described above with CPT and extracts were immunoblotted with chicken 1469 anti-VCP as described in text. Three different experiments are shown, one performed with K562 extracts (lanes 1–6) and two with MO59K extracts (lanes 8–13 and 15–20). Lanes 7 and 14, 0.5 μg of purified bovine liver VCP. (E) WRNp was detected in rabbit anti-Ku 80 precipitates (lanes 6–8) and Ku-80 was detected in rabbit anti-WRNp precipitates (lanes 2–4) of nuclear extracts. Lane 1, 20 μl of SW13 total lysate; lane 5, 20 μl of MO59K total lysate. (F) BRCA1 detected in VCP, but not WRNp IPs. MO59K cells were separated into cytoplasmic and nuclear extracts as described in MATERIALS AND METHODS and precipitated with rabbit anti-WRNp or Rb 5860 anti-VCP and probed for BRCA1.
To verify the specificity of the CPT effect on the VCP/WRNp interaction, we examined the effect of this drug on the abundance of these two proteins as well as on other multiprotein complexes that were reported to contain VCP or WRNp (Figure 5, C–F). As shown in Figure 5C, the WRNp signal did not diminish in immunoprecipitates with anti-WRNp antibodies (lane 1 versus 3), nor in total nuclear extracts (lane 7 versus 9) from cells treated with CPT. Similarly, we did not observe a decrease of total nuclear VCP after 4 h CPT treatment in most experiments (Figure 5D). Moreover, in contrast to the effect of CPT on the VCP/WRNp complex, CPT effected neither the nuclear abundance of the Ku-80 protein that is known to interact with WRNp (Cooper et al., 2000; Li and Comai, 2000), nor the ability of Ku and WRNp antibodies to immunoprecipitate these proteins (Figure 5E). Finally, we have also investigated complex formation between BRCA1 and VCP (Zhang et al., 2000a). We have confirmed this interaction, because BRCA1 was detected in VCP immunoprecipitates (IPs) from both nuclear and cytoplasmic extracts (Figure 5F). This complex was not disrupted by CPT, and BRCA1 was not recruited to the WRNp complex after CPT treatment (Figure 5C, lanes 4–6). BRCA1 was not detected in WRNp IPs, nor was WRNp found in BRCA1 IPs (Figure 5, C and F), indicating that the VCP/BRCA1 and VCP/WRNp were separate and distinct nuclear protein complexes.
We used indirect immunofluorescence to determine whether CPT dissociates the VCP/WRNp complex in the nucleolus. Figure 6 shows enlarged deconvolved images of representative nuclei from MO59K cells that were fixed with formaldehyde after CPT treatment, and stained with antibodies against VCP and WRNp. The NT nucleolus showed a similar fluorescence pattern for both anti-WRNp (green) and anti-VCP (red), with intense colocalization (merge, yellow) in the DAPI-negative central region of the nucleolus. After 1 h of CPT treatment, the nucleolar colocalization was diminished (Figure 6, 1 h). After 4 h of CPT treatment, very little colocalization remained in the central nucleolar area and both WRNp and VCP showed a different nucleolar staining pattern (Figure 6, 4 h). These data suggest that CPT treatment specifically disrupts the nucleolar complex formed between the VCP and the WRN proteins.
DISCUSSION
We have shown that VCP localizes to mammalian nuclei, where it physically associated with the Werner helicase in the nucleolus. This conclusion was based on the specific and reciprocal coimmunoprecipitation of these nucleolar proteins and precise colocalization by indirect immunofluorescence in deconvolved thin sections. Furthermore, treatment of cells with camptothecin causes the abrogation of the VCP–Werner complex. The dissociation of VCP and WRNp can be followed in high-resolution deconvolved fluorescent images of the nucleolus. Our data further suggest that VCP and WRNp both participate in other multiprotein complexes that are not disrupted by CPT.
We demonstrated that the AAA ATPase p97/VCP, known for its roles in homotypic membrane fusion and ubiquitin-directed proteolysis, was abundant in the nucleus. VCP/p97 showed a robust intranuclear signal, especially in nucleoli and was immunoprecipitated from nuclear extracts. Several AAA+ (but not AAA) proteins are present in the nucleus and participate in the nucleic acid metabolic pathways, such as DNA replication and damage repair (Ogura and Wilkinson, 2001) and ribosome biogenesis (Brown, 2001). We observed that nuclear VCP did not localize to PML bodies or replication factories, indicating that VCP probably does not participate in functions associated with these nuclear structures. Interestingly, a role in DNA repair for VCP has been implied by a report associating VCP with the nuclear protein BRCA1 (Zhang et al., 2000a). We confirmed this interaction and demonstrated that the VCP/BRCA1 complex is distinct from the VCP/WRNp complex, because WRNp was not observed in VCP/BRCA1 complexes and vice versa. These observations suggest that the nuclear role of VCP may involve regulating the abundance of its partner proteins, such as WRNp and BRCA1, modulating the availability of these proteins to participate in metabolic processes in response to DNA damage.
We found that similar to Cdc48p, the N-terminal domain of VCP was essential for nuclear localization. When the N-terminal portion was deleted, the ΔN-VCP-GFP fusion protein was unable to enter the nucleus and remained in the cytoplasm. When the C-terminal portion of VCP was deleted, the ΔC-VCP-GFP fusion protein was expressed throughout the cell, but it was no longer concentrated in the nucleus. This indicated that although the N-terminal portion of VCP was responsible for nuclear localization, the C-terminal domain has a role in retaining VCP in the nucleus, leading eventually to a high nuclear concentration of VCP. In yeast, a Cdc48p/VCP fusion protein showed that the C-terminal domain was functionally interchangeable between the orthologs, but not the N-terminal domain. Cdc48p containing the VCP N-terminal was unable to gain nuclear entry (Madeo et al., 1997). Frohlich and coworkers identified a bipartite NLS (Robbins et al., 1991) spanning residues 15–31 in Cdc48p that is mostly absent from VCP. However, as they had noted, a stretch of basic amino acid residues, KGKKRK, that resembles the SV40 T-antigen NLS (Kalderon et al., 1984) is identified at Cdc48p position 70–75 (Madeo et al., 1997). This sequence is highly conserved in VCP (KGKKRR, position 60–65) and can function as the VCP NLS. We should bear in mind, however, that the N-terminal domain of Cdc48p/p97/VCP is also thought to be involved in protein binding of adaptor molecules, such as p47 (Coles et al., 1999; Rouiller et al., 2000; Zhang et al., 2000b). Therefore, we cannot rule out the less likely possibility that the N-terminal deletion affected the interaction of VCP with a cofactor essential for its nuclear entry.
The VCP protein was associated with WRNp as seen by immunofluorescence and reciprocal coimmunoprecipitation. The Werner helicase has been shown to interact with multiple protein complexes: p53 (Blander et al., 1999; Spillare et al., 1999), Ku 80/76 (Cooper et al., 2000; Li and Comai, 2000), topoisomerase I (Lebel et al., 1999), and FEN1 (Brosh et al., 2001), among others. The VCP/WRNp complex described herein was dissociated by treatment of cells with the DNA-damaging agent camptothecin, whereas other WRN-associated complexes, such as WRNp/Ku, were not. CPT is a DNA topoisomerase I inhibitor that blocks topoisomerase I kinase activity (Rossi et al., 1996) and causes double-strand DNA breaks (Pommier et al., 1998; Pourquier and Pommier, 2001). Cells and cell lines derived from Werner Syndrome patients were shown to be sensitive to the genotoxins camptothecin and 4-nitroquinoline-1-oxide (Poot et al., 1992; Ogburn et al., 1997; Poot et al., 1999). We observed that other genotoxic agents, such as hydroxyurea and bleomycin, did not dissociate the VCP/WRNp complex as did CPT (Indig, unpublished data). In contrast, CPT had no effect on the interaction between WRNp and Ku 80 and on intranuclear levels of immunoprecipitated WRNp. Similarly, topoisomerase I was shown to dissociate from nucleoli after treatment with the CPT derivative topotecan, whereas hydroxyurea had no effect (Danks et al., 1996). In another WRNp-associated complex, the induction of p53 by CPT was reduced in WS cells (Blander et al., 2000). These molecular data are consistent with the observation that CPT has a specific effect on the survival of WS cells (Shen and Loeb, 2001) compared with other DNA-damaging agents, such as UV irradiation, hydroxyurea, bleomycin, and alkylating agents (Shen and Loeb, 2000).
We localized the VCP/WRNp interaction to the nucleolus. For many years, the nucleolus was largely considered to be a ribosome factory (Schwarzacher and Wachtler, 1993). With the localization to the nucleolus of proteins that are not involved in ribosome genesis, it is apparent that the nucleolus has other roles in the cell (Andersen et al., 2002). An example of novel nucleolar function is seen in the phosphatase Cdc14p, which is sequestered to the nucleolus by its inhibitor, Cfi1p/Net1p, and released into the nucleoplasm during anaphase (Visintin et al., 1999; Stegmeier et al., 2002). The Werner helicase has been localized to the nucleolus (Gray et al., 1998; Marciniak et al., 1998) and in fact possesses a nucleolar targeting sequence (von Kobbe and Bohr, 2002). However, the role of WRNp in the nucleolus is unclear. After treatment with CPT, WRNp translocated to intranuclear repair foci that included the repair proteins Rad50 and RPA (Sakamoto et al., 2001). WRNp also translocated from the nucleolus to the nucleoplasm after treatment with the genotoxic agent 4-nitroquinoline-1-oxide (Gray et al., 1998) and serum starvation (Suzuki et al., 2001). It has been proposed that tyrosine phosphorylation, either by direct modification of WRNp or of a putative “WRN-nucleolar carrier” may modulate the nucleolar trafficking of WRNp (Gray et al., 1998). Interestingly, VCP is known to be tyrosine-phosphorylated at its C-terminal domain and that hydrogen peroxide treatment greatly increases VCP tyrosine phosphorylation (Schulte et al., 1994). It has been suggested that another intranuclear structure, PML bodies, act as a nuclear depot of proteins, that are released upon viral attack (Negorev et al., 2001). Similarly, the sequestered WRNp is released upon accumulation of damaged DNA in the cell. Our observation that VCP and WRNp dissociate from each other and move away from the nucleolus after CPT treatment supports this suggestion. Together, we propose that VCP may play a role in the response to DNA damage by modulating the nucleolar trafficking of WRNp.
What role does the VCP ATPase play in Werner helicase biology? The P97/VCP hexamer is known to undergo a conformational change upon ATP binding that could be translated into mechanical work (Rouiller et al., 2000), such as protein complex dissociation. Other AAA proteins are thought to function in a similar manner (Ogura and Wilkinson, 2001). When DNA damage has occurred (from CPT, for example) that requires WRNp elsewhere (like in DNA repair complexes), VCP binds ATP and dissociates the protein complex, enabling WRNp to translocate from the nucleolus. We propose that VCP recruits or sequesters WRNp to the nucleolus, to be released as required through VCP action. It is probable that VCP is involved in more than one nuclear pathway and that the elucidation of the VCP nuclear interactions should provide important insights into normal nuclear function and nuclear response to DNA damage.
Acknowledgments
This article is dedicated to the memory of Professor Shmaryahu Blumberg, who suddenly passed away in December 2001. We thank Drs. Michael Gottesman, Vilhelm Bohr, and Mirit I. Aladjem for critical reading of the manuscript. This work was in part sponsored by grants GM-54729 from the National Institutes of Health and RG 183/1999-M from the Human Frontier Science Program, awarded to M.L.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–02–0111. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-02-0111.
Abbreviations used: AAA, ATPases associated with diverse cellular activities; CPT, camptothecin; DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride; ER, endoplasmic reticulum; GFP, green fluorescent protein; IP, immunoprecipitate; VCP, valosin-containing protein; WRNp, Werner helicase protein; WS, Werner Syndrome.
References
- Abmayr, S.M., and Workman, J.L. (2001). Unit 12.1 Preparation of nuclear and cytoplasmic extracts from mammalian cells. In: Current Protocols in Molecular Biology Online, ed. F.M. Ausubel, New York: John Wiley & Sons. [DOI] [PubMed]
- Acharya, U., Jacobs, R., Peters, J.M., Watson, N., Farquhar, M.G., and Malhotra, V. (1995). The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell 82, 895–904. [DOI] [PubMed] [Google Scholar]
- Andersen, J.S., Lyon, C.E., Fox, A.H., Leung, A.K., Lam, Y.W., Steen, H., Mann, M., and Lamond, A.I. (2002). Directed proteomic analysis of the human nucleolus. Curr. Biol. 12, 1–11. [DOI] [PubMed] [Google Scholar]
- Blander, G., Kipnis, J., Leal, J.F., Yu, C.E., Schellenberg, G.D., and Oren, M. (1999). Physical and functional interaction between p53 and the Werner's syndrome protein. J. Biol. Chem. 274, 29463–29469. [DOI] [PubMed] [Google Scholar]
- Blander, G., Zalle, N., Leal, J.F., Bar-Or, R.L., Yu, C.E., and Oren, M. (2000). The Werner syndrome protein contributes to induction of p53 by DNA damage. FASEB J. 14, 2138–2140. [DOI] [PubMed] [Google Scholar]
- Brosh, R.M., Jr., Orren, D.K., Nehlin, J.O., Ravn, P.H., Kenny, M.K., Machwe, A., and Bohr, V.A. (1999). Functional and physical interaction between WRN helicase and human replication protein A. J. Biol. Chem. 274, 18341–18350. [DOI] [PubMed] [Google Scholar]
- Brosh, R.M., Jr., von Kobbe, C., Sommers, J.A., Karmakar, P., Opresko, P.L., Piotrowski, J., Dianova, I., Dianov, G.L., and Bohr, V.A. (2001). Werner syndrome protein interacts with human flap endonuclease 1 and stimulates its cleavage activity. EMBO J. 20, 5791–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J.D. (2001). Ribosome biogenesis: stripping for AAAction? Curr. Biol. 11, R710–R712. [DOI] [PubMed] [Google Scholar]
- Coles, M., Diercks, T., Liermann, J., Groger, A., Rockel, B., Baumeister, W., Koretke, K.K., Lupas, A., Peters, J., and Kessler, H. (1999). The solution structure of VAT-N reveals a `missing link' in the evolution of complex enzymes from a simple betaalphabetabeta element. Curr. Biol. 9, 1158–1168. [DOI] [PubMed] [Google Scholar]
- Confalonieri, F., and Duguet, M. (1995). A 200-amino acid ATPase module in search of a basic function. Bioessays 17, 639–650. [DOI] [PubMed] [Google Scholar]
- Cooper, M.P., Machwe, A., Orren, D.K., Brosh, R.M., Ramsden, D., and Bohr, V.A. (2000). Ku complex interacts with and stimulates the Werner protein. Genes Dev. 14, 907–912. [PMC free article] [PubMed] [Google Scholar]
- Dai, R.M., Chen, E., Longo, D.L., Gorbea, C.M., and Li, C.C. (1998). Involvement of valosin-containing protein, an ATPase Co-purified with IkappaBalpha and 26 S proteasome, in ubiquitin-proteasome-mediated degradation of IkappaBalpha. J. Biol. Chem. 273, 3562–3573. [DOI] [PubMed] [Google Scholar]
- Danks, M.K., Garrett, K.E., Marion, R.C., and Whipple, D.O. (1996). Subcellular redistribution of DNA topoisomerase I in anaplastic astrocytoma cells treated with topotecan. Cancer Res. 56, 1664–1673. [PubMed] [Google Scholar]
- Frohlich, K.U., Fries, H.W., Rudiger, M., Erdmann, R., Botstein, D., and Mecke, D. (1991). Yeast cell cycle protein CDC48p shows full-length homology to the mammalian protein VCP and is a member of a protein family involved in secretion, peroxisome formation, and gene expression. J. Cell Biol. 114, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain-Lee, E.L., Obie, C., and Valle, D. (1997). NVL: a new member of the AAA family of ATPases localized to the nucleus. Genomics 44, 22–34. [DOI] [PubMed] [Google Scholar]
- Ghislain, M., Dohmen, R.J., Levy, F., and Varshavsky, A. (1996). Cdc48p interacts with Ufd3p, a WD repeat protein required for ubiquitin-mediated proteolysis in Saccharomyces cerevisiae. EMBO J. 15, 4884–4899. [PMC free article] [PubMed] [Google Scholar]
- Gray, M.D., Shen, J.C., Kamath-Loeb, A.S., Blank, A., Sopher, B.L., Martin, G.M., Oshima, J., and Loeb, L.A. (1997). The Werner syndrome protein is a DNA helicase. Nat. Genet. 17, 100–103. [DOI] [PubMed] [Google Scholar]
- Gray, M.D., Wang, L., Youssoufian, H., Martin, G.M., and Oshima, J. (1998). Werner helicase is localized to transcriptionally active nucleoli of cycling cells. Exp. Cell Res. 242, 487–494. [DOI] [PubMed] [Google Scholar]
- Huang, S., Li, B., Gray, M.D., Oshima, J., Mian, I.S., and Campisi, J. (1998). The premature ageing syndrome protein, WRN, is a 3′→5′ exonuclease. Nat. Genet. 20, 114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indig, F.E., Diaz-Gonzalez, F., and Ginsberg, M.H. (1997). Analysis of the tetraspanin CD9-integrin alphaIIbbeta3 (GPIIb-IIIa) complex in platelet membranes and transfected cells. Biochem. J. 327, 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon, D., Roberts, B.L., Richardson, W.D., and Smith, A.E. (1984). A short amino acid sequence able to specify nuclear location. Cell 39, 499–509. [DOI] [PubMed] [Google Scholar]
- Latterich, M., Frohlich, K.U., and Schekman, R. (1995). Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell 82, 885–893. [DOI] [PubMed] [Google Scholar]
- Latterich, M., and Schekman, R. (1994). The karyogamy gene KAR2 and novel proteins are required for ER-membrane fusion. Cell 78, 87–98. [DOI] [PubMed] [Google Scholar]
- Lebel, M., Spillare, E.A., Harris, C.C., and Leder, P. (1999). The Werner syndrome gene product co-purifies with the DNA replication complex and interacts with PCNA and topoisomerase I. J. Biol. Chem. 274, 37795–37799. [DOI] [PubMed] [Google Scholar]
- Li, B., and Comai, L. (2000). Functional interaction between Ku and the Werner syndrome protein in DNA end processing. J. Biol. Chem. 275, 28349–28352. [DOI] [PubMed] [Google Scholar]
- Lin, A., Patel, S., and Latterich, M. (2001). Regulation of organelle membrane fusion by Pkc1p. Traffic 2, 698–704. [DOI] [PubMed] [Google Scholar]
- Madeo, F., Schlauer, J., and Frohlich, K.U. (1997). Identification of the regions of porcine VCP preventing its function in Saccharomyces cerevisiae. Gene 204, 145–151. [DOI] [PubMed] [Google Scholar]
- Madeo, F., Schlauer, J., Zischka, H., Mecke, D., and Frohlich, K.U. (1998). Tyrosine phosphorylation regulates cell cycle-dependent nuclear localization of Cdc48p. Mol. Biol. Cell 9, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak, R.A., Lombard, D.B., Johnson, F.B., and Guarente, L. (1998). Nucleolar localization of the Werner syndrome protein in human cells. Proc. Natl. Acad. Sci. USA 95, 6887–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, H.H., Shorter, J.G., Seemann, J., Pappin, D., and Warren, G. (2000). A complex of mammalian ufd1 and npl4 links the AAA-ATPase, p97, to ubiquitin and nuclear transport pathways. EMBO J. 19, 2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir, D., Stewart, S.E., Osmond, B.C., and Botstein, D. (1982). Cold-sensitive cell-division-cycle mutants of yeast: isolation, properties, and pseudoreversion studies. Genetics 100, 547–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, J.M., Meyer, H.H., Ruhrberg, C., Stamp, G.W., Warren, G., and Shima, D.T. (1999). The mouse p97 (CDC48) gene. Genomic structure, definition of transcriptional regulatory sequences, gene expression, and characterization of a pseudogene. J. Biol. Chem. 274, 10154–10162. [DOI] [PubMed] [Google Scholar]
- Negorev, D., Ishov, A.M., and Maul, G.G. (2001). Evidence for separate ND10-binding and homo-oligomerization domains of Sp100. J. Cell Sci. 114, 59–68. [DOI] [PubMed] [Google Scholar]
- Neuwald, A.F., Aravind, L., Spouge, J.L., and Koonin, E.V. (1999). AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9, 27–43. [PubMed] [Google Scholar]
- Ogburn, C.E., Oshima, J., Poot, M., Chen, R., Hunt, K.E., Gollahon, K.A., Rabinovitch, P.S., and Martin, G.M. (1997). An apoptosis-inducing genotoxin differentiates heterozygotic carriers for Werner helicase mutations from wild-type and homozygous mutants. Hum. Genet. 101, 121–125. [DOI] [PubMed] [Google Scholar]
- Ogura, T., and Wilkinson, A.J. (2001). AAA+ superfamily ATPases: common structure-diverse function. Genes Cells 6, 575–597. [DOI] [PubMed] [Google Scholar]
- Opresko, P.L., von Kobbe, C., Laine, J.P., Harrigan, J., Hickson, I.D., and Bohr, V.A. (2002). Telomere-binding protein TRF2 binds to and stimulates the Werner and Bloom syndrome helicases. J. Biol. Chem. 277, 41110–41119. [DOI] [PubMed] [Google Scholar]
- Patel, S., and Latterich, M. (1998). The AAA team: related ATPases with diverse functions. Trends Cell Biol. 8, 65–71. [PubMed] [Google Scholar]
- Patel, S.K., Indig, F.E., Olivieri, N., Levine, N.D., and Latterich, M. (1998). Organelle membrane fusion: a novel function for the syntaxin homolog Ufe1p in ER membrane fusion. Cell 92, 611–620. [DOI] [PubMed] [Google Scholar]
- Peters, J.M., Harris, J.R., Lustig, A., Muller, S., Engel, A., Volker, S., and Franke, W.W. (1992). Ubiquitous soluble Mg(2+)-ATPase complex. A structural study. J. Mol. Biol. 223, 557–571. [DOI] [PubMed] [Google Scholar]
- Peters, J.M., Walsh, M.J., and Franke, W.W. (1990). An abundant and ubiquitous homo-oligomeric ring-shaped ATPase particle related to the putative vesicle fusion proteins Sec18p and NSF. EMBO J. 9, 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichierri, P., Franchitto, A., Mosesso, P., and Palitti, F. (2000). Werner's syndrome cell lines are hypersensitive to camptothecin-induced chromosomal damage. Mutat. Res. 456, 45–57. [DOI] [PubMed] [Google Scholar]
- Pommier, Y., Pourquier, P., Fan, Y., and Strumberg, D. (1998). Mechanism of action of eukaryotic DNA topoisomerase I and drugs targeted to the enzyme. Biochim. Biophys. Acta 1400, 83–105. [DOI] [PubMed] [Google Scholar]
- Poot, M., Gollahon, K.A., and Rabinovitch, P.S. (1999). Werner syndrome lymphoblastoid cells are sensitive to camptothecin-induced apoptosis in S-phase. Hum. Genet. 104, 10–14. [DOI] [PubMed] [Google Scholar]
- Poot, M., Hoehn, H., Runger, T.M., and Martin, G.M. (1992). Impaired S-phase transit of Werner syndrome cells expressed in lymphoblastoid cell lines. Exp. Cell Res. 202, 267–273. [DOI] [PubMed] [Google Scholar]
- Pourquier, P., and Pommier, Y. (2001). Topoisomerase I-mediated DNA damage. Adv Cancer Res. 80, 189–216. [DOI] [PubMed] [Google Scholar]
- Rabouille, C., Levine, T.P., Peters, J.M., and Warren, G. (1995). An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell 82, 905–914. [DOI] [PubMed] [Google Scholar]
- Robbins, J., Dilworth, S.M., Laskey, R.A., and Dingwall, C. (1991). Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell 64, 615–623. [DOI] [PubMed] [Google Scholar]
- Rossi, F., Labourier, E., Forne, T., Divita, G., Derancourt, J., Riou, J.F., Antoine, E., Cathala, G., Brunel, C., and Tazi, J. (1996). Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature 381, 80–82. [DOI] [PubMed] [Google Scholar]
- Rouiller, I., Butel, V.M., Latterich, M., Milligan, R.A., and Wilson-Kubalek, E.M. (2000). A major conformational change in p97 AAA ATPase upon ATP binding. Mol. Cell 6, 1485–1490. [DOI] [PubMed] [Google Scholar]
- Sakamoto, S., Nishikawa, K., Heo, S.J., Goto, M., Furuichi, Y., and Shimamoto, A. (2001). Werner helicase relocates into nuclear foci in response to DNA damaging agents and co-localizes with RPA and Rad51. Genes Cells 6, 421–430. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Schulte, R.J., Campbell, M.A., Fischer, W.H., and Sefton, B.M. (1994). Tyrosine phosphorylation of VCP, the mammalian homologue of the Saccharomyces cerevisiae CDC48 protein, is unusually sensitive to stimulation by sodium vanadate and hydrogen peroxide. J. Immunol. 153, 5465–5472. [PubMed] [Google Scholar]
- Schwarzacher, H.G., and Wachtler, F. (1993). The nucleolus. Anat. Embryol. 188, 515–536. [DOI] [PubMed] [Google Scholar]
- Shen, J., and Loeb, L.A. (2001). Unwinding the molecular basis of the Werner syndrome. Mech. Ageing Dev. 122, 921–944. [DOI] [PubMed] [Google Scholar]
- Shen, J.C., Gray, M.D., Oshima, J., and Loeb, L.A. (1998). Characterization of Werner syndrome protein DNA helicase activity: directionality, substrate dependence and stimulation by replication protein A. Nucleic Acids Res. 26, 2879–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, J.C., and Loeb, L.A. (2000). The Werner syndrome gene: the molecular basis of RecQ helicase-deficiency diseases. Trends Genet. 16, 213–220. [DOI] [PubMed] [Google Scholar]
- Spillare, E.A., Robles, A.I., Wang, X.W., Shen, J.C., Yu, C.E., Schellenberg, G.D., and Harris, C.C. (1999). p53-mediated apoptosis is attenuated in Werner syndrome cells. Genes Dev. 13, 1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmeier, F., Visintin, R., and Amon, A. (2002). Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase Cell 108, 207–220. [DOI] [PubMed] [Google Scholar]
- Suzuki, N., Shimamoto, A., Imamura, O., Kuromitsu, J., Kitao, S., Goto, M., and Furuichi, Y. (1997). DNA helicase activity in Werner's syndrome gene product synthesized in a baculovirus system. Nucleic Acids Res. 25, 2973–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, N., Shiratori, M., Goto, M., and Furuichi, Y. (1999). Werner syndrome helicase contains a 5′[arrow]3′ exonuclease activity that digests DNA and RNA strands in DNA/DNA and RNA/DNA duplexes dependent on unwinding. Nucleic Acids Res. 27, 2361–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T., Shiratori, M., Furuichi, Y., and Matsumoto, T. (2001). Diverged nuclear localization of Werner helicase in human and mouse cells. Oncogene 20, 2551–2558. [DOI] [PubMed] [Google Scholar]
- Visintin, R., Hwang, E.S., and Amon, A. (1999). Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature 398, 818–823. [DOI] [PubMed] [Google Scholar]
- von Kobbe, C., and Bohr, V.A. (2002). A nucleolar targeting sequence in the Werner syndrome protein resides within residues 949–1092. J. Cell Sci. 115, 3901–3907. [DOI] [PubMed] [Google Scholar]
- Yu, C.E., et al. (1996). Positional cloning of the Werner's syndrome gene. Science 272, 258–262. [DOI] [PubMed] [Google Scholar]
- Zhang, H., Wang, Q., Kajino, K., and Greene, M.I. (2000a). VCP, a weak ATPase involved in multiple cellular events, interacts physically with BRCA1 in the nucleus of living cells. DNA Cell Biol. 19, 253–263. [DOI] [PubMed] [Google Scholar]
- Zhang, X., et al. (2000b). Structure of the AAA ATPase p97. Mol. Cell 6, 1473–1484. [DOI] [PubMed] [Google Scholar]