Abstract
The common model for integrin mediated signaling is based on integrin clustering and the potential for that clustering to recruit signaling molecules including FAK and src. The clustering model for transmembrane signaling originated with the analysis of the EGF receptor signaling and remains the predominant model. The roles for substrate-bound ligand and ligand occupancy in integrin-mediated signaling are less clear. A kinetic model was established using HT1080 cells in which there was a linear relationship between the strength of adhesion, the proportion of α5β1 integrin that could be chemically cross-linked, and the number of receptor-ligand bonds. This graded signal produced a similarly graded response measured by the level of specific phosphorylation of FAK Y397. FAK Y397 phosphorylation could also be induced by antibody bound to the substrate. In contrast, clustering of α5β1 on suspended cells with either antibody to β1 or by clustering of soluble ligand bound to α5β1 induced the phosphorylation of FAK Y861 but not Y397. There were no differences in signaling when activating antibodies were compared with blocking antibodies, presence or absence of ligand. Only tethering of α5β1 to the substrate was required for induction of FAK Y397 phosphorylation.
INTRODUCTION
There are several classes of transmembrane receptors that are involved in the transmission of signals from the extracellular space across the plasma membrane. It is a logical requirement of these systems that ligand binding to the extracellular domain results in a change in the cytoplasmic domain. How the signal is transferred from the extracellular domain is basic to the generation of the intracellular down-stream signals. Allosteric proteins have been described in which the occupation of a binding site on one side of the protein can result in a conformational change that affects other binding sites. The interposition of a lipid bilayer between the receiving and effector domains of the transmembrane receptors poses limitations in the application of the allosteric model. Although a few of the receptor tyrosine kinase receptor systems have been analyzed in detail, the mechanism for transmembrane signal transduction by other receptor classes is less well understood.
The EGF receptor is the best understood model. Receptor dimerization is initiated by the binding of EGF to extracellular domain 1 altering the conformation of the extracellular domain to generate a dimerization of receptors mediated through domain 2. This dimerization brings the cytoplasmic domains of two EGF receptors into proximity and allows the cross-phosphorylation of cytoplasmic domains by the encoded tyrosine kinase (Schlessinger, 2000). The generation of signals through receptor dimerization is a general theme. Among the tyrosine kinase receptors the mechanisms of generating the dimer vary from the use of bivalent ligands for growth hormone and erythropoietin (Kossiakoff and de Vos, 1998; Jiang and Hunter, 1999), to dimeric ligands for PDGF and VEGF (Wiesmann et al., 1997), to complexes in which both receptor and ligand mediated the dimer binding as for FGF (Plotnikov et al., 2000). The other large class of transmembrane receptors for soluble ligands are the 7-transmembrane family. A recent model proposes that ligand binding results in the disruption of a salt bridge between TM domains at the cytoplasmic face and a displacement of one of the TM domains opening a binding site on the cytoplasmic side (Ballesteros et al., 2001; Pierce et al., 2002). For some of the 7-transmembrane receptors, receptor dimerization appears to play a role perhaps in the bringing together of JAKs to generate a cross-phosphorylation as described for the EGF receptors (Mellado et al., 2001).
Although it is clear that integrins serve as receptors for signal transduction as well as for cell adhesion (Menko and Boettiger, 1987; Guan et al., 1991; Schwartz, 2001), the mechanisms for transferring signals from the extracellular to the cytoplasmic domains are less clear. Because integrins do form clusters in the course of cell adhesion, a variation of the EGF receptor model has been proposed to explain this signal transduction (Schwartz et al., 1995; Miyamoto et al., 1995). This model is supported by experiments demonstrating that antibody-mediated clustering was sufficient to induce the phosphorylation of FAK (Kornberg et al., 1992) and was advanced by studies using ligand-coated beads showing that these beads could recruit signaling molecules including src family and the members of the canonical MAP kinase pathway to the binding site (Miyamoto et al., 1995). This concentration of signaling components could convert an energetically unfavorable reaction pathway to a favorable one. These integrin clustering models leave the role of the ligand open. Does ligand binding lead to a conformation change in integrin that promotes clustering? If the ligand is uniformly distributed on the surface and the receptor is uniformly distributed on the cell in suspension; how does plating the cell on the substrate induce clustering? This situation certainly initiates signaling. How does integrin clustering detect adhesion to a physically constrained substrate? How can soluble antibodies block myogenic differentiation, but the same antibody covalently linked to the substrate promote myogenic differentiation (Menko and Boettiger, 1987; Boettiger et al., 1989)? The integrin clustering models appear to separate the mechanical from the signaling functions of integrin. A role for integrins in the sensing of differences in tension applied to cells would appear to require some connection between its mechanical and signaling functions (Ishida et al., 1997; Tzima et al., 2001). In this report, we provide evidence for a tethering-induced signaling mode for α5β1 integrin that provides a means for mechanical sensing and results in distinct intracellular signaling.
MATERIALS AND METHODS
Cells and Reagents
Human fibrosarcoma HT1080 cells (CCL-121), human osteosarcoma HOS cells (CRL-1543), and normal human fibroblast strain IMR-90 (CCL-186; American Type Culture Collection ATCC, Manassas, VA) were grown in Dulbecco's modified Eagle's medium (DMEM) + 10% fetal bovine serum (FBS). Human plasma fibronectin and cell culture reagents were purchased from Life Technologies (Grand Island, NY). Fibronectin coating was done by incubation with different concentrations of fibronectin for 30 min at room temperature followed by incubation with 1% heat-inactivated BSA for 30 min. Levels of adsorbed fibronectin were determined using 125I-labeled fibronectin (Garcia et al., 1998a). Ethidium homodimer was obtained from Molecular Probes (Eugene, OR). Glass coverslips were purchased from Bellco (Vineland, NJ). Sulfo-BSOCOES cross-linker was purchased from Pierce (Rockford, IL). Cytochalasin D (CD) was obtained from Aldrich Chemical (Milwaukee, WI). All other reagents were purchased from Sigma (St. Louis, MO).
Antibodies
Polyclonal anti-α5, αv, β3 integrin subunits and LM609 anti-αvβ3 antibodies were purchased from Chemicon (Temecula, CA), whereas ployclonal no. 17 anti-β1 integrin subunit antibody was raised in this laboratory by standard procedures. AIIB2 and BIIG2 hybridomas were gift from C. Damsky, AG89 was a gift from J. Takagi, 13G12 was a gift from S. Akiyama (Akiyama et al., 1995), and 9E10 was obtained from the Genetics Core Facility (University of Pennsylvania). Monoclonal antibodies were affinity purified from supernatants on protein G-Sepharose columns. Goat anti-mouse IgG beads were purchased from Dynal (Great Neck, NY). Goat anti-rat polyclonal antibodies were purchased from Jackson ImmunoResearch (West Grove, PA). Polyclonal anti-FAKpY397, FAKpY407, FAKpY577, FAKpY861, and FAKpY925 phosphospecific antibodies were obtained from Biosource (Camarillo, CA), polyclonal anti-FAK (A-17) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and monoclonal anti-FAK antibody was purchased from Transduction Laboratories (Lexington, KY).
Quantitative Adhesion Assay
Cell adhesion to adsorbed fibronectin was measured using a spinning disk device (Garcia et al., 1998a). HT1080 cells were incubated in serum-free DMEM medium at 37°C for 15–18 h, dissociated with EDTA, washed, and resuspended in complete PBS plus 2 mM glucose. The cells were uniformly seeded (∼400 cells per mm2) onto fibronectin-coated glass coverslips mounted on the spinning disk device and allowed to attached for 60 min at 22°C in presence or absence of monoclonal antibodies (Garcia et al., 1998a). The chamber of the device was filled with buffer, and disks were spun for 10 min at constant speed with controlled acceleration rates. Adhesion cells were fixed in 3.7% formaldehyde, permeabilized with 1% Triton X-100, and stained with ethidium homodimer. Disks were analyzed by counting the number of nuclei per microscope field (∼0.8 mm2) using a motorized stage and image analysis software (Version 3.0; Phase 3 Imaging, Glen Mills, PA). Sixty-one fields were analyzed per disk and normalized to the nuclei count at the disk center for which the applied force is zero.
Integrin-binding Analysis
Cells were serum-starved and dissociated as described for adhesion analysis and seeded at 2 × 106 cells per 100-mm diameter dish in complete PBS plus 2 mM glucose for 60 min at 22°C on fibronectin-coated culture dishes. Experiments were also performed at 37°C for 20 min with similar results. Fibronectin-bound integrin was quantified using a modification of a cross-linking/extraction protocol (Enomoto-Iwamoto et al., 1993). The cultures were incubated in 1 mM Sulfo-BSOCOES for 10 min, quenched with 50 mM Tris, and extracted in ice-cold lysis buffer (0.1% SDS in complete PBS). Cross-linked integrins were recovered by incubation in 0.1% SDS + 50 mM NaHCO3 (pH 11.6) at 40°C for 2 h and concentrated by size-exclusion filtration (Microcon 30, Amicon, Beverly, MA). Integrin subunits were separated by 6% SDS-PAGE gels and transferred to PVDF membranes (Millipore, Bedford, MA). Bands were stained by Western blotting using polyclonal antibodies against specific integrin subunits and peroxidase-conjugated secondary antibodies (Promega, Madison, WI). Blots were developed with ECL substrate (Amersham-Pharmacia Biotech, Arlington Heights, IL) and quantified using a Fuji LAS-1000 using ScienceLab v 2.5 software (Fujifilm, Tokyo, Japan). This protocol provided a linear response to actual protein levels over a range of at least 103-fold. Control samples were plated either on suspension cell culture dishes that had been blocked with 1% BSA or on fibronectin-coated dishes in the absence of cross-linker. Integrins recovered from the soluble (non-cross-linked) fraction were used to normalize and calculate the proportion of integrin subunits in the cross-linked fraction. This corrects for differences in cell number and recovery efficiency.
FAK Phosphorylation
Cells were serum-starved and plated on different densities of fibronectin for 60 min at 22°C (as above). Incubation at 22°C provided for better synchronization because it slows the biochemical reactions relative to the settling of cells and establishing initial contact with ligand. For comparison, similar experiments performed at 37°C required ∼20 min to reach the same levels of FAK phosphorylation seen at 60 min at 22°C. For the determination of FAK phosphorylation, cells were extracted in ice-cold lysis buffer (100 mM NaCl, 10 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, 20 mM Na4P2O7, 1 mM NaF, 1% Triton X-100, 0.1% SDS) containing 2 mM Na3VO4 and protease inhibitors (350 μg/ml PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin) for 10 min. Protein concentration was determined (BCA Protein Assay Kit, Pierce). Twenty micrograms of protein from each cell extract was separated by electrophoresis on 7% SDS-PAGE gels and analyzed by Western blotting using primary antibodies listed above. Gels were quantified as described above. The extent of FAK tyrosine phosphorylation was normalized to the amount of total FAK protein recovered from each sample. To analyze the relative contributions of α5β1 and αvβ3 to FAK phosphorylation, cells were pretreated with either monoclonal antibodies BIIG2 (anti-α5) + AIIB2 (anti-β1), or LM609 (anti-αvβ3), or BIIG2 + AIIB2 + LM609 for 30 min and plated on fibronectin-coated (335 ng/cm2) plates.
Antibody- and Ligand-induced Clustering of Integrin
HT1080 cells were resuspended in DPBS + 2 mM glucose and incubated with 10 μg/ml purified mAb AIIB2 or AG89 for 15 min followed by 10 μg/ml purified goat anti-rat (for AIIB2) or anti-mouse (for AG89) IgG. Cells, 2 × 106, were seeded in 100-mm suspension cell culture dishes (Corning), which were preblocked with 1% BSA, and incubated at 22°C for 60 min. For ligand-induced clustering, HT1080 cells were incubated with 40 μg/ml FN7–10 (a fibronectin fragment consisting of the 7th to the 10th type III repeats purified from Escherichia coli, purified, and crystallized; Redick et al., 2000) in PBS plus glucose and 1 mM MnCl2 to induce binding. 13G12 mAb (10 μg/ml; a gift from S. Akiyama) followed by 10 μg/ml purified goat anti-mouse IgG to cluster the FN7–10.
Preparation of Antibody Substrates
Plates were coated with 0.1% bovine nasal collagen and allowed to dry and then coated with nitrocellulose, 1 cm2, dissolved in 1 ml methanol per 100-mm dish, and dried at 37°C overnight. Antibodies were added as described, bound for 30 min at room temperature, and blocked with 1% BSA for 30 min. Plates were washed with PBS as cells plated, as described above.
RESULTS
Mechanical Dose-Response Model for α5β1 Integrin-mediated Signaling
HT1080 cells express several integrin receptors that are potentially capable of binding to fibronectin; however, their adhesion to fibronectin is completely dependent on α5β1 integrin. Blocking with either BIIG2 antibody to α5 or AIIB2 antibody to β1 reduced the strength of adhesion measured using the spinning disk device to background levels (i.e., indistinguishable from BSA). The initial adhesion was followed by cell spreading and adhesion strengthening during which α5β1 was organized into focal adhesions.
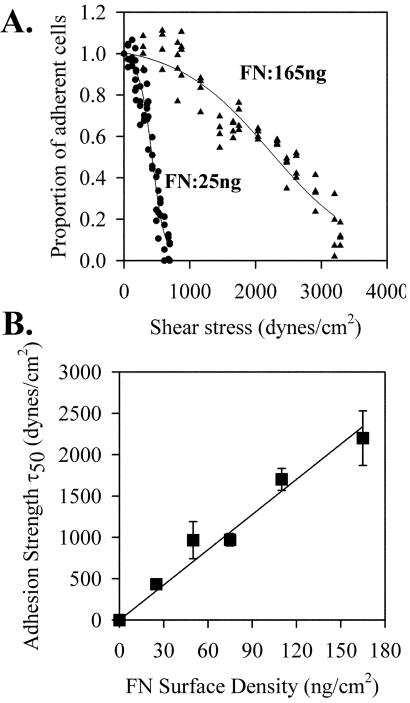
Because much of the analysis involves the 60-min time point and there is significant cell-spreading that take place during this period, it was necessary to examine the adhesion measurements at this time point. Figure 1A shows two adhesion strength profiles generated by plating HT1080 cells on different densities of fibronectin. Each plot was fit to a sigmoid curve based on the premise that the property of adhesion strength is normally distributed in this cell population. Increasing the fibronectin density resulted in a shift of the curve to the right showing that higher shear stresses were required for cell detachment. The inflection point for these plots (τ50) represents the mean of the normal distribution or the mean shear stress required to detach a cell. Figure 1B shows a plot of the τ50, or adhesion strength, for replicate analyses at different fibronectin densities. The linear relationship between τ50 and ligand density has been shown for α5β1 on other cells and for other integrin-ligand combinations (Garcia et al., 1998a; Garcia and Boettiger, 1999; Boettiger et al., 2001). This relationship provides a quantitative measure of adhesion strength that is directly related to the number of α5β1-fibronectin bonds. The data show that this measurement remains valid for HT1080 cells that have been allowed to spread for 60 min.
Figure 1.
Adhesion strength of HT1080 cells to fibronectin. HT1080 cells were incubated in serum-free medium for 18 h, washed, dissociated with EDTA, and plated on fibronectin-coated, BSA-blocked, surfaces in complete PBS plus 2 mM glucose for 1 h at room temperature. Cells were analyzed using the spinning disk system in adhesion buffer containing 5% Dextran: (A) Plated on fibronectin density 25 ng/cm2 (•) or 165 ng/cm2 (▴). (B) Adhesion strength defined as mean detachment shear stress (τ50) from spinning disk analyses as shown in A were plotted as a function of fibronectin surface density as determined by 125I adsorption (Garcia et al., 1998b). Error bars, SD; n = 3.
Proportion of Ligand-bound α5β1 Integrin as a Function of Fibronectin Density
Chemical cross-linking has been applied extensively in the analysis of the binding of soluble factors to their cell surface receptors (Brenner et al., 1985). The chemical cross-linking method used here takes advantage of the fact that fibronectin adheres strongly to polystyrene culture dishes and requires high concentrations of SDS and/or urea for solubilization (Hynes, 1990). Thus, fibronectin-bound integrins would not be extracted with 0.2% SDS buffer and could then recovered by cleaving the cross-linker after the other cell components had been removed. Sulfo-BSOCOES is a cell-impermeant, highly reactive homobifunctional N-hydroxy-succinimidyl ester cross-linker that reacts with extracellular secondary amines (lysines or arginines). The cross-linking requires that the spacing between secondary amines on α5β1 and fibronectin lie within 1.3 nm and are outside the α5β1-fibronectin bonding interface (because the cross-linker is added after the bonds are formed). Control experiments have demonstrated that i) cross-linked integrins are preferentially localized to focal adhesion-like structures, ii) cross-linked integrins contribute directly to an increase in adhesion strength, iii) cross-linking requires an activated form of α5β1 that can bind fibronectin; and iv) cross-linking requires that the integrin be bound to its ligand. (Enomoto-Iwamoto et al., 1993; Garcia et al., 1998a; Garcia and Boettiger, 1999).
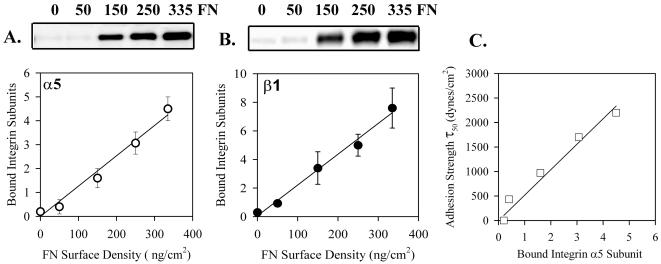
Chemical cross-linking of HT1080 cells at 60 min after plating on fibronectin substrates demonstrated a linear relationship between the number of receptor-ligand bonds and the proportion of α5 (Figure 2A) and β1 (Figure 2B), which could be cross-linked. Therefore the proportion of α5 and β1 integrin that can be cross-linked is a measure of the relative number of α5β1-fibronectin bonds. Note that the total proportion that was cross-linked was <4%, which would minimize the number of doubly bound α5β1 that were cross-linked both to fibronectin and to unbound α5β1. To analyze the relationship between the proportion of α5β1 that was cross-linked and adhesion strength, the values from the α5 blot were plotted as a function of τ50. The linear relationship shown in Figure 2C demonstrates that the chemical cross-linking method provides a measure of the proportion of α5β1 bonds that contribute directly to the mechanical adhesion of the cell to the substrate.
Figure 2.
Proportion of α5β1 bound to fibronectin as a function of density. HT1080 cells were incubated in serum-free medium for 18 h, washed, dissociated with EDTA, and plated on fibronectin-coated, BSA-blocked, surfaces in complete PBS plus 2 mM glucose for 1 h at room temperature. The cultures were then cross-linked with sulfo-BSCOES for 10 min and the cross-linked and non–cross-linked fractions were analyzed by Western blot. (A) Cross-linked α5; typical Western blot and combined quantification for three independent experiments. Bound integrin subunits represents the proportion of α5 cross-linked/total α5 × 100. Linear regression: R2 = 0.98, p = 0.001. (B) Cross-linked β1. Same as A. Error bars, SEM for n = 3 separate experiments. Linear regression: R2 = 0.99, p = 0.0005. (C) Adhesion strength τ50 taken from Figure 1B plotted against amount of cross-linked α5 from Figure 2A shows the linear relationship.
FAK Y397 Phosphorylation Is a Read-out of the Level of Fibronectin-bound α5β1
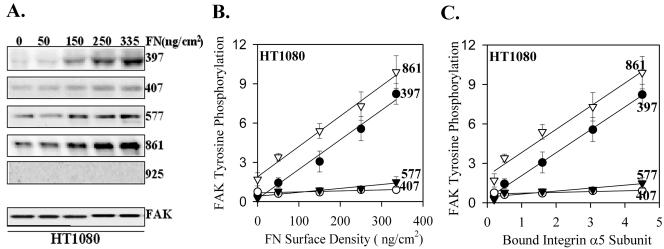
The phosphorylation of FAK appears to be an early event in α5β1-integrin–mediated signaling (Guan et al., 1991; Kornberg et al., 1992). There are at least six tyrosines in FAK that can be phosphorylated and distinct downstream signaling molecules have been shown to bind several of the phosphorylated tyrosines (Schlaepfer et al., 1999). Because the phosphorylation of different tyrosines could be involved in distinct signaling pathways, we chose to examine the phosphorylation of individual tyrosines to connect these events to the initial binding and activation of α5β1 integrin. The levels of specific FAK phosphorylation were measured as the ratio of the binding of the FAK phosphorylation site-specific antibody to the total FAK blot control. Figure 3 shows a linear increase in the phosphorylation of FAK Y397 and Y861 as a function of the number of receptor-ligand bonds, shown both as a function of ligand density (Figure 3B) and level of α5 cross-linked (Figure 3C). There were no significant increases in the levels of phosphorylation of Y407 or Y577 as a function of increased fibronectin-bound α5β1. No phosphorylation of Y925 was detected under these conditions although this antibody did detect pY925 in v-src–transformed cells (Datta et al., 2001).
Figure 3.
Phosphorylation of FAK by α5β1 binding to substrate fibronectin in HT1080 cells. HT1080 cells were incubated in serum-free medium for 18 h, washed, dissociated with EDTA, and plated on fibronectin-coated, BSA-blocked, surfaces in complete PBS plus 2 mM glucose for 1 h at room temperature. The cells were extracted with a RIPA-vanadate buffer and analyzed by Western blot using antibodies to specific FAK phosphorylation sites or a mAb to FAK protein. (A) A representative blot; (B) the quantified combined data from three independent experiments FAK tyrosine phosphorylation (level of FAK detected by the specific antiphosphotyrosine antibody/level of FAK protein for each data point ×10). Error bars, SEM for n = 3 separate experiments. Linear regression: pY397 R2 = 0.98, p = 0.0009; pY407 R2 = 0.66, p = 0.09; pY577 R2 = 0.87, p = 0.02; pY861 R2 = 0.99, p = 0.0004. (C) The data as a function of the level of cross-linked α5. Linear regression: pY397 R2 = 0.99, p <0.0001; pY407 R2 = 0.72, p = 0.07; pY577 R2 = 0.84, p = 0.03; pY861 R2 = 0.97, p = 0.0017.
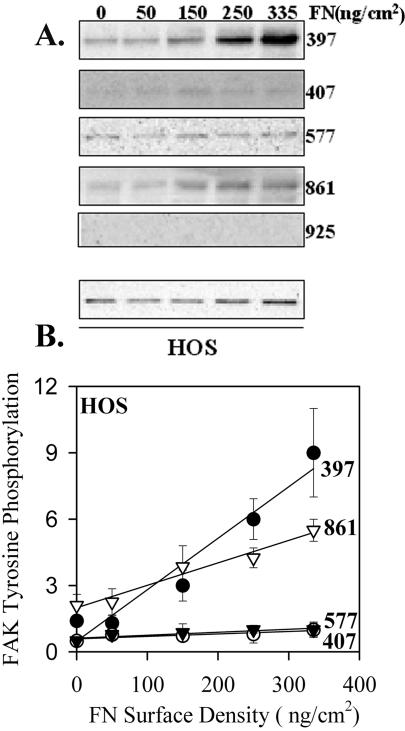
To determine the generality of these results, similar analyses were performed using IMR90 and HOS cells. Both these cell types gave essentially the same results, and those for HOS cells are shown in Figure 4. These results were similar to the HT1080 cells for each tyrosine except Y861. Although there was still a dose-dependent increase in Y861 phosphorylation, the proportional increase was decreased by about twofold. This decrease suggests a weaker coupling between α5β1 binding and phosphorylation of Y861. Overall, the data are consistent with a direct coupling between the binding of α5β1 and the phosphorylation of FAK Y397 with a 1:1 ratio for each of the cell systems analyzed. The level of phosphorylated Y397 in nonadhering cells was very low or undetected, whereas there was significant phosphorylated Y861 under these conditions.
Figure 4.
Phosphorylation of FAK by α5β1 binding to substrate fibronectin in HOS cells. Similar to Figure 3 except experiments shown are for HOS cells. (A) A representative blot. (B) The quantified combined data from three independent experiments FAK tyrosine phosphorylation. Error bars, SEM for n = 3 separate experiments. Linear regression: pY397 R2 = 0.94, p = 0.0059; pY407 R2 = 0.74, p = 0.06; pY577 R2 = 0.81, p = 0.0382; pY861 R2 = 0.97, p = 0.0027.
Control of FAK Y397 Phosphorylation by α5β1 Binding vs. αvβ3 Binding
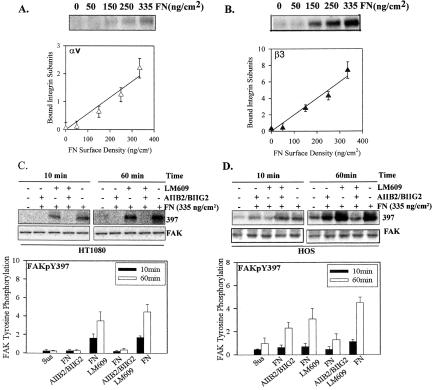
Both HT1080 and HOS cells express α5β1 and αvβ3 that have the potential to serve as fibronectin receptors. Treatment of HT1080 cells with monoclonal antibodies to α5β1 reduced adhesion to background levels (as described above), whereas antibody to αvβ3 (LM609) had no effect. In contrast, treatment of HOS cells with blocking antibodies to either α5β1 or αvβ3 only reduced adhesion by 50% and required a combination of both to reduce adhesion to background levels (Datta et al., 2002). In spite of the apparent absence of a contribution to the adhesion strength, αvβ3 on HT1080 cells could be chemically cross-linked at 60 min after plating (Figure 5, A and B). In previous analyses the ability to cross-link αvβ3 correlated with its contribution to total adhesion strength (Boettiger et al., 2001). Because of this apparent binding of αvβ3 to fibronectin, we tested the ability of function-blocking antibodies to inhibit FAK phosphorylation. Figure 5C shows that FAK Y397 phosphorylation was dependent of α5β1 but not on αvβ3 in the HT1080 cells. This difference could be seen at both 10 min and at 60 min. To determine whether αvβ3 could stimulate FAK Y397, we analyzed the contributions of α5β1 and αvβ3 to FAK phosphorylation in HOS cells. Figure 5D shows approximately equal contributions of α5β1 and αvβ3 to FAK Y397 phosphorylation. Thus, the specific connections between integrin and FAK can differ with cell type.
Figure 5.
FAK Y397 phosphorylation induction by α5β1 and αvβ3 is HT1080 cells and HOS cells is different. (A) Cross-linking of αv; linear regression: R2 = 0.95, p = 0.0046, and (B) β3 for HT1080 cells platted fibronectin using the protocol in Figure 2; linear regression: R2 = 0.96, p = 0.0032. (C) The inhibition of FAK Y397 phosphorylation by antibodies to α5β1 and/or αvβ3 at both 10 and 60 min after plating for HT1080 cells. (D) The inhibition of FAK Y397 phosphorylation by antibodies to α5β1 and/or αvβ3 at both 10 and 60 min after plating for HOS cells. Error bars, SEM for n = 3 for each.
Antibody-mediated Clustering of α5β1 Induces a Distinct FAK Phosphorylation Pattern
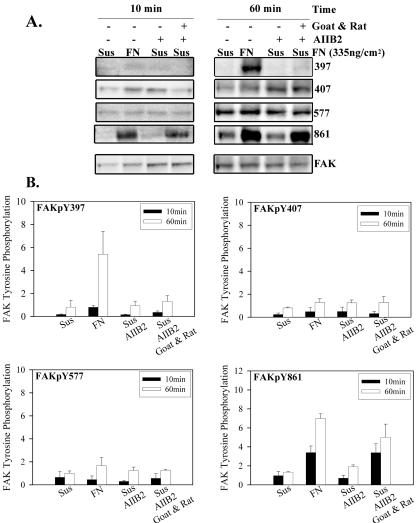
Integrin-mediated signals have been demonstrated following antibody-mediated clustering of integrin receptors in suspended cells (Kornberg et al., 1991). To examine the specific FAK phosphorylation in this simplified integrin clustering model, α5β1 integrin on HT1080 cells in suspension were clustered with a β1 integrin function-blocking antibody and clustered further with secondary antibody. The AIIB2 primary antibody was added at a level that would saturate the α5β1 integrin on the HT1080 cells, based on prior FACS analysis. Figure 6 shows that antibody-induced clustering had no effect on the phosphorylation of Y397, but the combined primary and secondary antibody-induced clustering produced levels of phosphorylated Y861 similar to plating the cells on fibronectin. Neither Y407 nor Y577 responded to antibody induced clustering of α5β1. The ability of the clustering protocol to induce phosphorylation of Y861 provides a positive control for the method and a reasonable explanation of previous demonstrations of integrin clustering-induced phosphorylation of FAK. To determine whether an early phosphorylation was missed using the 60-min time point, the analyses were repeated at both 10 and 30 min. Figure 6 shows that the level of Y397 phosphorylation for cells plated on fibronectin increased between 10 and 60 min, but there was no increase for cells in which α5β1 had been clustered by antibodies.
Figure 6.
Phosphorylation of FAK by clustering integrin. HT1080 cells were incubated in serum-free medium for 18 h, washed, and dissociated with EDTA. Some samples were mixed with the mAb AIIB2, or AIIB2 plus secondary goat anti-rat IgG and plated on fibronectin-coated (335 ng/cm2), BSA-blocked surfaces in complete PBS plus 2 mM glucose for 10 and 60 min at room temperature. (A) A typical Western blot; (B) the quantification of Western blots for specific phosphorylated FAK; (phospho-specific antibody/total FAK) for each. Values were normalized to the phosphorylation level of the suspended cells in the absence of cluster inducing antibodies. Error bars, SEM for n = 3.
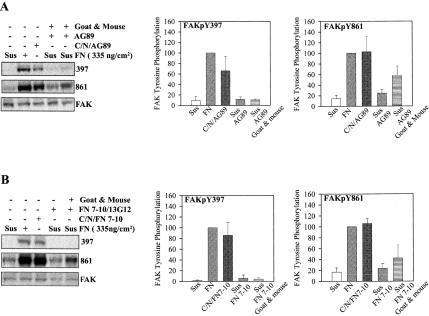
The failure of antibody-mediated clustering to induce phosphorylation of FAK Y397 could be that the antibodies held α5β1 in an inactive conformation. To test this possibility similar experiments were performed with α5β1 activating monoclonal antibodies that convert its conformation to the “activated” form. Figure 7A shows that clustering α5β1 with an activating mAb induced the same FAK phosphorylation pattern (increased pY861 but not pY397) as seen with the function blocking monoclonal antibodies. In contrast, immobilizing the mAb on the substrate induced phosphorylation of both Y397 and Y861. Thus, the tethered antibody acted like substrate-bound fibronectin and induced the phosphorylation of FAK Y397, whereas the soluble antibody did not.
Figure 7.
FAK stimulation by activated or ligand occupied α5β1. (A) Serum-starved HT1080 cells were treated is suspension with the β1 activating mAb AG89 or AG89 plus secondary anti-mouse IgG, or AG89 was bound to a nitrocellulose-coated surface. Cells kept in suspension or plated on fibronectin were used as controls. The specific phosphorylation is shown only for Y397 and Y861, there were no changes in Y407 or Y577. Data were normalized setting the specific phosphorylation for cells plated on fibronectin at 100%; error bars, SEM for n = 3. (B) Serum-starved HT1080 cells were treated in suspension with FN7–10 (40 μg/ml) and MnCl2 (1 mM) for 15 min; 13G12 (10 mg/ml) was added followed by secondary goat anti-mouse IgG (10 μg/ml) for 60 min. For controls, cells were maintained in suspension, plated on fibronectin, or plated on FN7–10 bound to 13G12 bound to nitrocellulose-coated surfaces. Quantification as for A, Error bars, SEM for n = 3.
However, antibodies are not the natural ligands for α5β1 and interact rather differently with it. To generate an integrin clustering model based on ligand binding, we used a fibronectin fragment consisting of the 7th through the 10th type III repeat (FN7–10) that contains the α5β1 binding domain spanning the 9th and 10th repeat (Leahy et al., 1996). FN7–10 was then clustered using mAb 13G12 that reacts with the surface of FN7–10 opposite to the cell binding domain (Akiyama et al., 1995). 13G12 was clustered in turn using a polyclonal secondary antibody. Figure 7B shows that 13G12 bound to the surface, bound FN7–10 and cells plated on the bound FN7–10 stimulated the phosphorylation of FAK Y397 to a level comparable to full-length fibronectin. In contrast, binding of FN7–10 to α5β1 on HT1080 cells in suspension and clustering the FN7–10 with 13G12 and secondary antibody resulted in an increase in phosphorylation of Y861, but not Y397. The increase on Y861 phosphorylation in this experiment demonstrates that the clustering protocol was effective. The combined experiments shown in Figure 7 demonstrate that ligand binding and conformational change in α5β1 is not sufficient to induce the phosphorylation of FAK Y397.
Induction of FAK Phosphorylation by Tethered Antibody
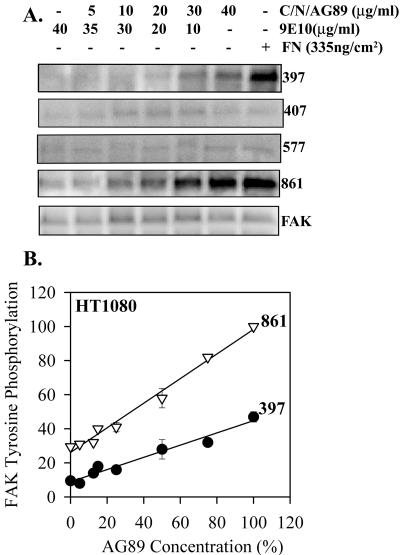
To test whether these signals were mediated through integrin tethering, an antibody substrate was generated. In initial experiments, adsorption of antiintegrin antibodies to tissue culture polystyrene did not result in a strong binding that resists incubation with cells. A more consistent antibody substrate was generated using a collagen coating followed by nitrocellulose to adsorb the antiintegrin monoclonal antibodies (Lee, Haskell, Charo, and Boettiger, et al., unpublished results). This system produces a density of 104 IgG molecules/μm2 with a active binding site density of 3.2 × 103/μm2. The density of AG89 mAb to β1 on the substrate was varied by mixing it with an irrelevant mAb (9E10, antimyc) in different proportions. This provided the foundation for a dose-dependent analysis of α5β1 stimulation of FAK phosphorylation analogous to the initial experiments with surface adsorbed fibronectin. Figure 8 shows a linear relationship between the proportion of α5β1 integrin linked to the substrate by AG89 antibody and the levels of phosphorylation of both Y397 and Y861. Thus, the antibody substrate reproduces the fibronectin substrate, except that the rate of increase in Y397 phosphorylation was reduced to 50%. Therefore, tethering of α5β1 is sufficient to induce a dose-dependent increase on phosphorylation of FAK Y397. The reduced efficiency of the AG89 substrate could be related to the differences in the sites on α5β1 that were directly tethered.
Figure 8.
Dose-dependent tethering stimulation of FAK phosphorylation. Serum-starved HT1080 cells were plated on antibody substrates formed from the binding of mixtures of AG89 diluted with different proportions of 9E10 that were bound to nitrocellulose-coated surfaces. Specific phosphorylation of Y861 and Y397 is shown as a function of antibody-ligand density. Error bars, SEM for n = 3. Linear regression: pY397 R2 = 0.96, p < 0.0001; pY861 R2 = 0.99, p < 0.0001.
DISCUSSION
These results address three issues in integrin-mediated signaling: 1) Is there a proportional dose-response relationship in signal initiation, and if so, how do intracellular processes read the extracellular signaling levels? 2) Can different integrin-ligand interactions elicit different intracellular signals? And 3) what are the contributions of integrin clustering, ligand occupancy, and integrin tethering to integrin-mediated signals? An experimental paradigm was developed that uses a kinetic approach in combination with technologies to quantify both the strength of adhesion and the number of ligated receptors to relate the proportion of integrin bound to the level of signaling. The analysis is focused on the phosphorylation of tyrosines Y397, Y407, Y577, Y861, and Y925. This may not represent the full output of α5β1-mediated signals.
If the binding of integrin to its ligand functions analogously to cytokine receptors or to receptor-tyrosine-kinases, then an increase in the number of bound receptors should generate a proportional increase in the level of downstream signals. As the signals proceed further downstream, some amplification of the signal is expected to generate a final all-or-none response (Ferrell, 1998). For the receptor tyrosine kinases, ligand binding results in the autophorylation of sites in the cytoplasmic domain that in turn provide sites for the binding of downstream effectors (Livneh et al., 1986). The total level of phosphorylation is proportional to the ligand concentration and the number of ligands bound. In this case and with other receptor systems, the internal cell read-out is provided by phosphorylation of the receptor itself or its associated proteins (Hunter and Cooper, 1981; Livneh et al., 1986). For α5β1 integrin a prime candidate for providing this read-out function is FAK. Previous analyses have identified FAK as an early target in integrin signaling (Guan et al., 1991; Kornberg et al., 1991, 1992; Schlaepfer et al., 1999) and have identified Y397 as a critical first step in the process (Owen et al., 1999).
The data demonstrate an increase in the level of both Y397 and Y861 phosphorylation that directly parallels the increase in the number of α5β1-fibronectin bonds. The relative number of α5β1 fibronectin bonds was correlated to both the proportion of α5 and β1 subunits that could be cross-linked and to the overall strength of adhesion measured using the spinning disk. Taken together, these results suggest that the level of phosphorylation of FAK Y397 is a direct consequence of the number of (mechanically strong) α5β1-fibronectin bonds. This provides a means for the cell to measure the mechanical and biochemical environment. The reason that we do not include Y861 as a primary measure of α5β1 bonds is that the coupling between Y861 phosphorylation and the number of bonds was weaker in other cell types: including, chicken embryo fibroblasts (Datta et al., 2001), IMR90 human fibroblasts, and HOS osteosarcoma cells, and because it responded to alternate signaling mechanisms. When HT1080 cells were plated on fibronectin, both tethering and clustering occur as part of the normal process. Thus, we cannot exclude clustering as an explanation for the increase in Y861 phosphorylation in those experiments.
The finding that the levels of phosphorylation of Y397 were directly proportional to the number of mechanically functional α5β1-fibronectin bonds provides a means for the cell to respond to integrin-mediated adhesion to a substrate. It is particularly significant that Y397 is the responding site because this site is central to the formation of complexes based on the binding of SH2 domains to its phosphorylated form. The SH2 domains of src, PI3-kinase, PLCγ, Grb7, and Shc have been reported to bind pY397 providing many potential downstream signaling consequences (Schaller et al., 1994; Schlaepfer and Hunter, 1997; Han and Guan, 1999; Reiske et al., 1999; Zhang et al., 1999).
Most current integrin signaling data has been interpreted in the context of the integrin-clustering model. The plating of fibroblasts on a fibronectin-coated surface induces not only the binding of α5β1 to fibronectin, but also the progressive organization of α5β1 into focal contacts and focal adhesions during the process of cell spreading (Singer et al., 1988; Pankov et al., 2000). Because the experiments described here do involve the plating of cells on a fibronectin substrate, a reorganization of integrin into focal contacts and focal adhesion did occur. Therefore, these data do not exclude a role for clustering in integrin-mediated signals, including the phosphorylation of Y397. The failure of clustering to stimulate Y397 phosphorylation in the absence of adhesion demonstrates that clustering alone is not a sufficient explanation for integrin-mediated signaling. Furthermore, the demonstrations that the same antibody and ligand combinations that elicited Y861 but not Y397 phosphorylation in the clustering protocol could stimulate phosphorylation Y397 when they were adsorbed to the substrate. This reinforces the conclusion that it is the tethering of α5β1 to the substrate, rather than its clustering, that provides the signal for phosphorylation of Y397.
In many transmembrane receptor signaling systems the role of the ligand is primarily to cluster or dimerize the receptor (Schlessinger, 2000). As long as the dimerization takes place, it does not matter whether the receptor is occupied or not. In the experiments described here, neither clustering integrins with an activating mAb to β1 integrin nor addition of a ligand and clustering through that ligand was able to induce the phosphorylation of Y397. Also, the use of antibodies bound to the substrate, in the absence of ligand, was still able to stimulate Y397 phosphorylation. These data imply that for stimulation of Y397 phosphorylation, it does not matter whether α5β1 is occupied by ligand or not, only that it is tethered to the substrate. In contrast to these results, there are other reports that suggest that ligand occupation itself could affect signaling; however, different signaling end points were used for those experiments and analysis of receptor occupancy effects was not the author's primary objective. Miyamoto et al. (1995) analyzed the recruitment of α-actinin, vinculin, and talin to beads coated with adhesion-blocking antibodies or noninhibitory integrin antibodies. In the presence of inhibitors, including cytochalasin D, these proteins were recruited to the bead sites only by the inhibitory antibodies. This was interpreted as a requirement for ligand occupancy for their recruitment. However, these antibodies also differ in other properties such as location of their binding epitope and ligand-binding affinity, which could influence the recruitment. Arthur et al. (2000) report the downregulation of RhoA activity in src-reconstituted SYF mouse fibroblasts in response to the addition of soluble RGD. But, the actual mechanism and possible receptors involved was not defined. Thus, it is possible that ligand occupancy can be detected by the intracellular signaling processes, but a clear answer is not yet available.
The data demonstrate a requirement for the tethering of α5β1 to substrate for the induction of phosphorylation or the formation of phosphorylated complexes, involving FAK Y397. Neither the clustering of α5β1, nor the binding of ligand to α5β1, was able to substitute for this requirement. Thus, this constitutes a novel mode for the transmission of signals from the extracellular environment across the plasma membrane to link with intracellular signaling pathways. The clustering of α5β1 and the tethering of α5β1 led to different patterns of FAK phosphorylation, raising the possibility that the downstream signals may depend, not only on the particular cell type, but also on the way in which the signal is delivered.
Acknowledgments
We thank Dr. C. Damsky and Dr. S. Akiyama for the gift of antibodies, Dr. H. Erickson for the gift of the FN7–10 expression vector and advice on protein purification and crystallization, Dr. R. Assoian for helpful comments on the manuscript, Dr. K. Yamada for discussions on integrin signaling, and L. Lynch for technical assistance. This work was supported by grants from the National Cancer Institute and The Institute for General Medical Sciences of the National Institutes of Health.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–01–0046. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-01-0046.
References
- Akiyama, S.K., Aota, S., and Yamada, K.M. (1995). Function and receptor specificity of a minimal 20 kilodalton cell adhesive fragment of fibronectin. Cell Adhes. Commun. 3, 13–25. [DOI] [PubMed] [Google Scholar]
- Arthur, W.T., Petch, L.A., and Burridge, K. (2000). Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10, 719–722. [DOI] [PubMed] [Google Scholar]
- Ballesteros, J.A., Jensen, A.D., Liapakis, G., Rasmussen, S.G., Shi, L., Gether, U., Javitch, J.A. (2001). Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 276, 29171–29177. [DOI] [PubMed] [Google Scholar]
- Boettiger, D., George-Weinstein, M., Menko, A. S. (1989). Triggering terminal myogenic differentiation. In: UCLA Symposia on Mol. Cell. Biol., New Series: Cellular and Molecular Biology of Muscle Development, ed. F. Stockdale, L. Kedes, New York: Alan R. Liss, Inc., 57–66.
- Boettiger, D., Huber, F., Lynch, L., and Blystone, S. (2001). Activation of alpha(v)beta3-vitronectin binding is a multistage process in which increases in bond strength are dependent on Y747 and Y759 in the cytoplasmic domain of beta3. Mol. Biol. Cell 12, 1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, M.B., Trowbridge, I.S., and Strominger, J.L. (1985). Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell 40, 183–190. [DOI] [PubMed] [Google Scholar]
- Datta, A., Huber, F., and Boettiger, D. (2002). Phosphorylation of beta3 integrin controls ligand binding strength. J. Biol. Chem. 277, 3943–3949. [DOI] [PubMed] [Google Scholar]
- Datta, A., Shi, Q., and Boettiger, D.E. (2001). Transformation of chicken embryo fibroblasts by v-src uncouples beta1 integrin-mediated outside-in but not inside-out signaling. Mol. Cell Biol. 21, 7295–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto-Iwamoto, M., Menko, A.S., Philp, N., and Boettiger, D. (1993). Evaluation of integrin molecules involved in substrate adhesion. Cell Adhes. Commun. 1, 191–202. [DOI] [PubMed] [Google Scholar]
- Ferrell, J.E., Jr. (1998). How regulated protein translocation can produce switch-like responses. Trends Biochem. Sci. 23, 461–465. [DOI] [PubMed] [Google Scholar]
- Garcia, A.J., and Boettiger, D. (1999). Integrin-fibronectin interactions at the cell-material interface: initial integrin binding and signaling. Biomaterials 20, 2427–2433. [DOI] [PubMed] [Google Scholar]
- Garcia, A.J., Huber, F., and Boettiger, D. (1998a). Force required to break alpha5beta1 integrin-fibronectin bonds in intact adherent cells is sensitive to integrin activation state. J. Biol. Chem. 273, 10988–10993. [DOI] [PubMed] [Google Scholar]
- Garcia, A.J., Takagi, J., and Boettiger, D. (1998b). Two-stage activation for alpha5beta1 integrin binding to surface-adsorbed fibronectin. J. Biol. Chem. 273, 34710–34715. [DOI] [PubMed] [Google Scholar]
- Guan, J.L., Trevithick, J.E., and Hynes, R.O. (1991). Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 2, 951–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, D.C., and Guan, J.L. (1999). Association of focal adhesion kinase with Grb7 and its role in cell migration. J. Biol. Chem. 274, 24425–24430. [DOI] [PubMed] [Google Scholar]
- Hunter, T., and Cooper, J.A. (1981). Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell 24, 741–752. [DOI] [PubMed] [Google Scholar]
- Hynes, R.O. (1990). Fibronectins. New York: Springer-Verlag.
- Ishida, T., Takahashi, M., Corson, M.A., and Berk, B.C. (1997). Fluid shear stress-mediated signal transduction: how do endothelial cells transduce mechanical force into biological responses? Ann. NY Acad. Sci. 811, 12–23. [DOI] [PubMed] [Google Scholar]
- Jiang, G., and Hunter, T. (1999). Receptor signaling: when dimerization is not enough. Curr. Biol. 9, R568–R571. [DOI] [PubMed] [Google Scholar]
- Kornberg, L., Earp, H.S., Parsons, J.T., Schaller, M., and Juliano, R.L. (1992). Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J. Biol. Chem. 267, 23439–23442. [PubMed] [Google Scholar]
- Kornberg, L.J., Earp, H.S., Turner, C.E., Prockop, C., and Juliano, R.L. (1991). Signal transduction by integrins: increased proteins tyrosine phosphorylation caused by clustering of β1 integrins. Proc. Natl. Acad. Sci. USA 88, 8392–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossiakoff, A.A., and de Vos, A.M. (1998). Structural basis for cytokine hormone-receptor recognition and receptor activation. Adv. Protein Chem. 52, 67–108. [DOI] [PubMed] [Google Scholar]
- Leahy, D.J., Aukhil, I., and Erickson, H.P. (1996). 2.0 A crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell 84, 155–164. [DOI] [PubMed] [Google Scholar]
- Livneh, E., Prywes, R., Kashles, O., Reiss, N., Sasson, I., Mory, Y., Ullrich, A., and Schlessinger, J. (1986). Reconstitution of human epidermal growth factor receptors and its deletion mutants in cultured hamster cells. J. Biol. Chem. 261, 12490–12497. [PubMed] [Google Scholar]
- Mellado, M., Vila-Coro, A.J., Martinez, C., and Rodriguez-Frade, J.M. (2001). Receptor dimerization: a key step in chemokine signaling. Cell. Mol. Biol. 47, 575–582. [PubMed] [Google Scholar]
- Menko, A.S., and Boettiger, D. (1987). Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell 51, 51–57. [DOI] [PubMed] [Google Scholar]
- Miyamoto, S., Teramoto, H., Coso, O.A., Gutkind, J.S., Burbelo, P.D., Akiyama, S.K., and Yamada, K.M. (1995). Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol. 131, 791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, J.D., Ruest, P.J., Fry, D.W., Hanks, S.K. (1999). Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 19, 4806–4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov, R., Cukierman, E., Katz, B.Z., Matsumoto, K., Lin, D.C., Lin, S., Hahn, C., and Yamada, K.M. (2000). Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J. Cell Biol. 148, 1075–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, K.L., Premont, R.T., and Lefkowitz, R.J. (2002). Seven-transmembrane receptors. Nat. Rev. Mol. Cell. Biol. 3, 639–650. [DOI] [PubMed] [Google Scholar]
- Plotnikov, A.N., Hubbard, S.R., Schlessinger, J., and Mohammadi, M. (2000). Crystal structures of two FGF-FGFR complexes reveal the determinants of ligand-receptor specificity. Cell 101, 413–424. [DOI] [PubMed] [Google Scholar]
- Redick, S.D., Settles, D.L., Briscoe, G., and Erickson, H.P. (2000). Defining fibronectin's cell adhesion synergy site by site-directed mutagenesis. J. Cell Biol. 149, 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiske, H.R., Kao, S.C., Cary, L.A., Guan, J.L., Lai, J.F., and Chen, H.C. (1999). Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J. Biol. Chem. 274, 12361–12366. [DOI] [PubMed] [Google Scholar]
- Schaller, M.D., Hildebrand, J.D., Shannon, J.D., Fox, J.W., Vines, R.R., and Parsons, J.T. (1994). Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell Biol. 14, 1680–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer, D.D., Hauck, C.R., and Sieg, D.J. (1999). Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71, 435–478. [DOI] [PubMed] [Google Scholar]
- Schlaepfer, D.D., and Hunter, T. (1997). Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J. Biol. Chem. 272, 13189–13195. [DOI] [PubMed] [Google Scholar]
- Schlessinger, J. (2000). Cell signaling by receptor tyrosine kinases. Cell 103, 211–225. [DOI] [PubMed] [Google Scholar]
- Schwartz, M.A. (2001). Integrin signaling revisited. Trends Cell Biol. 11, 466–470. [DOI] [PubMed] [Google Scholar]
- Schwartz, M.A., Schaller, M.D., and Ginsberg, M.H. (1995). Integrins: emerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. 11, 549–599. [DOI] [PubMed] [Google Scholar]
- Singer, I.I., Scott, S., Kawka, D.W., Kazazis, D.M., Gailit, J., and Ruoslahti, E. (1988). Cell surface distribution of fibronectin and vitronectin receptors depends on substrate composition and extracellular matrix accumulation. J. Cell Biol. 106, 2171–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima, E., Del Pozo, M.A., Shattil, S.J., Chien, S., and Schwartz, M.A. (2001). Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 20, 4639–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann, C., Fuh, G., Christinger, H.W., Eigenbrot, C., Wells, J.A., and de Vos, A.M. (1997). Crystal structure at 1.7 A resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell 91, 695–704. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Chattopadhyay, A., Ji, Q.S., Owen, J.D., Ruest, P.J., Carpenter, G., and Hanks, S.K. (1999). Focal adhesion kinase promotes phospholipase C-gamma1 activity. Proc. Natl. Acad. Sci. USA 96, 9021–9026. [DOI] [PMC free article] [PubMed] [Google Scholar]