Abstract
The small Ran GTPase, a key regulator of nucleocytoplasmic transport, is also involved in microtubule assembly and nuclear membrane formation. Herein, we show by immunofluorescence, immunoelectron microscopy, and biochemical analysis that a fraction of Ran is tightly associated with the centrosome throughout the cell cycle. Ran interaction with the centrosome is mediated by the centrosomal matrix A kinase anchoring protein (AKAP450). Accordingly, when AKAP450 is delocalized from the centrosome, Ran is also delocalized, and as a consequence, microtubule regrowth or anchoring is altered, despite the persisting association of γ-tubulin with the centrosome. Moreover, Ran is recruited to Xenopus sperm centrosome during its activation for microtubule nucleation. We also demonstrate that centrosomal proteins such as centrin and pericentrin, but not γ-tubulin, AKAP450, or ninein, undertake a nucleocytoplasmic exchange as they concentrate in the nucleus upon export inhibition by leptomycin B. Together, these results suggest a challenging possibility, namely, that centrosome activity could depend upon nucleocytoplasmic exchange of centrosomal proteins and local Ran-dependent concentration at the centrosome.
INTRODUCTION
The small GTPase Ran is a highly conserved protein that controls various cellular processes, the best characterized of which is the nucleocytoplasmic transport. Ran modulates the assembly and disassembly of import and export complexes, depending on its guanine nucleotide-bound state (Mattaj and Englmeier, 1998; Gorlich and Kutay, 1999). Nucleotide exchange and hydrolysis on Ran are catalyzed by regulator of chromosome condensation (RCC1) and Ran GTPase-activating protein (RanGAP), respectively; the rate of nucleotide turnover is further modulated by Ran binding protein 1 (RanBP1), a Ran-interacting protein that increases hydrolysis and inhibits nucleotide exchange (Bishoff and Ponstingl, 2001). During interphase, Ran regulators are localized in distinct subcellular compartments, i.e., RanGAP and RanBP1 in the cytoplasm and RCC1 in the nucleus, so that RanGTP is essentially generated in the nucleus and RanGDP in the cytoplasm (Kunzler and Hurt, 2001).
In the past few years, two additional roles of Ran have been identified: 1) nuclear envelope reconstitution at the mitosis-to-interphase transition (Hetzer et al., 2000; Zhang and Clarke, 2000); and 2) aster and spindle formation in mitosis. In Xenopus egg extract, Ran-GTP induces aster and spindle assembly even in the absence of centrosome and DNA (Carazo-Salas et al., 1999; Kalab et al., 1999; Ohba et al., 1999; Wilde and Zheng, 1999; Zhang et al., 1999).
Such distant functions as nucleocytoplasmic transport and microtubule array formation are ensured by a similar mechanism: during interphase, Ran-GTP destabilizes importin-containing complexes that regulate protein translocation through nuclear pores; in mitosis, upon nuclear envelope breakdown, Ran-GTP produced in the vicinity of the chromosomes, similarly dissociates complexes that contain aster-promoting factors, such as NuMA (Nachury et al., 2001; Wiese et al., 2001) and TPX2 (Gruss et al., 2001), which have been independently shown to act in spindle pole formation in Xenopus egg extracts (Merdes et al., 1996; Wittmann et al., 2000). In the same mitotic model system, Ran-GTP also acts on microtubule dynamics by stabilizing microtubule plus-ends (Carazo-Salas et al., 2001; Wilde et al., 2001). These regulatory roles of Ran have largely been characterized in egg extracts, whereas their physiological significance in cells is comparatively less understood. In mammalian cells, perturbation of the Ran activity by overexpressing RanBP1 yields multipolar spindles (Guarguaglini et al., 2000) by disrupting centrosome cohesion (Di Fiore et al., 2003). In Schizosaccaromyces pombe, a mutant allele of the Ran homolog, Spi1–25p, induces defects in the microtubular cytoskeleton in interphase (Fleig et al., 2000). These data suggest that Ran is implicated in the control of microtubule functions and spindle pole and/or centrosome-associated activities.
The centrosome is the major microtubule-organizing center in higher eukaryotes and consists of a pair of centrioles surrounded by the centrosomal matrix. The microtubule nucleation capacity is closely associated with the centrosome, where proteins such as γ-tubulin (Stearns et al., 1991; Moudjou et al., 1996) and γ-tubulin–associated proteins (Martin et al., 1998; Murphy et al., 1998; Tassin et al., 1998; Murphy et al., 2001) are located, as part of the γ-tubulin ring complex (γ-TuRC). Both centrioles in the centrosome can nucleate microtubules, but only the mother centriole maintains an array of focused microtubules (Piel et al., 2000), because it contains appendages, where ninein, a protein required for microtubule anchorage, is localized (Mogensen et al., 2000). Matrix proteins such as a kinase anchoring protein (AKAP450) and pericentrin/kendrin are huge scaf-folding proteins that anchor several regulatory proteins (Witczak et al., 1999; Takahashi et al., 1999).
Herein, we demonstrate that Ran is a core centrosomal component. Immunoprecipitation experiments demonstrate that Ran interacts with the centrosomal AKAP450 isoform. Accordingly, when AKAP450 is delocalized from the centrosome, Ran is also delocalized. In these cells, microtubule regrowth is altered, despite the persisting association of γ-tubulin with the centrosome. Moreover Ran is recruited to Xenopus sperm centrosome during activation of microtubule nucleation. Together, these results suggest that Ran–AKAP450 complex is required for crucial centrosomal functions such as microtubule nucleation and anchorage during interphase. We report also after blocking the nuclear export by leptomycin B that some centrosomal proteins such as centrin and pericentrin/kendrin accumulate into the nucleus. Together, the centrosomal localization of Ran and the nucleus cytoplasmic shuttling of some centrosomal proteins could provide the basis for coupling centrosome activity and nucleocytoplasmic exchange.
MATERIALS AND METHODS
Cell Cultures
HeLa cells were grown in DMEM supplemented with 10% fetal calf serum. Human lymphoblastic KE37 cells were grown in RPMI medium, supplemented with 7% fetal calf serum. Isolated pillar cells were obtained as described previously (Mogensen et al., 1997).
Microtubule stabilization experiments were carried out with HeLa cells incubated in 0.5 μM Taxol for 16 h at 37°C.
Centrin 1 green fluorescent protein (GFP) HeLa cells or HeLa cells were treated for 3 h with leptomycin B at 20 ng/ml (gift of M. Yoshida, University of Tokyo, Tokyo, Japan). Centrin GFP living cells were observed directly, whereas HeLa cells were fixed with paraformaldehyde (PAF) and processed for immunofluorescence (IF).
Antibodies
Monoclonal antibody (mAb) anti-Ran was purchased from BD Transduction Laboratories (Lexington, KY) and mAb α-tubulin from Amersham Biosciences (Piscataway, NJ). The rabbit polyclonal antibody directed against the native form of Ran-GTP was a gift of I. Macara (University of Virginia School of Medicine, Charlottesville, VA) (Richards et al., 1995). Two antibodies directed against γ-tubulin were used: a polyclonal (Tassin et al., 1998) or a mAb (GTU88; Sigma-Aldrich. St. Louis, MO). HsSpc98p was recognized with a polyclonal antibody (Tassin et al., 1998). The open reading frame of the AKAP450 cDNA corresponds to 50 exons and the protein is present in cells as several isoforms, two of them being recognized by two different antibodies obtained. A mAb, CTR453, that recognizes a sequence on exon 28 in the central domain of AKAP450 decorates strictly the centrosome (Bailey et al., 1989) such as rabbit serum 0013 (Keryer et al., 1993). Both antibodies are human specific. The polyclonal antibody a24 is directed against exons 24–27 and labels the centrosome proper like mAb CTR453 or serum 0013 but in addition reveals an extensive pericentrosomal Golgi-like network.
Polyclonal anti-ninein (Bouckson-Castaing et al., 1996) and polyclonal antipericentrin (Babco, Richmond,CA) were also used.
Immunofluorescence Microscopy and Data Processing
Cells were fixed in 4% PFA for 15 min at room temperature followed by either a permeabilization with Triton X-100 or postfixed by methanol for 6 min at –20°C Alternatively, cells were fixed with methanol for 6 min at –20°C or permeabilized for 1 min in PHEM buffer containing 1% Triton X-100 before fixation and processed for IF as described previously (Tassin et al., 1998). After immunostaining, cells were imaged on a Leica DMRXA microscope and fluorescence intensities measured automatically by MetaMorph software.
Permeabilized Xenopus sperm heads were prepared according to Murray (1991). For IF, sperm heads were sedimented on poly-lysine–coated coverslips, fixed in methanol, and processed for IF as described above. For sperm centrosome activation, sperm heads were incubated for 20 min in Xenopus egg extracts as described previously (Felix et al., 1994), centrifuged over a 25% glycerol cushion at 1700 × g for 10 min, and fixed with methanol.
Immunogold Electron Microscopy
Isolated nucleus–centrosomal complexes from KE37 cells (Maro and Bornens, 1980) were sedimented onto coverslips (400 × g, 15 min, 4°C) and fixed with 3% PFA/0.1% glutaraldehyde in phosphate-buffered saline (PBS). The coverslips were washed with PBS, incubated for 5 min in PBS/0.05M glycine, and blocked for 15 min in PBS/3% bovine serum albumin/0.1% Tween 20. Monoclonal anti-Ran in blocking buffer was applied for 1 h. After washing and reblocking (5 min), rabbit anti-mouse IgG was applied. For detection, we used protein A–10-nm gold for 40 min. After several washes with PBS/Tween 20, the coverslips were postfixed with 2% glutaraldehyde/1% OsO4, 30 min, incubated in 1% uranylacetate in 50% ethanol (10 min), dehydrated, and flat-embedded using standard protocols. In control experiments the primary antibody was omitted.
Cellular Fractionation
Proteins were fractionated from KE37 cells as described previously (Tassin et al., 1998).
Centrosomes were isolated from KE37 cells as described previously (Moudjou and Bornens, 1994). Pelleted centrosomes were incubated for 1 h at 4°C with extraction buffer (20 mM Tris-HCl pH 7.4, 2 mM EDTA) alone, or alternatively, in the same extraction buffer containing the following: 1) 0.5% NP-40 (1D buffer); (2) 0.5% NP-40 and 0.5% deoxycholate (DOC; 2D buffer); 3) 0.5% NP-40, 0.5% DOC, and 0.1% SDS (3D buffer); or 4) 8 M urea. Proteins were then fractionated into pellet (P) and supernatant (S) by centrifugation at 10,000 × g for 15 min.
Immunoprecipitation Experiments
Proteins from HeLa cells were extracted with 1D buffer (Tassin et al., 1998). After a centrifugation to eliminate trace aggregates, primary antibodies preadsorbed to protein G-Sepharose beads (Pharmacia, Peapack, NJ) were added to the sample, and the mixture was incubated for 2 h at 4°C. Protein G-Sepharose beads were then sedimented. The sedimented protein G-Sepharose beads were washed five times with buffer, and supernatants were precipitated with cold methanol. Proteins were then processed for SDS-PAGE electrophoresis and Western blotting.
Transfection of C-ter AKAP450-GFP
C-ter domain of human AKAP450 (aa 3707–3908) was amplified by polymerase chain reaction by using primers that incorporated restriction sites for cloning into GFP expression vector, the Advantage polymerase mix (BD Biosciences Clontech, Palo Alto, CA) and AKAP450 cDNA as template. The polymerase chain reaction products were subcloned into the corresponding sites of pEGFP-C3 (BD Biosciences Clontech) to yield vectors that direct expression of AKAP450 fragments fused to enhanced GFP. Exponentially growing HeLa cells were transfected by electroporation and seeded on collagen-fibronectin–coated coverslips for IF analyses. For microtubule regrowth experiments, microtubules were depolymerized using 5 μM nocodazole on ice. After 2 h, nocodazole was washed out, and microtubules were allowed to regrow for 5 min. Cells were fixed and processed for IF as described above.
RESULTS
Ran Localization at the Centrosome
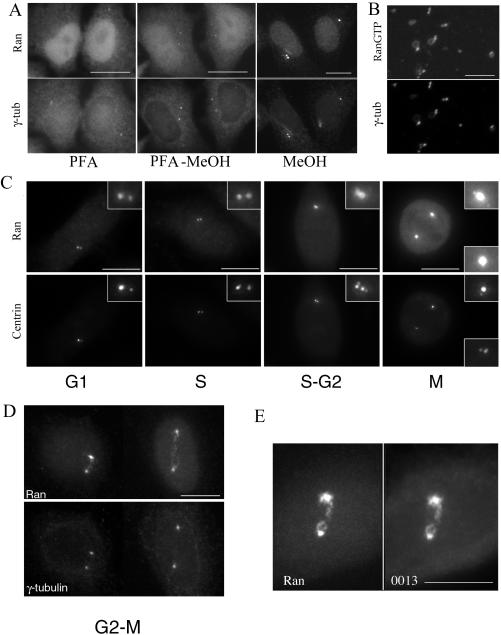
To assess whether Ran contributes to the control of microtubule organization during interphase, we reexamined its localization in human cells by IF with Ran mAb. Cells fixed with cold methanol or permeabilized with Triton X-100 before methanol or PFA fixation revealed a clear centrosomal staining in addition to the nuclear localization (Figure 1A). To confirm our centrosomal localization, various fixations were performed. Using PFA fixation, a bright Ran fluorescence staining was observed in the cytoplasm as well as in the nucleus as described previously (Ren et al., 1993; Zhang et al., 1999; Guarguaglini et al., 2000). However, no centrosomal staining could be distinguished, and the centrosomal γ-tubulin labeling was barely visible. PFA fixation followed by methanol allowed us to clearly visualize the centrosome with Ran mAb, whereas γ-tubulin staining seemed more conspicuous at the centrosome.
Figure 1.
Association of Ran with the centrosome. (A) Immunolocalization of Ran on HeLa cells by using the monoclonal Ran antibody after different fixation protocols. Note that after a PAF fixation, a bright fluorescent staining was observed both in the nucleus and in the cytoplasm. The γ-tubulin centrosome staining was poorly visible in these conditions. After PAF fixation followed by a postfixation with methanol at –20°C, the centrosome was detected with Ran antibody. The clearest centrosome staining was obtained using a direct methanol fixation (B) Decoration of nucleus–centrosome complexes from human lymphoblasts KE37 cells with the polyclonal AR12 antibody directed against RanGTP and a monoclonal anti-γ-tubulin antibody. Note that RanGTP was enriched at centrosomes while a faint staining around the nuclear remnant was also observed. (C and D) Ran was localized at the centrosome throughout the cell cycle as observed in centrin 1-GFP–expressing HeLa cells. Ran staining was larger than centrin staining, suggesting its presence in the pericentriolar matrix. In G2-M cells, Ran decorated both centrosomes and the link between them, whereas γ-tubulin was restricted to the centrosomes. (E) In G2-M cells, Ran colocalized perfectly with centrosomal AKAP450 as observed with serum 0013. Bars, 10 μm.
The centrosomal Ran staining was further confirmed using a polyclonal rabbit antibody that recognizes the Ran-GTP bound form (Richards et al.,1995; Figure 1B). The labeling was performed on nucleus–centrosome complexes before fixation to access directly the antigen. A strong centrosomal staining was observed with the antibody, as well as a faint staining of the nuclear membrane remnant (Figure 1B): these experiments indicate that Ran in the GTP-bound conformation can be detected in isolated centrosomes.
Using centrin-GFP as a centriole marker to monitor cell cycle progression (Piel et al., 2000), we observed that Ran localized at centrosomes throughout the cell cycle and was more intense in G2 and mitotic cells (Figure 1C). In G2/M cells, the mAb Ran distinctly labeled material connecting the two separating centrosomes, whereas γ-tubulin was restricted to the centrosome (Figure 1D). A similar distribution was previously observed for the centrosomal specific isoform of AKAP450 visualized by the mAb CTR453 or the serum 0013 (Keryer et al., 1993). Accordingly, in a double-labeling experiment, centrosomal Ran colocalized with the centrosome-specific AKAP450 isoform (Figure 1E), indicating its presence in the pericentriolar matrix, rather than restricted to centrioles.
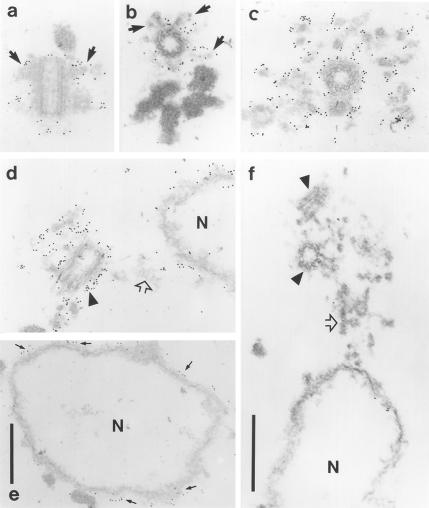
To confirm the pericentriolar localization of Ran, immunogold electron microscopy was performed on nucleus–centrosomal complexes isolated from KE37 cells (Figure 2). Gold particles were predominantly detected in the matrix surrounding centrioles (Figure 2, c and d) and on the centriolar subdistal appendages (see arrows in Figure 2, a and b). The density of gold particles at centrosomes was high compared with that observed on the surface of nuclear ghosts (Figure 2, d and e). Together, immunolocalization studies indicate that a fraction of Ran associates with the pericentriolar matrix.
Figure 2.
Immunogold electron microscopy of nucleus–centrosome complexes from KE37 cells. Anti-Ran (a–e) and control (f) omitting the primary antibody. Gold particles were observed on the centriolar appendages (a and b, arrows), the surface of centrioles (d, arrowhead), and in the pericentriolar matrix (c and d). Some gold particles were detected on the surface of the nuclear remnants, confirming the IF data (d, arrows in e). Open arrows in d and f, material-linking centrioles with the nucleus; arrowheads in f, centrioles. Bars, 0.5 μm (in f for a–d, and f).
Ran Is Strongly Associated with the Centrosome
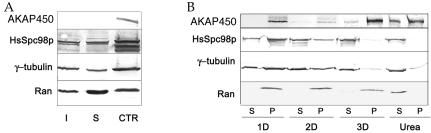
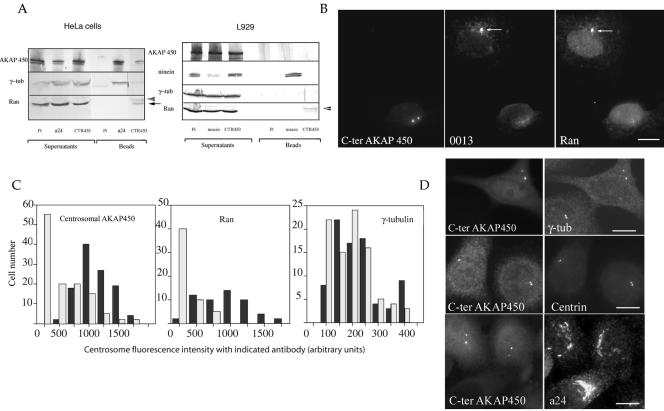
Triton X-100–soluble and –insoluble protein fractions from KE37 cells, as well as proteins from isolated centrosomes, were analyzed for their content in Ran. Although the major part of Ran was found in the soluble fraction, a significant amount was also associated with insoluble structures. Strikingly, Ran was as enriched in the centrosomal fraction as γ-tubulin or HsSpc98p (Figure 3A). Exposing isolated centrosomes to various solubilizing treatments, including detergents and chaotropic agents assessed the strength of this association. We found that only 8 M urea resulted in complete solubilization of Ran, whereas γ-tubulin and HsSpc98p can partly be solubilized by NP-40 and are completely extracted in 3D buffer (Figure 3B). Interestingly, the centrosome-specific AKAP450 isoform is also highly insoluble, about half of the protein being solubilized in 8 M urea (Figure 3B; Klotz et al., 1990).
Figure 3.
Ran is enriched at the centrosome. (A) Western blot analysis of Triton X-100–soluble (S) and insoluble (I) protein fractions from KE37 cells and a highly enriched centrosomal fraction (CTR). A significant fraction of Ran was associated with insoluble structures and Ran was enriched in the centrosomal fraction in the same proportion as γ-tubulin or HsSpc98p. Centrosomal AKAP450 detected by rabbit serum 0013 is observed only in the centrosome fraction. B: Biochemical extraction of the centrosome associated Ran. Soluble (S) and insoluble (P) centrosomal protein fractions obtained in different extraction conditions (see MATERIALS AND METHODS) were immunodetected with anti-Ran, anti-γ-tubulin, or anti-HsSpc98p antibodies or rabbit serum 0013. Only highly denaturing conditions (8 M urea) could solubilize centrosomal Ran and part of centrosomal AKAP450.
The presence of MgCl2 and of GTP or GDP nucleotides, in the extraction buffer, does not modify the solubilization properties of Ran. These results demonstrate a tight association of Ran with the centrosome, which can only be disrupted in denaturing conditions.
Ran Colocalizes with Microtubule Minus-Ends
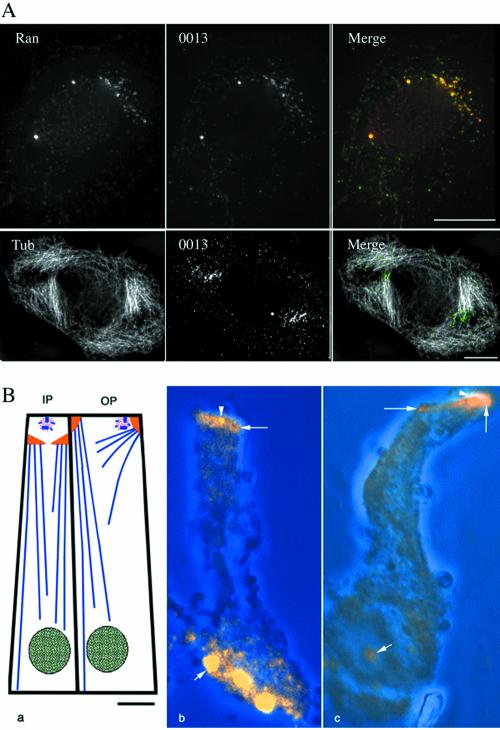
We then investigated whether the localization of Ran at centrosomes in vivo was affected by changes in microtubule organization. Cells treated with nocodazole (a microtubule-depolymerizing drug) showed a similar centrosomal and nuclear Ran staining as untreated cells (our unpublished data). Cells treated with Taxol (a microtubule-stabilizing drug) reorganized their interphase microtubules array into polarized bundles. Under Taxol treatment, Ran was present at the centrosome but also accumulated in one or several clouds of dots, which colocalized with AKAP450 (Figure 4, top row). These dots were found to be localized at one end of microtubule bundles induced by Taxol (Figure 4A, bottom row).
Figure 4.
Ran localizes with microtubule minus-ends. (A) HeLa cells treated with Taxol were stained with the rabbit serum 0013, and either with anti-Ran, or with anti-α-tubulin antibodies. In addition to the centrosome a cloud of dots was labeled using anti-Ran antibody. This labeling colocalized with rabbit serum 0013, which recognizes centrosomal AKAP450. Double staining with the rabbit serum 0013 and anti-α-tubulin antibodies revealed that the punctate staining was localized at one microtubule end. Bar, 10 μm. (B) Ran localization in isolated inner and outer pillar cells from organ of corti at day 6, when most of the microtubule minus-ends are concentrated at the noncentrosomal apical sites. a, schematic diagram of the microtubules organization in the inner and outer pillar cells with the centrosome in pink, microtubules in blue, apical sites in yellow, and the nuclei in green. b and c, projections of optical sections through the lateral length of an inner pillar cell (b) and an outer pillar cell (c) immunolabeled with the anti-Ran antibody. The phase contrast images (blue) have been superimposed on the fluorescent images. Ran was concentrated at centrosomes (arrowheads) and at apical sites (large arrows). Ran staining in the nucleus (small arrows) varied from very strong (b) to weak (c).
This result prompted us to investigate whether Ran localized at the minus-ends of microtubule. To address this question, we chose differentiated polarized epithelial cells, i.e., cochlear-supporting cells, in which microtubules minus-ends are anchored at apical sites (Mogensen, 1999). Moreover, in these cells the two functionally distinct domains specialized in nucleation and anchorage of microtubules, respectively, are spatially separated, thus providing a useful experimental system to determine whether Ran preferentially associates with either of these domains. Proteins responsible for microtubule nucleation such as γ-tubulin and pericentrin remain confined to the centrosome (Mogensen et al., 1997), whereas ninein (Mogensen et al., 2000) and dynactin (Schroer., 2001) are present at the centrosome and at the apical noncentrosomal sites in these polarized epithelial cells.
In these cells, Ran was found at the centrosome and at the apical sites where thousands of microtubule minus-ends are concentrated (Figure 4B), suggesting that Ran may have a role in anchoring or capping microtubule minus-ends that is independent of microtubule nucleation.
Ran Associates with Centrosomal AKAP450
To confirm the potential association of Ran with centrosomal AKAP450 suggested by IF experiments, we carried out coimmunoprecipitation (IP) experiments by using purified immunoglobulins directed against the centrosomal-specific isoform of AKAP450 (CTR453). Part of Ran and a tiny amount of γ-tubulin coprecipitated with centrosomal-specific isoform of AKAP450 (Figure 5A, lane CTR453). This coprecipitation was specific: CTR453, which does not recognize AKAP450 in the mouse cell line L929, coprecipitated neither AKAP450 nor Ran. In these cells, an antibody directed against the other centrosomal protein ninein, associated with microtubule minus-ends, precipitated large amounts of ninein but no Ran (Figure 5, lane ninein). These results suggest that Ran is part of a complex containing the centrosomal-specific AKAP450 isoform. Because AKAP450 has recently been shown to be in complex with γ-tubulin (Takahashi et al., 2002), IPs were carried out with polyclonal a24 IgGs directed against another isoform of AKAP450, which is not restricted to the centrosome (Keryer et al., 2003). Interestingly, this antibody precipitated γ-tubulin in addition to AKAP450, whereas no Ran was detected in the immunoprecipitate. These results suggest that two distinct AKAP450-containing complexes are present at the centrosome: a conspicuous complex containing a specific AKAP450 isoform and γ-tubulin and a minor complex containing one another AKAP450 isoform and Ran.
Figure 5.
Ran is associated with centrosomal AKAP450. (A) IP experiments were carried out on HeLa or L929 cells by using preimmune (PI), anti-AKAP450 (mAb CTR453 or polyclonal a24), or anti-ninein IgGs. The immunoprecipitated proteins are indicated as “beads”, whereas nonassociated components are referred as “supernatants”. IP results were analyzed by using antibodies directed against Ran, γ-tubulin, ninein, or AKAP450. CTR453 IgGs precipitated AKAP450 protein and a small fraction of Ran (arrow), but only tiny amount of γ-tubulin. In contrast, a24 precipitated large amount of AKAP450 and γ-tubulin, but no Ran. Arrowhead, IgGs light chains. In L929 cells, where CTR453 did not recognize AKAP450, no AKAP450 or Ran was precipitated. This suggested that the fraction of Ran that precipitated with AKAP450 in HeLa cells was not a contaminant. (B) In C-ter AKAP450-GFP–expressing cells, the endogenous centrosomal AKAP450 stained by rabbit serum 0013 and Ran concomitantly disassociated from the centrosome. The arrow pointed to a centrosome costained by serum 0013 and anti-Ran in a nontransfected cell. Bar, 10 μm. (C) Quantitative analysis of centrosomal AKAP450, Ran, and γ-tubulin immunostainings. Cells (250) expressing the C-ter AKAP450 (gray bars) or not (black bars) were scored for each indicated antibody. Centrosome staining was measured and expressed as fluorescence intensities. Note that the number of cells presenting centrosomal AKAP450 and Ran decreased concomitantly in C-ter-AKAP450–expressing cells, whereas it remained stable for γ-tubulin. (D) Cells expressing the C-ter domain of AKAP450 were stained for the centrosome either with anti-γ-tubulin, polyclonal a24 antibody, or centrin. Note that γ-tubulin and centrin still decorated centrosomes suggesting that the targeting of the C-terminal domain of AKAP450 to centrioles did not disrupt the centrosome. The Golgi-like network of AKAP450 was still present. Bars, 10 μm.
To establish whether this interaction could be demonstrated in vivo, we took advantage of an experimental situation in which the endogenous centrosomal AKAP450 was delocalized from the centrioles. The C-terminal domain of AKAP450 (C-ter AKAP450-GFP) when overexpressed is targeted to the centrosome and induces a delocalization of the endogenous centrosomal AKAP450 (Keryer et al., 2003). As expected, in C-ter AKAP450-GFP–expressing cells, the centrosomal-specific AKAP450 isoform and Ran were no longer associated with the centrosome after 24 h (Figure 5, B and C). Interestingly, in these conditions γ-tubulin and centrin remained associated with the centrosome as well as the AKAP450 isoform recognized by a24 antibody (Figure 5D). This result indicates that the dissociation of Ran and AKAP450-centrosomal specific isoform from the centrosome does not lead to the complete disruption of the centrosomal structure. When centrosomal AKAP450 and Ran were dissociated from the centrosome, γ-tubulin remained at the centrosome. This confirms the immunoprecipitation results and indicates that the Ran-centrosomal AKAP450 containing complex is distinct from the γ-TuRC.
Function of the Centrosomal Ran-AKAP450-containing Complex
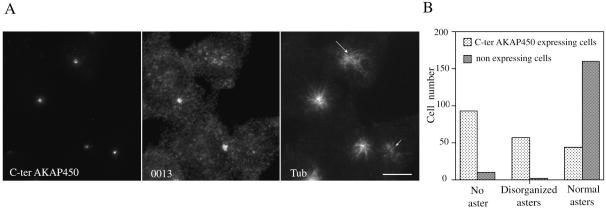
We first studied microtubule regrowth after nocodazole treatment in cells in which the centrosomal Ran–AKAP450 complex was delocalized from the centrosome by expressing the C-ter AKAP450-GFP. In these transfected cells, no asters or disorganized asters were observed in 75% of cells, whereas untransfected cells showed a typical microtubule regrowth pattern (Figure 6B). A triple-labeling experiment allowed us to demonstrate that in C-ter AKAP450-GFP–transfected cells, very few microtubules were observed in the absence of Ran and centrosomal-specific AKAP450 isoform (Figure 6A, small arrow) despite the presence of γ-tubulin at the centrosome (Figure 5, C and D). Other cells, which presented disorganized endogenous centrosome-specific AKAP450 isoform, showed disorganized microtubule regrowth pattern. Conversely, transfected cells that presented a control centrosomal AKAP450 pattern presented typical microtubule asters. These results argue for an involvement of the centrosomal Ran–AKAP450 complex in microtubule anchorage rather than in nucleation.
Figure 6.
Function of AKAP450-Ran–containing complex at the centrosome. (A) Microtubule regrowth was observed after microtubule depolymerization in expressing-C-ter-AKAP450 cells. Centrosomes were stained using rabbit serum 0013 and microtubules by using anti-α-tubulin antibody. Dependent on centrosomal AKAP450 localization, three regrowth patterns were observed: typical microtubule asters (in cells presenting a typical 0013 centrosomal staining), disorganized asters (long arrow, AKAP450 unfocused), and no microtubule asters (small arrow, absence of centrosomal AKAP450). Bars, 10 μm. (B) Quantification of the three patterns of microtubule regrowth. About 200 expressing and nonexpressing C-ter AKAP 450 cells were scored for the presence of asters.
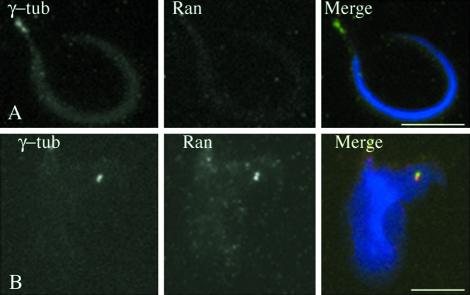
Previous observations by Nachury et al. (2001) showed that in Ran-depleted Xenopus egg extract, nucleation activity of sperm head centrosomes is inhibited. Importantly they demonstrated that the addition of bacterially WT Ran loaded with GDP was able to switch on sperm centrosome activity, suggesting that Ran by itself is important for the sperm centrosome nucleation activity. Sperm centrosomes are incompetent for microtubule nucleation and need to be activated by egg extract to become competent (Felix et al., 1994; Stearns and Kirschner 1994). We wondered whether the inhibition of nucleation activity in Ran-depleted extract reflected a lack of Ran at the sperm centrosome, and hence a requirement for Ran recruitment to induce nucleation activity. Therefore, we reinvestigated the presence of Ran at the centrosome of sperm heads before and after egg extract activation. Sperm cells were double labeled with γ-tubulin and Ran antibodies. As previously described, sperm centrosomes contained γ-tubulin, AKAP450 (our unpublished data) and other centrosomal markers before activation (Stearns and Kirschner 1994; Tassin et al., 1998), whereas no Ran staining was observed (Figure 7A; Zhang et al., 1999). After 20-min incubation in egg extract, Ran was detected at the centrosome (Figure 7B). This result indicates that Ran is specifically recruited from the extract to the centrosome and suggests that it is a necessary step to achieve full activation of the sperm centrosome activity. Together, these results suggest that Ran plays a specific role in the centrosomal activity.
Figure 7.
Xenopus sperm centrosomes recruit Ran from Xenopus egg extract. Decoration of Xenopus sperm centrosomes before (A) or after (B) activation in Xenopus egg extracts by using anti-Ran and anti-γ-tubulin antibodies. Before activation (A), Ran was not observed at the centrosome, whereas γ-tubulin was present. After activation, Ran was recruited at centrosomes and codistributed with γ-tubulin (B). Bars, 10 μm.
Some Centrosomal Proteins Shuttle between the Cytoplasm and the Nuclear Compartment
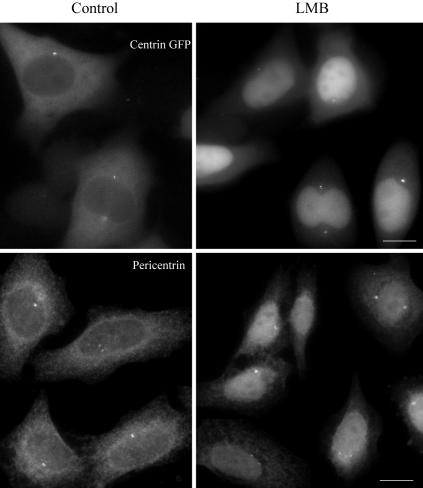
An attractive possibility is that centrosome-associated RanGTP could locally activate centrosomal proteins by dissociating them from a complex with importins/karyopherins. This would allow microtubule nucleation or anchorage to take place in the vicinity of the centriole pair, where Ran is concentrated. Such a mechanism is however difficult to test experimentally by a biochemical approach since candidate centrosomal factors are likely to be minor cellular components. But one prediction is that these centrosomal factors would be bound to importins/karyopherins in the cytoplasm and therefore submitted also to nuclear shuttling. To test this hypothesis, we thought of looking at the cellular distribution of centrosomal proteins when CRM1-dependent protein nuclear export is blocked by leptomycin B (Kudo et al., 1999). Cells were treated with leptomycin B for 3 h and the cellular distribution of a whole series of centrosomal proteins (γ-TuRC components, ninein, AKAP450, and centrin) was monitored by immunofluorescence. Leptomycin B-treated cells accumulated centrin 1-GFP and pericentrin/kendrin into the nucleus (Figure 8), whereas ninein, γ-tubulin, or AKAP450 were not redistributed (our unpublished data). We conclude from these experiments that among the known bona fide centrosomal proteins, at least centrin and pericentrin/kendrin can be regarded as potential cargoes in Ran-dependent nucleocyto-plasmic transport.
Figure 8.
Leptomycin B treatment accumulated centrin and pericentrin into the nucleus. HeLa centrin-GFP cells or HeLa cells were treated with leptomycin B (LMB) for 3 h. Centrin and pericentrin was observed. Bars, 10 μm.
DISCUSSION
Ran is a highly abundant GTPase that is largely localized in the nucleus in a variety of species. Here, we demonstrate that a small fraction of Ran is tightly associated with the centrosome throughout the cell cycle. This was demonstrated by IF using two different antibodies and different fixation protocols as well as by ultrastructural and biochemical analysis of isolated centrosomes. Surprisingly, whereas tagged versions of Ran were targeted to the nucleus they were not observed at the centrosome (our unpublished data), suggesting that the docking to the centrosome was not possible with the tagged version of Ran. As a matter of fact, a Saccharomyces cerevisiae Ran GFP was unable to substitute for the endogenous Ran protein (Quimby et al., 2000).
Cytoplasmic Ran Is Concentrated at the Centrosome
Because this result had not been reported before in spite of numerous studies on Ran distribution, we were eager to rule out the possibility of an artifactual staining of the centrosome. We first confirmed that the PFA fixation used in previous reports did not reveal any centrosomal staining, but instead a strong staining in the nucleus and in the cytoplasm. This high background could suffice to preclude detection of centrosome staining. We then could eliminate that the centrosomal staining observed with three other fixation protocols was artifactual, because anti-Ran antibodies did not reveal any centrosome staining when we overexpressed the C-terminal domain of AKAP450. We demonstrated that in these conditions endogenous centrosomal isoform of AKAP450 was dissociated from the centrosome (see also below), whereas other pericentriolar or centriole markers such as γ-tubulin, centrin, or polyglutamylated tubulin (Bobinnec et al., 1998) were not significantly affected, indicating that the overall centrosome structure was not destroyed. Moreover, after Taxol treatment, we showed that Ran can associate with the ends of the Taxol-induced microtubule bundles.
We further demonstrated biochemically that Ran was enriched in isolated centrosomes and was tightly associated with them, as only highly denaturing conditions could dissociate it. This behavior is that of a core centrosomal component, thus definitively demonstrating that part of cytoplasmic Ran is concentrated at the centrosome. Interestingly, in S. pombe, Ran has been shown to genetically interact with at least one component of the spindle pole body and to control microtubule integrity (Fleig et al., 2000).
The Docking of Ran at the Centrosome
Ran centrosomal staining colocalizes with the centrosomal isoform of AKAP450. In agreement with this observation, Ran and centrosomal AKAP450 are extracted from the isolated centrosomes by similar chemical treatments. Moreover, Ran was dissociated in situ from the centrosome when centrosomal AKAP450 was displaced by overexpression of C-ter AKAP450-GFP, suggesting that Ran might be docked within the pericentriolar matrix through AKAP450. Such a possibility was supported by IP experiments, which indicated that Ran and centrosomal AKAP450 participate in the same protein complex.
Function of Ran-AKAP450-containing Complex at the Centrosome
Ran localization in the centrosomal matrix but also at the mother centriole-associated subdistal appendages, at microtubule ends of Taxol-treated cells as well as at the microtubule-anchoring apical domains in inner ear pillar cells, suggested that Ran might participate in the control of microtubule anchorage at the centrosome. Accordingly, cells in which the Ran-AKAP450–containing complex was displaced from the centrosome have defects in microtubule regrowth pattern, despite the persisting association of γ-tubulin with the centrosome. This suggests that the complex containing the centrosomal specific AKAP450 isoform and Ran plays a role in microtubule regrowth or anchorage. In this experiment, it is difficult to distinguish the function of AKAP450 from that of Ran. AKAP450 is a large coiled-coil protein that anchors numerous regulatory factors at the centrosome (Witczak et al., 1999; Takahashi et al., 1999, 2002). Therefore, delocalizing this specific isoform could by itself influence microtubule nucleation. On the other hand, the important role played by Ran during mitosis in destabilizing importin/karyopherin-containing complexes of proteins necessary for spindle formation (for reviews, see Melchior, 2001; Dasso, 2002) argues for a specific role of Ran on microtubule organization during interphase.
Furthermore, sperm centrosomes, which are unable to nucleate microtubules, contain most centrosomal proteins, including AKAP450, pericentrin, γ-tubulin, and Spc98 but are devoid of Ran. We show that Ran is recruited at the centrosome in egg extract. Moreover, it was previously demonstrated that sperm centrosome activation was inhibited by depletion of Ran from the extracts and could be restored when bacterially expressed Ran is added (Nachury et al., 2001). The functional data obtained by Nachury et al. (2001) and our results show a correlation between Ran accumulation at the centrosome and the sperm centrosome nucleation activity. A definitive demonstration of Ran function at the centrosome will require further work. It has been observed that the nucleation activity of the centrosome increased in a Ran Q69L-containing extract, whereas it decreases slowly with time in a RanT24N-containing extract (Carazo-Salas et al., 2001).
In the present work, RanGTP was detected on unfixed centrosomes by using a specific antibody (Richards et al., 1995). Interestingly, in an independent set of experiments, we have detected a fraction of RanBP1 at the centrosome; when present in excess, centrosome splitting was observed (Di Fiore et al., 2003). A full guanine nucleotide cycle of Ran may take place at the centrosome, despite the absence of Ran GAP1 (Joseph et al., 2002) or RCC1 (Moore et al., 2002) at the centrosome. Noteworthy, the activation of sperm centrosomes in Ran-depleted extract was restored by RanGDP, suggesting that RanGTP was produced at the centrosome (Nachury et al., 2001). The identification of Ran effectors at the centrosome will require further studies. Proteins presenting RCC1 domains have been reported previously (Roig et al., 2002), and it is possible that a centrosomal protein with RCC1 domain acts as a guanine nucleotide exchange factor.
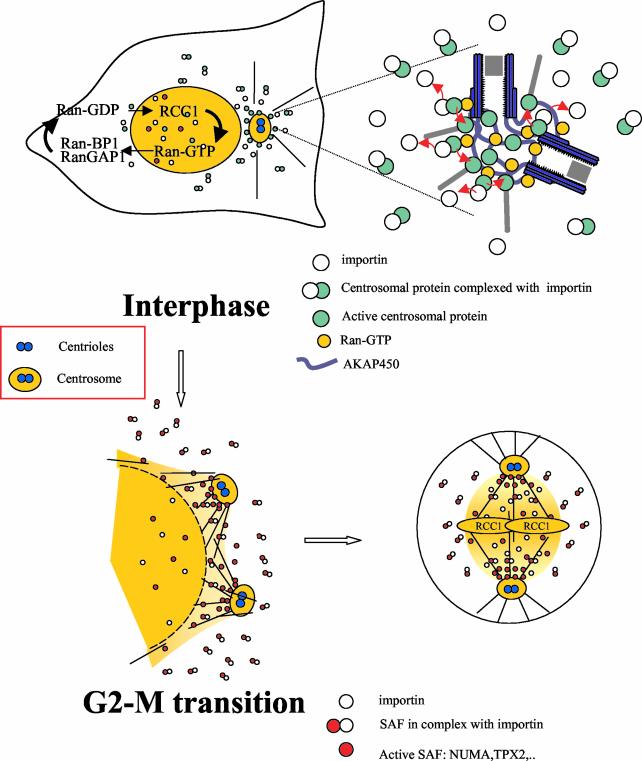
How may Ran control centrosome activity? An attractive possibility, is that centrosome-associated RanGTP could locally destabilize, and therefore activate, complexes between importins/karyopherins and centrosomal proteins important for centrosome activity. This mechanism could control the delivery of proteins involved either in microtubule assembly itself (such as αβ-tubulin dimers or Stathmin), in the control of microtubule nucleation (such as γ-TuRC components), or in the control of microtubule dynamics or anchorage (such as e.g., ninein, AKAP450, and XMAP215). As a result, microtubule nucleation or anchorage would take place in the vicinity of the centriole pair, where Ran is concentrated. It would explain why these activities are not taking place elsewhere in the cytoplasm, where the same components would be in an inactive importin/karyopherin-associated form. This model implies that centrosomal proteins, which are bound to importin/karyopherin, cycle between the cytoplasm and the nucleus. Such a behavior has indeed been observed in this work for two centrosomal proteins: centrin and pericentrin/kendrin. It is interesting to note that pericentrin/kendrin and AKAP450 are two large divergent coiled-coil centrosomal proteins sharing some common domains, including a C-terminal targeting domain (PACT domain; Gillingham and Munro, 2000). Interestingly, pericentrin/kendrin has been shown to be involved in the control of microtubule organization at the centrosome (Doxsey et al., 1994) and is suspected to anchor the γ-TuRC (Flory et al., 2000) in addition to AKAP450 (Takahashi et al., 2002) at the centrosome. We propose from all these data a working model (Figure 9). Whether the nuclear cycling of centrin and pericentrin/kendrin is a futile cycle or is necessary for other functions, will require further investigations.
Figure 9.
A working model for the Ran-dependent control of centrosome activity Top, during interphase, RanGTP is concentrated in the nucleus due to the asymmetric distribution of its regulators allowing the nucleocytoplasmic transport. RanGTP is also concentrated at the centrosome. We propose that some centrosomal proteins important for microtubule nucleation or anchorage are present in the cytoplasm in an inactive form due to their binding to importins/karyopherins. These complexes are transported to the centrosome where they are dissociated from importins/karyopherins by RanGTP allowing their activation (see blowup on the right). This would explain that microtubule nucleation or anchorage is restricted at the centrosome, although a large pool of centrosomal proteins exists in the cytoplasm. Bottom, at prometaphase, nuclear membrane and separating centrosomes are close by. At NEB, RanGTP produced in the vicinity of the chromosome where RCC1 is localized, diffuses in the cytoplasm as a steep gradient. In addition, the concentration of Ran at separating centrosomes increases at the G2-M transition (Figure 1, B and D). Many new microtubules are nucleated at the centrosome, whereas some polymerize close to the nuclear envelope. Spindle assembly factors (SAFs) sequestered in the nucleus also diffuse in the cytoplasm and migrate to the centrosome due to a dynein based-movement. These SAFs are either bound to β-importin (inactive state) or free from β-importin (active state) in the immediate vicinity of the chromosomes and centrosomes. The preferential activation of the SAFs at centrosomes due to Ran concentration would explain the dominant role of the centrosome during bipolar spindle formation.
The accumulation of Ran at the centrosome may also shed some light on how Ran could control mitotic spindle assembly in somatic cells. At the G2-M transition, the nuclear envelope breakdown is initiated close to the centrosome (Beaudouin et al., 2002; Salina et al., 2002) as dynein redistributes at the nuclear membrane (Busson et al., 1998; Gonczy et al., 1999). At nuclear envelope breakdown, two nuclear proteins, NuMA and TPX2 (Carazo-Salas et al., 2001; Nachury et al., 2001; Wiese et al., 2001), are released from a complex containing importin β in a Ran GTP-dependent manner and are able to assemble or stabilize microtubules, thus explaining that the spindle arises in the vicinity of chromosomes (Kalab et al., 2002). Interestingly, NuMA and TPX2 require cytoplasmic dynein for their localization to spindle poles (Merdes et al., 2000; Wittmann et al., 2000). If importin β–NuMA or –TPX2 complexes have a higher probability to be destabilized in the vicinity of the separating centrosomes where Ran is concentrated (Figure 1B), this would kinetically favor the correct assembly of a bipolar spindle on centrosomes over the self-assembly pathway, thus explaining the dominant role of the centrosomes in spindle formation (Heald et al., 1997).
In summary, we have shown that a small fraction of Ran is tightly associated with the centrosome throughout the cell cycle. In addition, cytoplasmic Ran redistributes at microtubule minus-end after Taxol treatment of HeLa cells and concentrates in polarized epithelial cells at apical domains where microtubules are anchored. We also provided the first evidence that some centrosomal proteins shuttle between the cytoplasm and the nucleus. Together, our results strongly suggest that Ran might participate in the control of centrosome activity.
Acknowledgments
We thank J. DeMey, F. Coquelle, and J.B. Sibarita for microscopy facilities and deconvolution; I. Macara for Ran-GTP antibody; and A Paoletti for critical reading of the manuscript. B.D. was supported by a doctoral fellowship from Ministero dell' Istrúzione dell' Università e della Ricerca and Consiglio Nazionale delle Ricerche. This work has been supported by the Centre National de la Recherche Scientifique (Unité Mixte Recherche 144) and Institut Curie, by grants human frontier science program RG0319 to M.B. and by ARC5998 to A.-M.T. M.M. was supported by the Wellcome Trust (grant 049616/Z/96/Z/WRE/MK/JAT) and thanks Birgit Lane for support and use of research facilities.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–11–0773. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-11-0773.
References
- Bailly, E., Doree, M., Nurse, P., and Bornens, M. (1989). p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 8, 3985–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudouin, J., Gerlich, D., Daigle, N., Eils, R., and Ellenberg, J. (2002). Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell 108, 83–96. [DOI] [PubMed] [Google Scholar]
- Bishoff, F.R., and Ponstingl, H. (2001). Ran regulation by RanGEF and Ran-GAP. In: The small GTPase Ran, ed. M. Rush and P. D'Eustachio, Norwell, MA: Kluwer Academic Publishers, 163–176.
- Bobinnec, Y., Khodjakov, A., Mir, L.M., Rieder, C.L., Edde, B., and Bornens, M. (1998). Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 143, 1575–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouckson-Castaing, V., Moudjou, M., Ferguson, D.J., Mucklow, S., Belkaid, Y., Milon, G., and Crocker, P.R. (1996). Molecular characterisation of ninein, a new coiled-coil protein of the centrosome. J. Cell Sci. 109, 179–190. [DOI] [PubMed] [Google Scholar]
- Busson, S., D. Dujardin, et al. (1998). Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 8, 541–544. [DOI] [PubMed] [Google Scholar]
- Carazo-Salas, R.E., Gruss, O.J., Mattaj, I.W., and Karsenti, E. (2001). Ran-GTP coordinates regulation of microtubule nucleation and dynamics during mitotic-spindle assembly. Nat. Cell Biol. 3, 228–234. [DOI] [PubMed] [Google Scholar]
- Carazo-Salas, R.E., Guarguaglini, G., Gruss, O.J., Segref, A., Karsenti, E., and Mattaj, I.W. (1999). Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature 400, 178–181. [DOI] [PubMed] [Google Scholar]
- Dasso, M. (2002). The Ran GTPase: theme and variations. Curr. Biol. 12, R502. [DOI] [PubMed] [Google Scholar]
- Di Fiore, B., Ciciarollo, M., Mangiacasale, R., Palona, A., Tassin, A.M., Cundari, E., Lavia, P. (2003). Mammalian Ran BP1 regulates centrosome cohesion during mitosis. J. Cell Sci. 116, 3399–3411. [DOI] [PubMed] [Google Scholar]
- Doxsey, S.J., P. Stein, et al. (1994). Pericentrin, a highly conserved centrosome protein involved in microtubule organization. Cell 76, 639–650. [DOI] [PubMed] [Google Scholar]
- Felix, M.A., Antony, C., Wright, M., and Maro, B. (1994). Centrosome assembly in vitro: role of gamma-tubulin recruitment in Xenopus sperm aster formation. J. Cell Biol. 124, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleig, U., Salus, S.S., Karig, I., and Sazer, S. (2000). The fission yeast Ran GTPase is required for microtubule integrity. J. Cell Biol. 151, 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory, M.R., Moser, M.J., Monnat, R.J., Jr., and Davis, T.N. (2000). Identification of a human centrosomal calmodulin-binding protein that shares homology with pericentrin. Proc. Natl. Acad. Sci. USA 97, 5919–5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham, A.K., and Munro, S. (2000). The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 1, 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy, P., Pichler, S., Kirkham, M., and Hyman, A.A. (1999). Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J. Cell Biol., 147, 135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlich, D., and Kutay, U. (1999). Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell. Dev. Biol. 15, 607–660. [DOI] [PubMed] [Google Scholar]
- Gruss, O.J., Carazo-Salas, R.E., Schatz, C.A., Guarguaglini, G., Kast, J., Wilm, M., Le Bot, N., Vernos, I., Karsenti, E., and Mattaj, I.W. (2001). Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 104, 83–93. [DOI] [PubMed] [Google Scholar]
- Guarguaglini, G., Renzi, L., D'Ottavio, F., Di Fiore, B., Casenghi, M., Cundari, E., and Lavia, P. (2000). Regulated Ran-binding protein 1 activity is required for organization and function of the mitotic spindle in mammalian cells in vivo. Cell Growth Differ. 11, 455–465. [PubMed] [Google Scholar]
- Heald, R., Tournebize, R., Habermann, A., Karsenti, E., and Hyman, A. (1997). Spindle assembly in Xenopus egg extracts: respective roles of centrosomes and microtubule self-organization. J. Cell Biol. 138, 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzer, M., Bilbao-Cortes, D., Walther, T.C., Gruss, O.J., and Mattaj, I.W. (2000). GTP hydrolysis by Ran is required for nuclear envelope assembly. Mol. Cell 5, 1013–1024. [DOI] [PubMed] [Google Scholar]
- Joseph, J., Tan, S.H., Karpova, T.S., McNally, J.G., and Dasso, M. (2002). SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles. J. Cell Biol. 156, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalab, P., Weis, K., and Heald, R. (2002). Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science 295, 2452–2456. [DOI] [PubMed] [Google Scholar]
- Kalab, P., Pu, R.T., and Dasso, M. (1999). The ran GTPase regulates mitotic spindle assembly. Curr. Biol. 9, 481–484. [DOI] [PubMed] [Google Scholar]
- Keryer, G., Rios, R.M., Landmark, B.F., Skalhegg, B., Lohmann, S.M., and Bornens, M. (1993). A high-affinity binding protein for the regulatory subunit of cAMP-dependent protein kinase II in the centrosome of human cells. Exp. Cell Res. 204, 230–240. [DOI] [PubMed] [Google Scholar]
- Keryer, G., Witczak, O., Delouvée, A., Kemmner, W., Rouillard, D., Tasken, K., and Bornens, M. (2003). Dissociating the centrosomal matrix protein AKAP450 from centrioles impairs centriole duplication and cell cycle progression. Mol. Biol. Cell 14, 2436–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz, C., Dabauvalle, M.C., Paintrand, M., Weber, T., Bornens, M., and Karsenti, E. (1990). Parthenogenesis in Xenopus eggs requires centrosomal integrity. J. Cell Biol. 110, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo, N., Matsumori, N., Taoka, H., Fujiwara, D., Schreiner, E.P., Wolff, B., Yoshida, M., and Horinouchi, S. (1999). Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 96, 9112–9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzler, M., and Hurt, E. (2001). Targeting of Ran: variation on a common theme? J. Cell Sci. 114, 3233–3241. [DOI] [PubMed] [Google Scholar]
- Maro, B., and Bornens, M. (1980). The centriole-nucleus association: effects of cytochalasine B and nocodazole. Biol. Cell 39, 287–290. [Google Scholar]
- Martin, O.C., Gunawardane, R.N., Iwamatsu, A., and Zheng, Y. (1998). Xgrip 109, a gamma tubulin-associated protein with an essential role in gamma tubulin ring complex (gammaTuRC) assembly and centrosome function. J. Cell Biol. 141, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj, I.W., and Englmeier, L. (1998). Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem. 67, 265–306. [DOI] [PubMed] [Google Scholar]
- Melchior, F. (2001). Ran GTPase cycle: one mechanism—two functions. Curr. Biol. 11, R257–R260. [DOI] [PubMed] [Google Scholar]
- Merdes, A., Heald, R., Samejima, K., Earnshaw, W.C., and Cleveland, D.W. (2000). Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J. Cell Biol. 149, 851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes, A., Ramyar, K., Vechio, J.D., and Cleveland, D.W. (1996). A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 87, 447–458. [DOI] [PubMed] [Google Scholar]
- Mogensen, M.M. (1999). Microtubule release and capture in epithelial cells. Biol. Cell 91, 331–341. [PubMed] [Google Scholar]
- Mogensen, M.M., Mackie, J.B., Doxsey, S.J., Stearns, T., and Tucker, J.B. (1997). Centrosomal deployment of gamma-tubulin and pericentrin: evidence for a microtubule-nucleating domain and a minus-end docking domain in certain mouse epithelial cells. Cell Motil. Cytoskeleton 36, 276–290. [DOI] [PubMed] [Google Scholar]
- Mogensen, M.M., Malik, A., Piel, M., Bouckson-Castaing, V., and Bornens, M. (2000). Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J. Cell Sci. 113, 3013–3023. [DOI] [PubMed] [Google Scholar]
- Moore, W., Zhang, C., and Clarke, P. (2002). Targeting of RCC1 to chromosomes is required for proper mitotic spindle assembly in human cells. Curr. Biol. 12, 1442. [DOI] [PubMed] [Google Scholar]
- Moudjou, M., Bordes, N., Paintrand, M., and Bornens, M. (1996). gamma-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J. Cell Sci. 109, 875–887. [DOI] [PubMed] [Google Scholar]
- Moudjou, M., and Bornens, M. (1994). Isolation of centrosomes from cultured animal cells. In: Cell Biology: A Laboratory Handbook, ed. J.E. Celis, San Diego: Academic Press, 595–604.
- Murphy, S.M., Preble, A.M., Patel, U.K., O'Connell, K.L., Dias, D.P., Moritz, M., Agard, D., Stults, J.T., and Stearns, T. (2001). GCP5 and GCP 6, two new members of the human gamma-tubulin complex. Mol. Biol. Cell 12, 3340–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, S.M., Urbani, L., and Stearns, T. (1998). The mammalian gamma-tubulin complex contains homologues of the yeast spindle pole body components spc97p and spc98p. J. Cell Biol. 141, 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, A.W. (1991). Cell cycle extracts. Methods Cell Biol. 36, 581–605. [PubMed] [Google Scholar]
- Nachury, M.V., Maresca, T.J., Salmon, W.C., Waterman-Storer, C.M., Heald, R., and Weis, K. (2001). Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell 104, 95–106. [DOI] [PubMed] [Google Scholar]
- Ohba, T., Nakamura, M., Nishitani, H., and Nishimoto, T. (1999). Self-organization of microtubule asters induced in Xenopus egg extracts by GTP-bound Ran. Science 284, 1356–1358. [DOI] [PubMed] [Google Scholar]
- Piel, M., Meyer, P., Khodjakov, A., Rieder, C. L., and Bornens, M. (2000). The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 149, 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quimby, B.B., Wilson, C.A., and Corbett, A.H. (2000). The interaction between Ran and NTF2 is required for cell cycle progression. Mol. Biol. Cell 11, 2617–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, M., Drivas, G., D'Eustachio, P., and Rush, M.G. (1993). Ran/TC 4, a small nuclear GTP-binding protein that regulates DNA synthesis. J. Cell Biol. 120, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, S.A., Lounsbury, K.M., and Macara, I.G. (1995). The C terminus of the nuclear RAN/TC4 GTPase stabilizes the GDP-bound state and mediates interactions with RCC1, RAN-GAP, and HTF9A/RANBP1. J. Biol. Chem. 270, 14405–14411. [DOI] [PubMed] [Google Scholar]
- Roig, J., Mikhailov, A., Belham, C., and Avruch, J. (2002). Nercc1, a mammalian NIMA-family kinase, binds the Ran GTPase and regulates mitotic progression. Genes Dev. 16, 1640–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salina, D., Bodoor, K., Eckley, D.M., Schroer, T.A., Rattner, J.B., and Burke, B. (2002). Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell 108, 97–107. [DOI] [PubMed] [Google Scholar]
- Schroer, T.A. (2001). Microtubules don and doff their caps: dynamic attachments at plus and minus ends. Curr. Opin. Cell Biol. 13, 92–96. [DOI] [PubMed] [Google Scholar]
- Stearns, T., Evans, L., and Kirschner, M. (1991). Gamma-tubulin is a highly conserved component of the centrosome. Cell 65, 825–836. [DOI] [PubMed] [Google Scholar]
- Stearns, T., and Kirschner, M. (1994). In vitro reconstitution of centrosome assembly and function: the central role of gamma-tubulin [see comments]. Cell 76, 623–637. [DOI] [PubMed] [Google Scholar]
- Takahashi, M., et al. (2002). Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol. Biol. Cell 13, 3235–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, M., Shibata, H., Shimakawa, M., Miyamoto, M., Mukai, H., and Ono, Y. (1999). Characterization of a novel giant scaffolding protein, CG-NAP, that anchors multiple signaling enzymes to centrosome and the Golgi apparatus. J. Biol. Chem. 274, 17267–17274. [DOI] [PubMed] [Google Scholar]
- Tassin, A.M., Celati, C., Moudjou, M., and Bornens, M. (1998). Characterization of the human homologue of the yeast Spc98p and its association with gamma-tubulin. J. Cell Biol. 141, 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese, C., Wilde, A., Moore, M.S., Adam, S.A., Merdes, A., and Zheng, Y. (2001). Role of importin-beta in coupling Ran to downstream targets in microtubule assembly. Science 291, 653–656. [DOI] [PubMed] [Google Scholar]
- Wilde, A., Lizarraga, S.B., Zhang, L., Wiese, C., Gliksman, N.R., Walczak, C.E., and Zheng, Y. (2001). Ran stimulates spindle assembly by altering microtubule dynamics and the balance of motor activities. Nat. Cell Biol. 3, 221–227. [DOI] [PubMed] [Google Scholar]
- Wilde, A., and Zheng, Y. (1999). Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science 284, 1359–1362. [DOI] [PubMed] [Google Scholar]
- Witczak, O., Skalhegg, B.S., Keryer, G., Bornens, M., Tasken, K., Jahnsen, T., and Orstavik, S. (1999). Cloning and characterization of a cDNA encoding an A-kinase anchoring protein located in the centrosome, AKAP450. EMBO J. 18, 1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann, T., Wilm, M., Karsenti, E., and Vernos, I. (2000). TPX2, A novel Xenopus MAP involved in spindle pole organization. J. Cell Biol. 149, 1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C., and Clarke, P.R. (2000). Chromatin-independent nuclear envelope assembly induced by Ran GTPase in Xenopus egg extracts. Science 288, 1429–1432. [DOI] [PubMed] [Google Scholar]
- Zhang, C., Hughes, M., and Clarke, P.R. (1999). Ran-GTP stabilises microtubule asters and inhibits nuclear assembly in Xenopus egg extracts. J. Cell Sci. 112, 2453–2461. [DOI] [PubMed] [Google Scholar]