Abstract
We describe the isolation and characterization of a homologous pair of proteins, Pex25p (YPL112c) and Pex27p (YOR193w), whose C-termini are similar to the entire Pex11p. All three proteins localize to the peroxisomal membrane and are likely to form homo-oligomers. Deletion of any of the three genes resulted in enlarged peroxisomes as revealed by fluorescence and electron microscopy. The partial growth defect on fatty acids of a pex25Δ mutant was not exacerbated by the additional deletion of PEX27; however, when PEX11 was deleted on top of that, growth was abolished on all fatty acids. Moreover, a severe peroxisomal protein import defect was observed in the pex11Δpex25Δpex27Δ triple mutant strain. This import defect was also observed when cells were grown on ethanol-containing medium, where peroxisomes are not required, suggesting that the function of the proteins in peroxisome biogenesis exceeds their role in proliferation. When Pex25p was overexpressed in the triple mutant strain, growth on oleic acid was completely restored and a massive proliferation of laminar membranes and peroxisomes was observed. Our data demonstrate that Pex11p, Pex25p, and Pex27p build a family of proteins whose members are required for peroxisome biogenesis and play a role in the regulation of peroxisome size and number.
INTRODUCTION
Peroxisomes are ubiquitous eucaryotic organelles that harbor a wide variety of metabolic processes. Foremost of these is a complete β-oxidation system able to metabolize very long, long, and medium-chain (MCFA) fatty acids, which is exclusively peroxisomal in higher plants and yeast (van den Bosch et al., 1992; Kunau et al., 1995). Peroxisomes constitute a dynamic compartment whose biogenesis is inducible when the specialized functions contained are most needed. In the yeast Saccharomyces cerevisiae for instance, fatty acids as sole carbon source elicit a response that causes not only a transcriptional up-regulation of genes encoding the peroxisomal β-oxidation machinery, but also a massive increase in peroxisomal numbers (Veenhuis et al., 1987). This response is mediated by the oleate response element (ORE) and involves the transcription factor Pip2p-Oaf1p, the activity of which is strictly dependent on fatty acids (Karpichev et al., 1997; Rottensteiner et al., 1997; Baumgartner et al., 1999).
According to the peroxisomal growth and division model, proliferation of peroxisomes occurs by division of existing organelles once a threshold size has been overstepped (Lazarow and Fujiki, 1985). This model probably suffices to explain peroxisome dynamics and inheritance under normal circumstances; however, the existence of a second and independent mechanism was postulated to explain the reappearance of peroxisomal structures in mutant cells apparently completely lacking peroxisomal membranes upon introduction of the affected gene (Kunau and Erdmann, 1998; Titorenko and Rachubinski, 2001). The formation of peroxisomes may therefore conceptually be divided into the processes of peroxisomal membrane synthesis, protein transport, and peroxisome proliferation. Genes controlling peroxisome biogenesis are collectively named PEX genes, the majority of which are linked to matrix protein import (Holroyd and Erdmann, 2001; Purdue and Lazarow, 2001). By contrast, genes that are considered as being specifically involved in peroxisome proliferation are scarce. PEX11 was the first such gene to be identified (Erdmann and Blobel, 1995; Marshall et al., 1995), and it was long regarded as being the key in driving peroxisome proliferation.
In mammals, three isoforms of Pex11p have been identified to date (Abe et al., 1998; Passreiter et al., 1998; Schrader et al., 1998; Li et al., 2002). Pex11β is able to significantly induce peroxisome proliferation upon ectopic expression; however, knock-out mice devoid of both Pex11α and Pex11β still contain numerous peroxisomes, are only mildly affected in several key biochemical activities, and exhibit no obvious peroxisomal protein import defect (Li et al., 2002). Nonetheless, these mice die early after birth because of severe neurological defects, thereby challenging the idea of a major role for Pex11p in peroxisome proliferation.
Peroxisome abundance seems also to be modulated by a phenomenon known as metabolic control. This process might be governed by a signal derived from the oxidation of fatty acids (Chang et al., 1999) or possibly more specifically of MCFA (van Roermund et al., 2000); however, whether active peroxisomal β-oxidation can be the predominant factor controlling proliferation is currently under debate (Smith et al., 2000; van Roermund et al., 2000; Li and Gould, 2002). Other proteins implicated in peroxisome dynamics include the dynamin-like proteins Vps1p of S. cerevisiae and DLP1 of rat, which are necessary for peroxisomal fission in interphase cells and the Pex25p of S. cerevisiae, whose absence gives rise to enlarged peroxisomes and noticeable a partial protein import defect (Hoepfner et al., 2001; Smith et al., 2002; Koch et al., 2003).
In this study, we functionally characterize Pex25p along with its newly identified homologue Pex27p and analyze their roles in peroxisome proliferation. We further link the function of this pair of proteins to that of Pex11p and suggest that these proteins form a family of common function, the Pex11p family. We show that in the concurrent absence of all three proteins, defects in peroxisome proliferation, protein import and fatty acid utilization appear. We discuss our findings in terms of the functions of the members of this protein family in peroxisome biogenesis, especially in regulating the size and number of peroxisomes.
MATERIALS AND METHODS
Strains, Media, and Growth Conditions
Escherichia coli strain DH5α was used for plasmid amplifications and isolations. BL21 (DE3) was used to express the 6× His-Pex25p fragment from pETak. The S. cerevisiae strains used are listed in Table 1. Standard media for the cultivation of yeast and bacterial strains were prepared as described (Erdmann et al., 1989; Sambrook et al., 1989; Palmieri et al., 2001). Wild-type strain BY4742 and the otherwise isogenic gene deletion strains were obtained from EUROSCARF (Frankfurt, Germany). PEX25 (YPL112c) was deleted using a PCR fragment that had been obtained by PCR amplification from plasmid pUC45Kan with primer pair RE39/40. Plasmid pUC45Kan was constructed by replacing the EcoRV-BamHI fragment of the PEX25 reading frame with the kanMX4 cassette from plasmid pFA6 (Wach et al., 1994). Correct gene replacement of G418-resistant transformants was verified by PCR. PEX27 (YOR193w) was similarly deleted using a loxP-flanked kan-MX4 cassette that had been obtained by PCR using primer pair RE332/333 and plasmid pUG6 as template. The resistance marker was subsequently removed by the action of Cre recombinase as described (Güldener et al., 1996). PEX11 was deleted by transformation with a LEU2-based gene deletion cassette that had been amplified with primer pair RE383/384 from pex11Δ (Erdmann and Blobel, 1995). Genomic TAP-tagging was achieved by transforming BY4742 with DNA fragments that had been obtained by PCR reactions using plasmid pBS1539 (Rigaut et al., 1999) as template. Specifically, the following primers were used: RE736/737 for PEX27 (yHPR349), RE738/739 for PEX25 (yHPR348), and RE443/444 for PEX11 (yHPR345).
Table 1.
S. cerevisiae strains used
| Strain | Description | Source or Reference |
|---|---|---|
| S. cerevisiae | ||
| UTL7-A | MATα leu2-3, 112 ura3-52 trp1 | Erdmann et al. (1989) |
| UTL7-Apex11Δ | UTL7-A pex11Δ::LEU2 | Erdmann and Blobel (1995) |
| yKat2 | UTL7-A pex25Δ::kanMX4 | This study |
| yKat6 | UTL7-A pex27Δ::loxP | This study |
| yKat53 | UTL7-A pex25Δ::kanMX4 pex27Δ::loxP | This study |
| yHPR347 | UTL7-A pex11Δ::LEU2 pex25Δ::kanMX4 pex27Δ::loxP | This study |
| yHPR251 | UTL-7A [PTS2-DsRed] | Stein et al. (2002) |
| PJ69-4A | MATa ura3-52 his3-200 trp1-901 leu2-3/112gal4Δ gal80Δ LYS2::GAL1-HIS3 met2::GAL7-lacZ GAL2-ADE2 | P. James, Madison |
| BY4742 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Brachmann et al. (1998) |
| BY4742pex11Δ | pex11Δ::kanMX4 | Euroscarf, Frankfurt |
| BY4742pex25Δ | pex25Δ::kanMX4 | Euroscarf, Frankfurt |
| BY4742pex27Δ | pex27Δ::kanMX4 | Euroscarf, Frankfurt |
| BY4742fox3Δ | fox3Δ::kanMX4 | Euroscarf, Frankfurt |
| BY4742pxa1Δ | pxa1Δ::kanMX4 | Euroscarf, Frankfurt |
| yHPR345 | PEX11-TAP::URA3 | This study |
| yHPR348 | PEX25-TAP::URA3 | This study |
| yHPR349 | PEX27-TAP::URA3 | This study |
Plasmid Constructions
All plasmids generated in this study are listed in Table 2. PEX25, PEX27, and PEX11 were amplified from S. cerevisiae genomic DNA and cloned into the various expression vectors using the restriction sites and primer pairs as indicated in Table 2. Overexpression was achieved by cloning PEX25 (RE39/40), PEX27 (RE330/331), and PEX11 (RE383/384) under control of their own promoters into episomal plasmids; PEX25 was cloned into YEp352 (Hill et al., 1986), and PEX27 and PEX11 into YEplac195 (Gietz and Sugino, 1988).
Table 2.
Plasmids and oligonucleotides used
| Plasmid or oligonucleotide | Sequence | Primer pair | Source or Reference | ||
|---|---|---|---|---|---|
| Plasmid | |||||
| pRS416 | URA3-marked centromeric plasmid | Sikorski and Hieter (1989) | |||
| YCplac33 | URA3-marked centromeric plasmid | Gietz and Sugino (1988) | |||
| pUG35 | vector for C-terminal GFP fusions | Mumberg et al. (1994) | |||
| pUG36 | vector for N-terminal GFP fusions | Mumberg et al. (1994) | |||
| pPC86 | Gal4p-AD two-hybrid vector | Chevray and Nathans (1992) | |||
| pPC97 | Gal4p-BD two-hybrid vector | Chevray and Nathans (1992) | |||
| pLW100 | MLS1pr-GFP-SKL | Lametschwandtner et al. (1998) | |||
| pHPR129 | ANT1-GFP-prA | Palmieri et al. (2001) | |||
| pSK45 | PEX25 in pSK/EcoRI-XbaI | RE39/40 | This study | ||
| pSL45 | PEX25 in pRS416/EcoRI-XbaI | RE39/40 | This study | ||
| pSH45 | PEX25 in YEp352/EcoRI-XbaI | RE39/40 | This study | ||
| P45-GFP | PEX25 in pUG35/EcoRI-XbaI | RE41/56 | This study | ||
| GFP-P45 | PEX25 in pUG36/EcoRI-SalI | RE41/55 | This study | ||
| PETak | PEX25173-248 in pET21b/NdeI-XhoI | RE43/44 | This study | ||
| PSK45-KanMX4 | pSK45::kanMX4/EcoRV-BamHI | This study | |||
| pPC97/45 | PEX25 in pPC97/BglII-SalI | RE57/55 | This study | ||
| pPC86/45 | PEX25 in pPC86/BglII-SalI | RE57/55 | This study | ||
| pKat78 | PEX27 in pUG35/BamHI-SalI | RE242/244 | This study | ||
| pKat84 | PEX27 in pUG36/BamHI-SalI | RE242/243 | This study | ||
| pKat101 | PEX27 in pPC86/SalI-BglII | RE337/338 | This study | ||
| pKat115 | PEX27 in pPC97/SalI-BglII | RE337/338 | This study | ||
| pKat107 | PEX27 in YCplac33/SmaI | RE330/331 | This study | ||
| pKat108 | PEX27 in YEplac195/SmaI | RE330/331 | This study | ||
| pKat56 | PEX11 in pPC86/EcoRI-SacI | RE167/168 | This study | ||
| pKat71 | PEX11 in pPC97/EcoRI-SacI | RE167/168 | This study | ||
| pHPR167 | PEX11 in YEplac195/SphI-BamHI | RE383/384 | This study | ||
| Oligonucleotide | |||||
| RE39 | 5′-ATCGAATTCACCCTGATGTCCTCGGATCG-3′ | ||||
| RE40 | 5′-TGCATCTAGATAGATCTGCGTCAGTGTCAG-3′ | ||||
| RE41 | 5′-CAGGAATTCCATATGAGTCAGTTTGGCACGAC-3′ | ||||
| RE43 | 5′-CAGGAATTCCATATGGGCGGCACACCATTCCG-3′ | ||||
| RE44 | 5′-GGATCTAGACTCGAGAGACTCTTGTCTACTGACA-3′ | ||||
| RE55 | 5′-GGATCTAGAGTCGACTCAATCTTTTGAAGAGCAAAGTG-3′ | ||||
| RE56 | 5′-GGATCTAGAGTCGACATCTTTTGAAGAGCAAAGTGAC-3′ | ||||
| RE57 | 5′-CAGGAATTCAGATCTCAATGAGTCAGTTTGGCACG-3′ | ||||
| RE167 | 5′-CGGAATTCATATGGTCTGTGATACACTG-3′ | ||||
| RE168 | 5′-GCGAGCTCCTATGTAGCTTTCCACAT-3′ | ||||
| RE242 | 5′-GGATCCATGACATCCGATCCTGTT-3′ | ||||
| RE243 | 5′-GTCGACTCAAACAGCGCTTGTATG-3′ | ||||
| RE244 | 5′-GTCGACAACAGCGCTTGTATGTTC-3′ | ||||
| RE330 | 5′-TCAACAGATTCACATTCCAA-3′ | ||||
| RE331 | 5′-TCGGTTCAGAACTAGATGAT-3′ | ||||
| RE332 | 5′-GTAGACTATGACCTTTGTGTTAACTTGGACAATCGTTTTATCATGCAGCTGAAGCTTCGTACGCT-3′ | ||||
| RE333 | 5′-AAACGAAATAAAGAGGGATGCAACGAACTTGGTCATCTGTTGTCAATAGGCCACTAGTGGATCTG-3′ | ||||
| RE337 | 5′-CGGAATTCGTCGACCATGACATCCGATCCTGTT-3′ | ||||
| RE338 | 5′-CCCTCGAGAGATCTTCAAACAGCGCTTGTATG-3′ | ||||
| RE383 | 5′-AATTGCATGCTCGTTATGGGTCGTCCTGGG-3′ | ||||
| RE384 | 5′-AATTGGATCCCTAGCGGCCGCATGTAGCTTTCCACATGTCTT-3′ | ||||
| RE443 | 5′-GGTGTTGTCACATCTATCCTTGGTATGCAAGACATGTGGAAAGCTACATCCATGGAAAAGAGAAG-3′ | ||||
| RE444 | 5′-AAATAAATTATAAAGAAGGGTCGAATCAAACATAAGCGGAGAATAGCCTACGACTCACTATAGGG-3′ | ||||
| RE736 | 5′-AACCGAGCCAAAGTCACTTCGGCTAATGAACATACAAGCGCTGTTTCCATGGAAAAGAGAAG-3′ | ||||
| RE737 | 5′-AGTGGAAGCGGAGTGGGTATAACTAAATCTTTAGACGTAAAGAAGTACGACTCACTATAGGG-3′ | ||||
| RE738 | 5′-AAACTTTGGATAACAACAAAGAGGTCACTTTGCTCTTCAAAAGATTCCATGGAAAAGAGAAG-3′ | ||||
| RE739 | 5′-GAGGTAAAACATTATTCGCCACATATATATGTACATATCTATATGTACGACTCACTATAGGG-3′ | ||||
Antibodies and Immunoblotting
Antibodies against Fox3p, Mir1p, Pex11p (Erdmann and Blobel, 1995), Kar2p (Rose et al., 1989), protein A (Sigma, St. Louis, MO), green fluorescent protein (GFP; W.H. Kunau, Bochum), Cta1p (Gurvitz et al., 1997), and monoclonal anti-yeast 3-phosphoglycerate kinase, Pgk1p (Molecular Probes, Eugene, OR) have been described previously. To obtain polyclonal antibodies against Pex25p, a fragment of PEX25 coding for amino acids 173–248 was cloned into pET21b (Novagen, Madison, WI). The resulting plasmid pETak was used to express the 6× His-tagged fragment in E. coli strain BL21 (DE3). The protein fragment was purified under denaturing conditions by affinity chromatography using a nickel-nitrilotriacetic acid (Ni-NTA) matrix (Invitrogen, De-Schelp, The Netherlands) and was subsequently used to immunize rabbits at Eurogentech (Serain, Belgium). Immunoreactive complexes were visualized using anti-rabbit or anti-mouse IgG-coupled horseradish peroxidase in combination with the ECL system from Amersham Biosciences (Freiburg, Germany).
Biochemical Procedures
The separation and subsequent sequence analysis of peroxisomal membrane proteins have been described previously (Erdmann and Blobel, 1995; Henke et al., 1998). Differential centrifugation of fractionated yeast lysates at 25,000 × g was performed as described (Erdmann et al., 1989). Protease protection assays were carried out by treating crude organellar pellets with 100 μg/ml proteinase K in the presence or absence of Triton X-100. Protein extraction of organellar pellets with low salt, high salt, and carbonate, pH 11, was performed as described (Erdmann and Blobel, 1995). Peroxisomes were separated from mitochondria in a vertical rotor (SV-288, Sorvall RCB5, DuPont, BadNauheim, Germany) on continuous 20–53% (wt/wt) sucrose gradients (run for 1.5 h at 48,000 × g).
Fluorescence and Electron Microscopy
Microscopy of live cells for DsRed and GFP fluorescence was performed according to Westermann and Neupert (Westermann and Neupert, 2000). PTS1- and PTS2-dependent protein import was analyzed by expressing GFP-SKL from plasmid pLW100 (Lametschwandtner et al., 1998) and PTS2-DsRed from pHPR131 (Stein et al., 2002), respectively. Electron microscopy of oleic acid–induced cells, fixed in 2% paraformaldehyde and 0.5% glutaraldehyde, was performed as described (Erdmann et al., 1989; Höhfeld et al., 1992).
Miscellaneous
The following procedures were performed according to published methods: nucleic acid manipulations, yeast transformation, determination of protein concentration, and electrophoresis (Sambrook et al., 1989), the preparation of yeast whole-cell extracts (Yaffe and Schatz, 1984), and the activity measurements of catalase and fumarase (Moreno de la Garza et al., 1985).
RESULTS
Identification and Subcellular Localization of Pex25p (Ypl112cp)
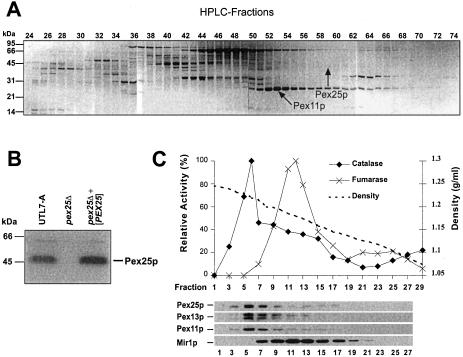
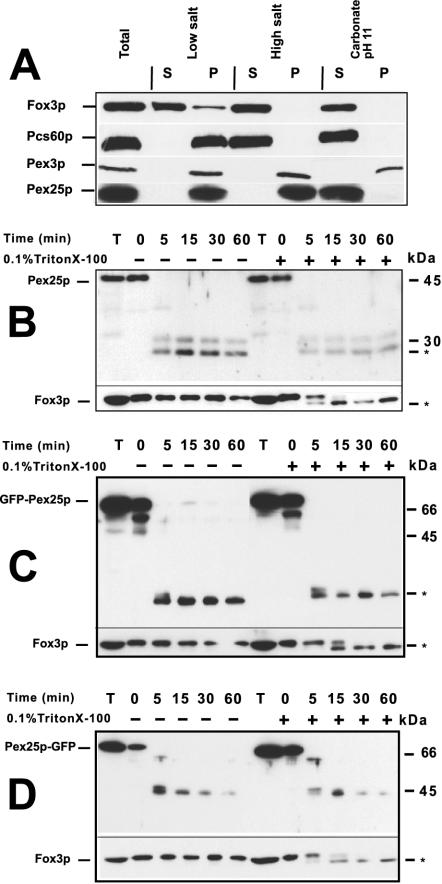
A preparation of S. cerevisiae peroxisomal membranes was solubilized by SDS and subjected to reversed-phase high-performance liquid chromatography. The resulting fractions were further separated by SDS-PAGE and analyzed as described (Erdmann and Blobel, 1995; Henke et al., 1998). A distinct band with an apparent molecular weight of 45 kDa was identified as Ypl112cp (arrow in Figure 1A) by sequencing of the N-terminal and Lys-C derived internal peptides. Recently, YPL112c was also identified in a screen for oleic acid–induced genes and renamed as PEX25 (Smith et al., 2002). An antibody directed against a bacterially expressed fragment of Pex25p turned out to recognize Pex25p in yeast lysates of a wild-type strain, but not of a pex25Δ strain (Figure 1B). The specific anti-Pex25p antibody was used to demonstrate cosedimentation of Pex25p with peroxisomes in a sucrose density gradient (Figure 1C). Treatment of a 25,000 × g organellar pellet with low-salt, high-salt, or carbonate (pH 11) revealed that Pex25p was only extractable by carbonate (Figure 2A), which was in agreement with data that had used a protein A–tagged version of Pex25p (Smith et al., 2002).
Figure 1.
Identification of Pex25p (YPL112c) as a peroxisomal protein. (A) Reversed-phase HPLC separation of peroxisomal membrane proteins. A protein band of 45 kDa appearing in fractions 57–63 (marked by the arrow) was analyzed by Edman sequencing of N-terminal and Lys-C–derived peptide fragments. The peptide sequences obtained (SRSDDEDSQAKTKMV, xLVYLDIARLSFDCMANTS) matched the translation product of the S. cerevisiae open reading frame YPL112c/PEX25. (B) Specificity of a polyclonal antibody against Pex25p. Whole-cell extracts of the indicated oleic acid–induced strains were analyzed for the appearance of a Pex25p-dependent band by immunoblotting with antiserum that had been raised against a bacterially expressed fragment of Pex25p. (C) Cosedimentation of Pex25p with peroxisomes in a sucrose density gradient. A cell-free extract of an oleic acid–induced wild-type strain (UTL7-A) was separated on a 20–53% (wt/vol) sucrose density gradient. The resulting fractions were subjected to immunoblot analysis using antibodies against Pex25p, the peroxisomal Pex11p and Pex13p, and mitochondrial Mir1p (bottom). The enzyme activities of peroxisomal catalase and mitochondrial fumarase were also determined (top).
Figure 2.
Pex25p is a peripheral membrane protein with both termini exposed to the cytosol. (A) Subperoxisomal fractionation analysis. A 25,000 × g organellar pellet of an oleic acid–induced wild-type strain (UTL7-A) was split into three parts and extracted with low salt (10 mM Tris/Cl pH 7.5), high salt (10 mM Tris/Cl, pH 7.5; 0.5 M KCl), or 100 mM Na2CO3 (pH 11.0) for 30 min on ice. The subsequent separation of membrane-bound (P) and soluble proteins (S) was achieved by a 200,000 × g centrifugation step. Equal amounts of each fraction were analyzed for the distribution of Pex25p, the peroxisomal matrix protein Fox3p, the integral membrane protein Pex3p, and the membrane-associated Pcs60p (Fat2p). (B) Protease protection assay. Such an organellar pellet was also treated with 100 μg/ml proteinase K in the presence or absence of 0.1% Triton X-100. Samples were removed at the indicated time points, precipitated, and subjected to immunoblot analysis using antibodies against intraperoxisomal Fox3p and Pex25p. Asterisks denote stable proteolytic fragments. (C and D) Protease protection assays of GFP-Pex25p and Pex25p-GFP. Organellar pellets of a pex25Δ strain expressing either GFP-Pex25p (C) or Pex25p-GFP (D) were assayed for accessibility to externally added protease as described in B, except that anti-GFP antibodies were used.
Treatment of such an organellar pellet with proteinase K did result in the degradation of Pex25p in the absence of Triton X-100, whereas the peroxisomal matrix protein Fox3p was protected under these conditions (Figure 2B), indicating that Pex25p is accessible to externally added protease. To address the topology of the N- and C-termini of Pex25p, the GFP was fused to either end of Pex25p and expressed in a pex25Δ mutant (see below). Organellar pellets were prepared and treated with proteinase K. In this set of experiments, anti-GFP antibodies were used to analyze the fate of the GFP moieties. In the absence of Triton X-100, degradation products were detected for both fusion proteins, but not for Fox3p (Figure 2, C and D). These fragments did also form in the presence of detergent, indicating that they represented protease-resistant fragments rather than protected remains of an integral protein. A protease-resistant fragment is also observed for Fox3p. Taken together, these results suggested that Pex25p is a peripheral peroxisomal membrane protein localized to the cytosolic side of the peroxisomal membrane.
Identification of PEX27 (YOR193w), a Homologue of PEX25
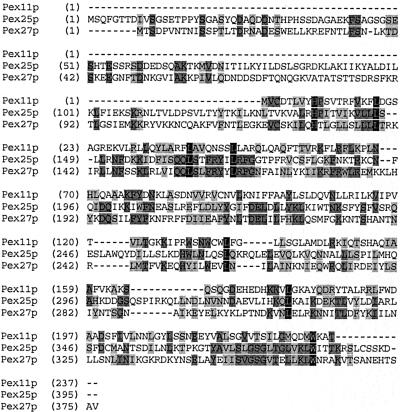
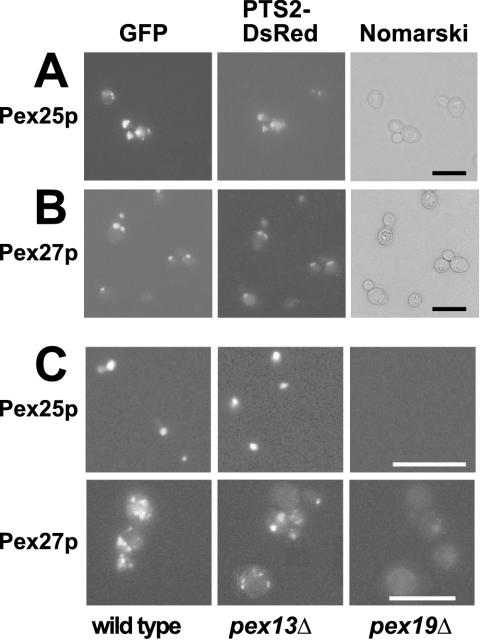
Scrutiny of the SGD database revealed an open reading frame, YOR193w, designated here as PEX27, that is highly similar to PEX25 over its entire sequence (Figure 3). The subcellular location of the novel homologous protein was examined by fluorescence microscopy using fusions of Pex27p with GFP at its N- and C-terminus, respectively. In parallel, the localization of Pex25p was investigated. The fusion proteins were coexpressed with PTS2-DsRed, a fluorescent peroxisomal marker protein (Stein et al., 2002). In oleic acid–induced wild-type strains, punctate staining patterns were observed for both Pex25p and Pex27p (Figure 4, A and B). These punctate structures clearly colocalized with those of PTS2-DsRed, demonstrating that Pex25p and Pex27p are peroxisomal proteins. Expression of GFP-Pex25p and Pex27p-GFP in pex13Δ mutant strains retained the punctate staining patterns, whereas in pex19Δ strains staining appeared diffuse (Figure 4C). These observations indicate that Pex25p and Pex27p are targeted to peroxisomes via the transport route for peroxisomal membrane proteins, as only in the pex19Δ mutant strain membrane protein insertion is compromised (Hettema et al., 2000).
Figure 3.
Sequence alignment of the S. cerevisiae Pex11p family. The amino acid sequences of Pex11p, Pex25p, and Pex27p were aligned by using the Vector NTI Advance Software package (Infor-Max Inc., Oxford, UK). Residues that are identical in all three sequences are shaded in black, and those in two out of three in dark gray. Blocks of similar residues are shaded in light gray. Similarity rule A = G, S; D = E; F = W = Y; H = Y; I = L = M = V; Q = N; R = K; S = A, T.
Figure 4.
Pex27p (YOR193w) and Pex25p are peroxisomal membrane proteins. Wild-type strains (UTL7-A) coexpressing PTS2-DsRed with either Pex25p-GFP (A) or GFP-Pex27p (B) were grown on solid oleic acid–containing medium for 2 d and were subsequently examined for GFP and DsRed fluorescence, respectively. Colocalization with PTS2-DsRed indicates peroxisomal targeting of the GFP fusion proteins. Nomarski images demonstrate structural integrity of the cells. Bar, 10 μm. (C) Targeting of Pex25p and Pex27p depends on the peroxisomal membrane protein route. Both GFP fusion proteins were expressed in pex13Δ and pex19Δ strains, which are impaired in matrix and membrane protein import, respectively, and examined for GFP fluorescence. Bar, 10 μm.
PEX27 Is a High-copy Suppressor of a pex25 Mutant on Oleic Acid
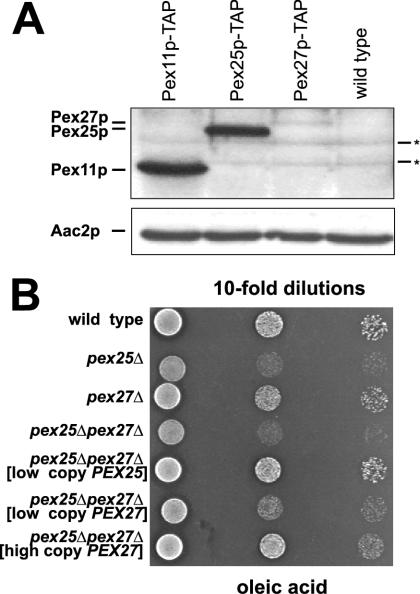
Mutations that affect the integrity of the peroxisomal compartment in S. cerevisiae will lead to a growth defect on media containing fatty acids as sole carbon source, because their breakdown obligatorily depends on peroxisomal fatty acid β-oxidation (Erdmann et al., 1989). It was therefore tested whether deletions in PEX25, PEX27 or in both genes together would affect growth on oleic acid plates. A partial growth defect was observed for the pex25Δ mutant strain, whereas the absence of Pex27p did not cause any retardation of growth. The growth phenotype of the pex25Δ mutant was also not exacerbated by additionally deleting PEX27 (Figure 5B). These data suggested that Pex25p represents the major isoform of this pair of proteins.
Figure 5.
Pex25p and Pex27p are functionally conserved (A) Comparative expression of Pex11p, Pex25p, and Pex27p. Equal amounts of whole-cell extracts of the oleic acid–induced strains yHPR345 (PEX11-TAP), yHPR348 (PEX25-TAP), yHPR349 (PEX27-TAP), and the untagged wild-type control were analyzed for the amounts of the corresponding tagged proteins by immunoblot analysis using anti-protein A antibodies. Asterisks indicate nonspecific cross-reactions of the antibody. (B) Overexpression of Pex27p suppresses the growth phenotype of a pex25Δ mutant. Together with the control strains, transformants of the pex25Δpex27Δ mutant strain harboring plasmids SL45 (low copy PEX25), pKat107 (low copy PEX27), or pKat108 (high copy PEX27) were spotted on an oleic acid plate in 10-fold dilutions and incubated for 5 d at 30°C.
Pex25p is oleic acid inducible (Smith et al., 2002) via an unusual ORE in its promoter (Rottensteiner et al., 2003), whereas Pex27p lacks an obvious ORE. Although a fusion of the PEX25 promoter with the lacZ gene revealed high β-galactosidase activities in oleic acid medium (Rottensteiner et al., 2003) a similar fusion of the PEX27 promoter failed to yield enzyme activities above background levels under this condition (our unpublished results). We therefore opted to genomically tag Pex27p with the TAP tag (Rigaut et al., 1999), which is composed of two successive epitope tags including protein A. To directly compare the abundance of Pex27p with that of Pex25p, strains harboring chromosomally tagged versions of PEX25 or PEX11 (see below) were also generated. The transformed strains were grown in the presence of oleic acid, and whole-cell extracts were subjected to immunoblot analysis. Decoration of the blots with anti–protein A antibodies revealed that the amount of the oleic acid–inducible Pex11p exceeded that of Pex25p, which in turn was significantly more abundant than Pex27p (Figure 5A). The specificity of the detected bands was established by a comparison with the untransformed wild-type control (lane 4). The signal intensities of Pex27p-TAP were similarly low in samples obtained from ethanol- or glucose-grown cells (our unpublished results), indicating that Pex27p is constitutively expressed on a low level. We therefore tested whether increasing amounts of Pex27p would reveal functional conservation with Pex25p. This possibility was examined by introducing PEX27 on a low- and a high-copy number plasmid and, as control, PEX25 on a low-copy number plasmid into the pex25Δpex27Δ double mutant. The resulting transformants were subjected to growth analysis on oleic acid plates. In low doses, PEX25 but not PEX27 restored wild-type growth behavior of the double-mutant strain (Figure 5B). However, in high doses, also PEX27 was capable of doing so, indicating that Pex27p shares a common function with Pex25p.
Pex25p and Pex27p Are Similar to Pex11p
Pex25p shows sequence similarities to Pex11p, as pointed out previously (Smith et al., 2002). Aligning Pex25p, Pex27p, and Pex11p revealed that the entire region of Pex11p matches with the C-terminal parts of the other two proteins, whereas Pex25p and Pex27p harbor an N-terminal extension of ∼100 amino acids (Figure 3). It is worth noting that the shared homologous region shows similarity to the ligand-binding domain of nuclear hormone receptors, particularly to that of PPARs (Barnett et al., 2000).
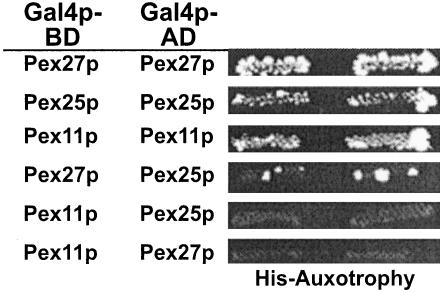
Another feature of Pex11p reported to be important for function is its ability to form homodimers (Marshall et al., 1996). Pex25p, Pex27p, and Pex11p were therefore tested for possible mutual interactions in a yeast two-hybrid assay. Clearly, all three proteins did interact with themselves (Figure 6). On the other hand, Pex25p interacted only very weakly with Pex27p, and Pex11p failed to interact with Pex25p or Pex27p. These data indicate that all three proteins probably act at least temporarily as homo-oligomers.
Figure 6.
Mutual two-hybrid interactions of Pex11p, Pex25p, and Pex27p. Full-length PEX11, PEX25, and PEX27 were fused to the GAL4 activation domain (Gal4p-AD) in vector pPC86 (Gal4p-AD) and GAL4 binding domain (Gal4p-BD) in vector pPC97, respectively, and tested pairwise in strain PJ69–4A for interaction in a yeast two-hybrid assay. Prototrophs on histidine dropout plates containing 1 mM aminotriazole were considered positive for interaction.
A pex11Δpex25Δpex27Δ Triple Deletion Is Unable to Use Fatty Acids as an Energy Source
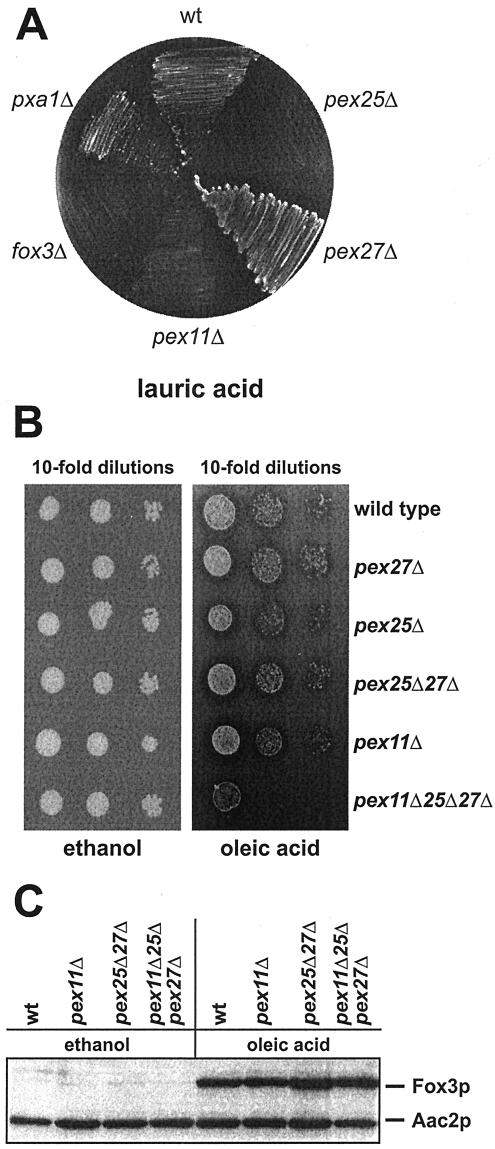
Loss of Pex11p results in a drastic phenotype on medium-chain fatty acids such as lauric acid, whereas the defect on long-chain fatty acids (i.e., oleic acid) is only partial. This observation was taken as evidence that Pex11p plays a primary role in the metabolism of MCFA (van Roermund et al., 2000). To assess whether the sequence similarity of Pex25p and Pex27p with Pex11p would be reflected in a conserved function, growth on lauric acid plates was analyzed. As anticipated, the wild-type strain BY4742, which is proficient in utilizing this fatty acid (Palmieri et al., 2001), grew well, whereas the fatty acid nonutilizing fox3Δ control strain failed to form colonies on this medium (Figure 7A). The inability of the pex11Δ mutant to grow on lauric acid was shared by the strain devoid of Pex25p, whereas a pex27Δ mutant grew normally. This observation rather pointed to a nonredundant function of Pex11p and Pex25p; however, because of the toxicity of lauric acid, even subtle impairments in fatty acid degradation might work out badly.
Figure 7.
Requirement of the Pex11p family for growth on fatty acids, but not for fatty acid signaling. (A) Growth analysis of the single mutants on lauric acid plates. The indicated BY4742-derived mutant strains were streaked on a lauric acid plate and incubated for 7 d at 30°C. (B) Growth analysis of the pex11Δpex25Δpex27Δ triple knock out strain. Tenfold dilutions of the indicated strains were spotted on ethanol and oleic acid plates and incubated for 5 d at 30°C. (C) Fatty acid signaling is not impaired in the triple mutant. The indicated strains were grown under induced (oleic acid) and noninduced (ethanol) conditions for 16 h. Whole-cell extracts were prepared and equal amounts subjected to immunoblot analysis with antisera against the oleic acid–inducible Fox3p and the constitutively expressed Aac2p.
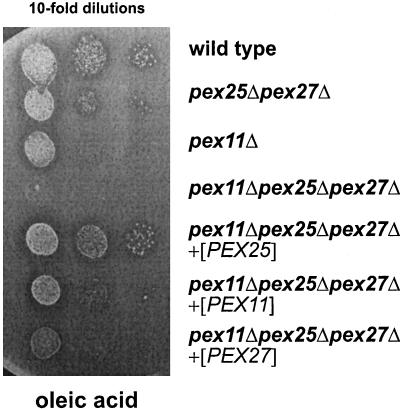
To better analyze a possible redundancy of Pex11p, Pex25p, and Pex27p, growth assays were carried out in the presence of oleic acid as sole carbon source. This fatty acid is well utilizable, and only a partial growth defect is observed for the pex11Δ single mutant or the pex25Δpex27Δ double mutant. A strain deleted in all three genes was therefore generated and its growth behavior compared with that of the single mutant strains. Strikingly, the triple mutant grew normally on ethanol plates, but did not grow at all on oleic acid (Figure 7B). These data clearly show an essential role for Pex11p, along with Pex25p and Pex27p, in the metabolism of both long-chain and medium-chain fatty acids.
The synthetic lethal effect of the triple knock out strain on oleic acid medium allowed for the first time an assessment of the role of this novel protein family in fatty acid signaling. This idea was particularly intriguing in light of the proteins' similarity to the ligand-binding domain of PPAR (Barnett et al., 2000), which would make them good candidates as lipid sensors. In that case, the absence of all three proteins should result in an inability to induce the fatty acid-triggered expression of peroxisomal β-oxidation enzymes (Karpichev et al., 1997; Rottensteiner et al., 1997). Whole-cell extracts from noninduced and oleic acid–induced cells were therefore analyzed for expression of 3-ketoacyl-CoA thiolase (Fox3p). The typical oleic acid-triggered increase in the amount of Fox3p was evident in the wild-type strain (Figure 7C, lanes 1 and 2), but also the tested mutant strains, foremost the triple knock-out strain (lane 8), had comparably induced amounts of Fox3p. Thus, the β-oxidation machinery is synthesized normally in the absence of the Pex11p family, indicating that these proteins are not involved in fatty acid signaling.
Peroxisomes Are Similarly Enlarged in All Mutant Strains
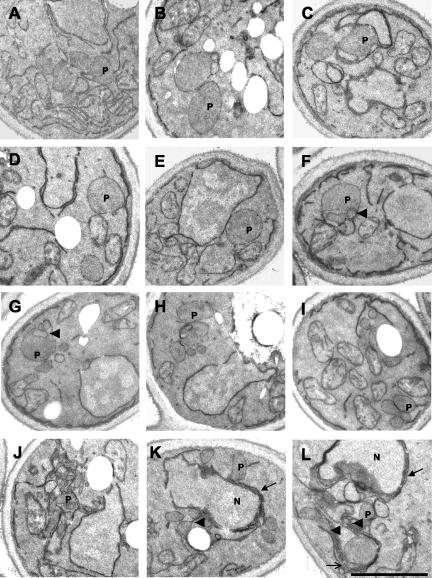
The appearance of giant peroxisomes in pex11Δ cells prompted an inspection of peroxisomal profiles in the analyzed mutant strains by means of electron microscopy (Figure 8). Cells were induced in oleic acid–containing medium and inspected for peroxisome size and number. The average peroxisomal size was significantly increased not only in the pex11Δ mutant (Figure 8B) but also in strains lacking Pex25p, Pex27p, or both proteins (Figure 8, C–E). Oleic acid-induction of the triple mutant resulted in a large number of cells lacking clear subcellular structures as is typical for dead cells (our unpublished results). Notwithstanding that, some cells did contain one or two peroxisomes that were about the same size than those of the single mutant strains, albeit these giant peroxisomes frequently contained intraperoxisomal membranes (arrowheads in Figure 8, F and G). Thus, a simple correlation between the mutant strains' peroxisome morphology and the severity of their growth phenotype on fatty acids did not emerge.
Figure 8.
Morphological analysis of cells devoid of Pex11p family members. The ultrastructure of the following strains was determined by electron microscopy: wild-type (A), pex11Δ (B), pex25Δ (C), pex27Δ (D), pex25Δpex27Δ (E), pex11Δpex25Δpex27Δ (F and G), pex11Δpex25Δpex27Δ + [PEX27] (H), pex11Δpex25Δpex27Δ + [PEX11] (I), and pex11Δpex25Δpex27Δ + [PEX25] (J–L). Strains were induced with oleic acid for 16 h before fixation. P, peroxisome; N, nucleus. Bar, 1 μm. See RESULTS for a description of arrows and arrowheads.
A pex11Δpex25Δpex27Δ Triple Deletion Is Impaired in Peroxisomal Protein Import
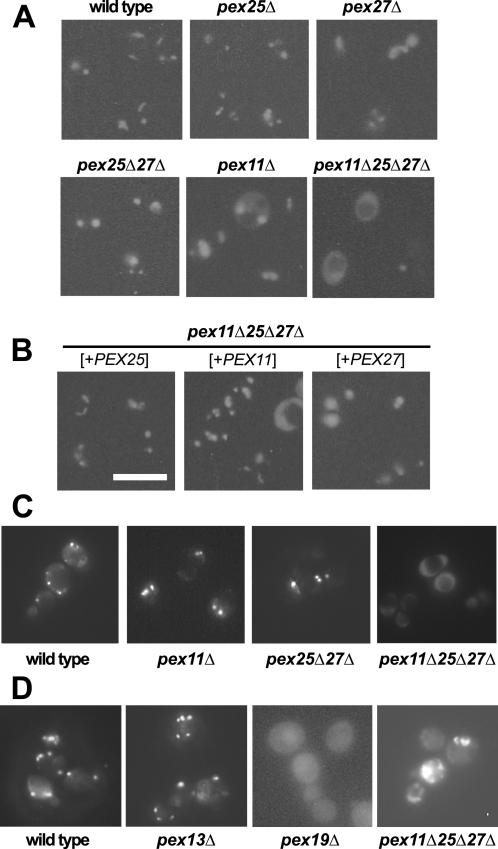
Smith et al. (2002) reported that a subpopulation of pex25Δ mutant cells exhibited a partial matrix protein import defect, whereas cells devoid of Pex11p were not affected. To that end, the strains described above were scrutinized for a possible defect in the import of a peroxisomally targeted GFP (GFP-SKL). Fluorescence microscopy of live cells that had been induced by oleic acid revealed a puncate staining pattern in the wild-type strain that is typical for peroxisomes (Figure 9A). The size of the fluorescent spots was somewhat larger in pex25Δ or pex27Δcells, and significantly larger in cells lacking either Pex11p or both Pex25p and Pex27p. Although in rare cases partial mislocalization of GFP-SKL was observed in the strains lacking Pex25p (our unpublished results), only in the absence of all three proteins, the majority of cells showed a diffuse staining pattern, the remaining cells contained giant fluorescing spots. Apparently, the peroxisomal compartment of the triple mutant failed to properly import GFP-SKL.
Figure 9.
Influence of the Pex11p family on peroxisomal protein import and peroxisome morphology. (A) Matrix protein import in oleic acid–induced cells. The indicated strains expressing peroxisomally targeted GFP-SKL from plasmid pLW100 were grown on solid oleic acid–containing medium for 2–3 d. Subsequently, live cells were inspected for GFP fluorescence. (B) Transformants of the pex11Δpex25Δpex27Δ triple mutant harboring in addition episomal plasmids designed to overexpress PEX11 (pHPR167), PEX27 (pKat108), or PEX25 (pSH45) were also analyzed. (C) Matrix protein import under noninducing conditions. The indicated strains all harboring plasmid pLW100 were grown in the presence of ethanol as sole carbon source, where S. cerevisiae peroxisomes are dispensable and were similarly inspected microscopically for GFP fluorescence. (D) Peroxisomal membrane protein import in the triple mutant. Live cells of the indicated oleic acid–induced strains were analyzed for the intracellular distribution of the peroxisomal membrane protein Ant1p-GFP (pHPR129) by GFP fluorescence. Bar, 10 μm.
To analyze whether also endogenous peroxisomal enzymes are mislocalized in the pex11Δpex25Δpex27Δ strain, postnuclear supernatants (PNS) from oleic acid–induced cells were subjected to differential centrifugation at 25,000 × g. Peroxisomal catalase as well as mitochondrial fumarase activities were assayed in the resulting organellar pellet and supernatant fractions. Comparable amounts of fumarase activity were found in the pellet fractions of all strains tested (Table 3), demonstrating that mitochondria remained intact upon preparing the organelles. The fractions of catalase that were pelletable from the PNS of the pex27Δ and the pex11Δ strains were reduced to ∼ 75% in relation to that of the wild-type strain and were further reduced in the strain lacking Pex25p. In the absence of both Pex25p and Pex27p, the value dropped to 30%. Finally, when Pex11p was absent on top of that, only 10% of the wild-type catalase activity was recovered in the pellet fraction (Table 3), suggesting that in the absence of all three factors matrix protein import is largely disabled.
Table 3.
Distribution of organellar enzyme activities
| Relative enzyme activities
|
||||||
|---|---|---|---|---|---|---|
| Catalase
|
Fumarase
|
|||||
| Strains | P | SN | Rel. level (%) | P | SN | Rel. level (%) |
| Wild type | 55 | 45 | 100 | 50 | 50 | 100.0 |
| pex11Δ | 42 | 58 | 76.3 | 47 | 53 | 94.0 |
| pex25Δ | 24 | 76 | 43.6 | 50 | 50 | 100.0 |
| pex27Δ | 41 | 59 | 74.5 | 49 | 51 | 98.0 |
| pex25Δpex27Δ | 15 | 85 | 27.2 | 53 | 47 | 106.0 |
| pex11Δpex25Δpex27Δ | 5 | 95 | 9.1 | 50 | 50 | 100.0 |
| Wild type | 59 | 41 | 100 | 49 | 51 | 100.0 |
| pex25Δpex27Δ | 16 | 84 | 27.1 | 48 | 52 | 97.9 |
| pex11Δpex25Δpex27Δ | 5 | 95 | 8.4 | 48 | 52 | 97.9 |
| +Pex11p | 34 | 66 | 57.6 | 55 | 45 | 112.2 |
| +Pex25p | 38 | 62 | 64.4 | 47 | 53 | 95.9 |
| +Pex27p | 17 | 83 | 28.8 | 48 | 52 | 97.9 |
Postnuclear supernatants (PNS) of the indicated strains were separated into a 25,000 × g organellar pellet (P) and a supernatant (SN) fraction. The sum of the enzyme activities measured in P and SN were taken as 100%. Relative level (Rel. level) refers to the activities of the indicated P fractions in relation to that of the wild type.
Because this defect might have been secondary to the triple mutant's inability to utilize fatty acids, we analyzed peroxisomal protein import also under conditions where peroxisomes are dispensable for yeast growth, i.e., in the presence of ethanol as a sole carbon source. The triple mutant grew comparable to the wild-type strain on ethanol medium (Figure 7B); nevertheless, it exhibited a diffuse staining pattern for GFP-PTS1 for most cells (Figure 9C). On the other hand, predominantly punctate staining patterns were observed for the pex11Δ, the pex25Δpex27Δ, and the wild-type strains, with the average size of peroxisomes appearing to be increased in the mutant strains. The phenotype of the mutants under these noninducing conditions is therefore similar to the phenotypes of oleic-acid induced mutant cells. Thus, the members of the Pex11p-family are already required for peroxisome biogenesis under conditions where peroxisomes are not needed for growth.
We also addressed whether targeting of peroxisomal membrane proteins would be affected in that mutant. To that end, we expressed a GFP fusion of the peroxisomal adenine nucleotide transporter Ant1p in the triple mutant. This protein has previously been shown to be targeted to peroxisomes in a Pex19p-dependent manner (Palmieri et al., 2001). The strains were induced with oleic acid and inspected for GFP fluorescence. The punctate staining pattern typical of peroxisomes was obtained for the wild-tpe and the pex13Δ strain, whereas pex19Δ strains were diffusely labeled (Figure 9D). The fluorescing pattern of the pex11Δpex25Δpex27Δ strain was heterogeneous, but clearly a significant number of cells exhibited a diffuse staining. A similar picture was obtained when cells were grown under noninducing conditions (our unpublished results). Thus, also the membrane protein Ant1p failed to become properly targeted to peroxisomes in the absence of the Pex11p family of proteins.
High Doses of PEX25 Substitute PEX11
To obtain direct evidence for the conserved function of Pex11p, Pex25p, and Pex27p, each protein was overexpressed in the pex11Δpex25Δpex27Δ triple deletion strain and analyzed for its ability to restore growth on oleic acid plates. The pex25Δpex27Δ and the pex11Δ mutant strains were included in the assay to compare the degree of complementation in the transformed strains. Interestingly, overexpression of Pex25p in the triple mutant (Figure 10) and in a pex11Δ single mutant strain (our unpublished results) resulted in normal utilization of oleic acid, as halo formation and colony size appeared similar to the spotted wild-type strain. On the other hand, growth was only partially restored upon overexpression of Pex11p and Pex27p, with Pex27p being less efficient than Pex11p. Similar results were obtained when the proteins were overexpressed in the triple mutant from the oleic acid-inducible FOX3 promoter (our unpublished results). Excess Pex25p was apparently proficient in substituting Pex11p, whereas Pex11p failed to fully compensate for a deficiency in Pex25p.
Figure 10.
Suppression of the growth defect of a pex11Δ pex25Δpex27Δ triple mutant upon overexpression of Pex11p family members. Growth of the indicated strains was analyzed by spotting 10-fold dilutions on oleic acid plates, followed by an incubation for 5 d at 30°C.
The transformed triple mutant strains were inspected for peroxisome morphology by electron microscopy. Giant peroxisomes were regularly retained upon overexpression of Pex27p, but on average, the numbers per cell increased and also small peroxisomes were visible (Figure 8H). Pex11p overexpression often caused a wild-type like appearance of peroxisomes, but extensive membrane sheets were never observed (Figure 8I). Most interestingly, the presence of a surplus on Pex25p resulted in the formation of karmellae around the nucleus (arrows in Figure 8, K and L) and elsewhere (open arrow in Figure 8L), out of which peroxisomes seem to emerge as bulbous structures (arrowheads in Figure 8, K and L). In some instances a large number of small vesicles was observed (Figure 8J). These data clearly demonstrate the ability of Pex25p to promote peroxisome proliferation in the absence of Pex11p.
To analyze whether matrix protein import resumed in the transformed triple mutants, GFP-SKL was coexpressed in these strains. Fluorescence microscopy revealed that a punctate staining pattern indeed reappeared in more than 90% of the mutant cells overexpressing Pex25p. Furthermore, both the size and the number of peroxisomes appeared normal (Figure 9). When Pex11p was overexpressed in the triple mutant strain, a significant fraction of cells remained import deficient. Cells that had imported the fluorescing protein showed peroxisomes of normal size and numbers in most cases and giant peroxisomes were rarely observed. Overexpression of Pex27p reduced the fraction of diffusely stained cells, but these cells usually showed fluorescing spots that were larger than those of the wild-type strain. Finally, the fractions of catalase activities reappearing in the organellar pellets of the transformed pex11Δpex25Δpex27Δ mutant strains were determined. Again, none of the tested strains showed significant deviations in the distribution of mitochondrial fumarase. The fractions of organellar-bound catalase in the strains overexpressing Pex11p and Pex25p were measured as being 57 and 64% of the wild-type value, respectively (Table 3). Also overexpression of Pex27p caused a significant increase in organellar-bound catalase when compared with that of the untransformed triple mutant. These data indicate a conserved function for Pex11p, Pex25p, and Pex27p that is important in peroxisome biogenesis.
DISCUSSION
We have identified a homologous pair of peroxisomal membrane proteins, Pex25p and Pex27p, whose function is partially redundant to that of Pex11p. We therefore propose that these three peroxins form a family of low homology, the Pex11p family. Cells lacking Pex11p, Pex25p, or Pex27p still contain peroxisomes that seem to be at least partially functional, indicating that the members of this family are not required for peroxisome assembly per se. However, a common feature exhibited by cells lacking members of the family is the occurrence of peroxisomes that are fewer but significantly larger than wild-type peroxisomes. These data clearly indicate that the members of the family including the newly identified Pex27p are involved in the regulation of size and number of peroxisomes as has been proposed previously for Pex11p and Pex25p (Erdmann and Blobel, 1995; Marshall et al., 1995; Smith et al., 2002). Deletion of two or even all three members of the family leads to giant peroxixomes and is accompanied by a growth defect on fatty acids as well as a partial import defect for peroxisomal matrix and membrane proteins. The import defect for matrix proteins is also observed under noninducing conditions (Figure 9), demonstrating that the defect is not limited to induced and proliferated peroxisomes. This result is especially interesting, as it suggests that the members of the family might perform a more general function in peroxisome biogenesis that exceeds the well-established role in peroxisome proliferation.
The molecular function of the members of the Pex11p-family, especially of the dominant peroxisomal membrane protein, Pex11p was hitherto still unclear. A pex11Δ gene deletion strain still contains peroxisomes and an abundant and correctly localized peroxisomal β-oxidation machinery (Erdmann and Blobel, 1995; Marshall et al., 1995). The reason for a pex11Δ mutant to be negatively affected for growth on fatty acids on first sight is therefore not obvious. Interestingly, β-oxidation measurements in pex11Δ mutant cells demonstrated that MCFA β-oxidation rates are reduced in intact cells but not in homogenates (van Roermund et al., 2000). In light of these observations, it has been proposed that Pex11p plays a role in metabolic exchange of intermediates in MCFA metabolism and that peroxisome proliferation is only indirectly controlled by Pex11p via the generation of a signaling molecule, which is generated by the degradation of medium chain fatty acids. However, this is in contrast to Li and Gould (2002), who recently showed that metabolic activity is not required to trigger Pex11p-induced peroxisome proliferation.
We show here that in the concomitant absence of Pex25p, Pex27p, and Pex11p also the utilization of long-chain fatty acids is blocked. The observed synthetic lethality of the triple mutant on oleic acid in addition to the striking morphological alterations and the peroxisomal import defect strongly argues for a general role of the Pex11p family in peroxisome biogenesis, which when abrogated finally blocks metabolic functions, including growth on fatty acids. The rather weak phenotypes of pex11Δ or pex25Δpex27Δ cells on oleic acid might now best be explained by a redundancy of the three proteins. Each of them, likely to varying extents, contributes to a single function that is essential for peroxisome function. This conclusion is supported by the similarity of the amino acid sequences of the three proteins (Figure 3). Furthermore, it proved possible to at least partially restore growth of the pex11Δpex25Δpex27Δ triple mutant on oleic acid by overexpressing any of the three family members (Figure 10). We also performed a two-hybrid analysis to identify interactions among the members of the Pex11p family. Our data indicate that all three members of the family can homooligomerize, a feature that previously has been reported for Pex11p and also has been associated with its function in peroxisome proliferation (Marshall et al., 1996). Interestingly, Pex25p weakly interacts with Pex27p, indicating that these proteins act in the same pathway in regulating peroxisome proliferation. As Pex11p can be functionally replaced by Pex25p and, although to a lesser extent, also by Pex27p, all the members of the family might control peroxisome proliferation in a similar way.
Deletion of Pex27p did not reveal such a strong phenotype as observed in pex25Δ and pex11Δ cells and in contrast to the other two members of the family, its expression level is not induced by the presence of oleic acid. Nevertheless, when overexpressed, Pex27p was able to improve growth on oleic acid of a pex25Δpex27Δ mutant and to some extent of a pex11Δpex25Δpex27Δ triple mutant (Figures 5 and 10). It is worth noting that overexpressed Pex11p was less efficient in restoring growth of the triple mutant on oleic acid than Pex25p, indicating that Pex11p lacks a key feature contained within Pex25p (Figure 10). Because the most obvious difference is the N-terminal extension of Pex25p (and Pex27p), future work is aimed to study the functional role of this region.
In light of the similarity of the members of the Pex11p-family, it is tempting to speculate that each member of the yeast Pex11p family has its counterpart in the mammalian Pex11α, β, and γ (Abe and Fujiki, 1998; Abe et al., 1998; Passreiter et al., 1998; Schrader et al., 1998; Li et al., 2002; Tanaka et al., 2003). As Pex27p, the mammalian Pex11β and Pex11γ are constitutively expressed. In analogy to the clear phenotype of the yeast triple mutant, knocking-out all three murine Pex11 isoforms might also reveal more severe defi-ciencies in peroxisomal biochemical pathways than the rather mild ones of a Pex11αPex11β knock-out mouse (Li et al., 2002).
The increase in peroxisome numbers upon Pex11p overexpression has been amply recorded (Erdmann and Blobel, 1995; Marshall et al., 1996; Schrader et al., 1998; Li and Gould, 2002). We have shown here that peroxisome proliferation can resume in the absence of Pex11p by overexpressing Pex25p or Pex27p, which unequivocally also links the latter proteins to peroxisome proliferation. Remarkably, PEX25 overexpression was accompanied by a massive proliferation of membranes, leading to karmellae around the nucleus in some cells. Despite the suggestive appearance of peroxisomes in close connection to these membranes, which seem to become inflated at a certain stage, it is probably premature to read out an origin of peroxisomes from these structures. Electron microscopy data on overexpressed membrane proteins have to be interpreted with care, because mistargeting to unrelated compartments, particularly the endoplasmic reticulum, is frequently observed. A surplus on Pex15p for instance, also induces the formation of karmellae, but even in a pex3Δ mutant, where peroxisomal remnants are considered to be absent (Hettema et al., 2000). Nonetheless, overexpressed Pex25p was able to rescue the growth defect of the pex11Δpex25Δpex27Δ mutant strain. Consequently, at least some of the observed peroxisomal structures must have been biochemically active. Evidence for the nuclear membrane acting as the donor membrane for peroxisomal vesicles was provided recently also in the yeast Hansenula polymorpha (Faber et al., 2002).
How does the Pex11p family promote peroxisome proliferation? Recent work clearly implicates the dynamin-like proteins Vps1p of S. cerevisiae (Hoepfner et al., 2001) and mammalian DLP1 in peroxisomal fission (Koch et al., 2003). Accordingly, the members of the Pex11p-family are at least not self-sufficient in constricting vesicles from peroxisomal tubules. Recent work by Li and Gould (2003) suggests that Pex11p might be part of the machinery that connects peroxisomes to the cytoskeleton and recruits dynamin-like proteins to appropriate sites at the peroxisomal membrane as has been suggested previously (Erdmann and Blobel, 1995; Li et al., 2002). Ectopic expression of a dominant negative mutant of DLP1 results in a network of tubular peroxisomes, whose length increases significantly when Pex11β is additionally overexpressed (Koch et al., 2003). Consequently, Pex11p and its partners could well be directly involved in the elongation of peroxisomal structures. The resemblance of the yeast Pex11p family to the ligand-binding domain of nuclear hormone receptors might point to a role in phospholipid-binding, upon which the peroxisomal membrane might be deformed and stretched.
On the basis of similarities in their amino acid sequences and the mutant phenotypes as well as cross-complementation studies and physical interactions, we propose that Pex25p along with its newly identified homologue Pex27p and Pex11p form the Pex11p family of proteins. The concurrent absence of all three proteins leads to striking morphological alterations and a peroxisomal import defect and strongly argues for an important role of the Pex11p family in peroxisome biogenesis, which when abrogated finally blocks metabolic functions, including growth on fatty acids. Our results point to a role of the members of this family in the regulation of the size and number of peroxisomes. Pex25p and Pex27p form complexes with additional functions that comprise but exceed the role of Pex11p in peroxisome division.
Acknowledgments
We thank Prof. Wolf-Hubert Kunau and Henk Tabak for antibodies, Katja Nau for strains, Andreas Hartig for plasmid pLW100, and Jan Drouven, Annette Schell-Steven and Michael Schneider for their help. Our gratitude also goes to Monika Bürger and Wolfgang Girzalsky for their help with the electron microscopical work. This work was supported by the Deutsche Forschungsgemeinschaft Grant ER178/2–4 (R.E.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–03–0153. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-03-0153.
Abbreviations used: MCFA, medium-chain fatty acids; PTS, peroxisomal targeting signal.
References
- Abe, I., and Fujiki, Y. (1998). cDNA cloning and characterization of a constitutively expressed isoform of the human peroxin Pex11p. Biochem. Biophys. Res. Commun. 252, 529–533. [DOI] [PubMed] [Google Scholar]
- Abe, I., Okumoto, K., Tamura, S., and Fujiki, Y. (1998). Clofibrate-inducible, 28-kDa peroxisomal integral membrane protein is encoded by PEX11. FEBS Lett. 431, 468–472. [DOI] [PubMed] [Google Scholar]
- Barnett, P., Tabak, H.F., and Hettema, E.H. (2000). Nuclear receptors arose from pre-existing protein modules during evolution. Trends Biochem. Sci. 25, 227–228. [DOI] [PubMed] [Google Scholar]
- Baumgartner, U., Hamilton, B., Piskacek, M., Ruis, H., and Rottensteiner, H. (1999). Functional analysis of the Zn(2)Cys(6) transcription factors Oaf1p and Pip2p. Different roles in fatty acid induction of β-oxidation in Saccharomyces cerevisiae. J. Biol. Chem. 274, 22208–22216. [DOI] [PubMed] [Google Scholar]
- Brachmann, C.B., Davies, A., Cost, G.J., Caputo, E., Li, J., Hieter, P., and Boeke, J.D. (1998). Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14, 115–132. [DOI] [PubMed] [Google Scholar]
- Chang, C.C., South, S., Warren, D., Jones, J., Moser, A.B., Moser, H.W., and Gould, S.J. (1999). Metabolic control of peroxisome abundance. J. Cell Sci. 112, 1579–1590. [DOI] [PubMed] [Google Scholar]
- Chevray, P.M., and Nathans, D. (1992). Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc. Natl. Acad. Sci. USA 89, 5789–5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann, R., and Blobel, G. (1995). Giant peroxisomes in oleic acid-induced Saccharomyces cerevisiae lacking the peroxisomal membrane protein Pmp27p. J. Cell Biol. 128, 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann, R., Veenhuis, M., Mertens, D., and Kunau, W.H. (1989). Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 86, 5419–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber, K.N., Haan, G.J., Baerends, R.J., Kram, A.M., and Veenhuis, M. (2002). Normal peroxisome development from vesicles induced by truncated Hansenula polymorpha Pex3p. J. Biol. Chem. 277, 11026–11033. [DOI] [PubMed] [Google Scholar]
- Gietz, R.D., and Sugino, A. (1988). New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74, 527–534. [DOI] [PubMed] [Google Scholar]
- Güldener, U., Heck, S., Fielder, T., Beinhauer, J., and Hegemann, J.H. (1996). A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24, 2519–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvitz, A., Rottensteiner, H., Kilpelainen, S.H., Hartig, A., Hiltunen, J.K., Binder, M., Dawes, I.W., and Hamilton, B. (1997). The Saccharomyces cerevisiae peroxisomal 2, 4-dienoyl-CoA reductase is encoded by the oleate-inducible gene SPS19. J. Biol. Chem. 272, 22140–22147. [DOI] [PubMed] [Google Scholar]
- Henke, B., Girzalsky, W., Berteaux-Lecellier, V., and Erdmann, R. (1998). IDP3 encodes a peroxisomal NADP-dependent isocitrate dehydrogenase required for the β-oxidation of unsaturated fatty acids. J. Biol. Chem. 273, 3702–3711. [DOI] [PubMed] [Google Scholar]
- Hettema, E.H., Girzalsky, W., van Den Berg, M., Erdmann, R., and Distel, B. (2000). Saccharomyces cerevisiae Pex3p and Pex19p are required for proper localization and stability of peroxisomal membrane proteins. EMBO J. 19, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, J.E., Myers, A.M., Koerner, T.J., and Tzagoloff, A. (1986). Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast 2, 163–167. [DOI] [PubMed] [Google Scholar]
- Hoepfner, D., van den Berg, M., Philippsen, P., Tabak, H.F., and Hettema, E.H. (2001). A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J. Cell Biol. 155, 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhfeld, J., Mertens, D., Wiebel, F.F., and Kunau, W.-H. (1992). Defining components required for peroxisome biogenesis in Saccharomyces cerevisiae. In: New Comprehensive Biochemistry Series, vol. Membrane Biogenesis and Protein Targeting, ed. E. S. P. Co, New York: Elsevier Science Publishing Co., 185–207.
- Holroyd, C., and Erdmann, R. (2001). Protein translocation machineries of peroxisomes. FEBS Lett. 501, 6–10. [DOI] [PubMed] [Google Scholar]
- Karpichev, I.V., Luo, Y., Marians, R.C., and Small, G.M. (1997). A complex containing two transcription factors regulates peroxisome proliferation and the coordinate induction of β-oxidation enzymes in Saccharomyces cerevisiae. Mol. Cell. Biol. 17, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, A., Thiemann, M., Grabenbauer, M., Yoon, Y., McNiven, M.A., and Schrader, M. (2003). The dynamin-like protein DLP1 is involved in peroxisomal fission. J. Biol. Chem. 278, 8597–8605. [DOI] [PubMed] [Google Scholar]
- Kunau, W.H., Dommes, V., and Schulz, H. (1995). β-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog. Lipid Res. 34, 267–342. [DOI] [PubMed] [Google Scholar]
- Kunau, W.H., and Erdmann, R. (1998). Peroxisome biogenesis: back to the endoplasmic reticulum? Curr. Biol. 8, 299–302. [DOI] [PubMed] [Google Scholar]
- Lametschwandtner, G., Brocard, C., Fransen, M., Van Veldhoven, P., Berger, J., and Hartig, A. (1998). The difference in recognition of terminal tripeptides as peroxisomal targeting signal 1 between yeast and human is due to different affinities of their receptor Pex5p to the cognate signal and to residues adjacent to it. J. Biol. Chem. 273, 33635–33643. [DOI] [PubMed] [Google Scholar]
- Lazarow, P.B., and Fujiki, Y. (1985). Biogenesis of peroxisomes. Annu. Rev. Cell Biol. 1, 489–530. [DOI] [PubMed] [Google Scholar]
- Li, X., Baumgart, E., Dong, G.X., Morrell, J.C., Jimenez-Sanchez, G., Valle, D., Smith, K.D., and Gould, S.J. (2002). PEX11α is required for peroxisome proliferation in response to 4-phenylbutyrate but is dispensable for peroxisome proliferator-activated receptor α-mediated peroxisome proliferation. Mol. Cell. Biol. 22, 8226–8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., and Gould, S.J. (2002). PEX11 promotes peroxisome division independently of peroxisome metabolism. J. Cell Biol. 156, 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., and Gould, S.J. (2003). The Dynamin-like GTPase DLP1 is essential for peroxisome division and is recruited to peroxisomes in part by PEX11. J. Biol. Chem. 278, 17012–17020. [DOI] [PubMed] [Google Scholar]
- Marshall, P.A., Dyer, J.M., Quick, M.E., and Goodman, J.M. (1996). Redox-sensitive homodimerization of Pex11p: a proposed mechanism to regulate peroxisomal division. J. Cell Biol. 135, 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, P.A., Krimkevich, Y.I., Lark, R.H., Dyer, J.M., Veenhuis, M., and Goodman, J.M. (1995). Pmp27 promotes peroxisomal proliferation. J. Cell Biol. 129, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno de la Garza, M., Schultz-Borchardt, U., Crabb, J.W., and Kunau, W.H. (1985). Peroxisomal β-oxidation system of Candida tropicalis. Eur. J. Biochem. 148, 285–291. [DOI] [PubMed] [Google Scholar]
- Mumberg, D., Muller, R., and Funk, M. (1994). Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 22, 5767–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri, L., Rottensteiner, H., Girzalsky, W., Scarcia, P., Palmieri, F., and Erdmann, R. (2001). Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J. 20, 5049–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passreiter, M., Anton, M., Lay, D., Frank, R., Harter, C., Wieland, F.T., Gorgas, K., and Just, W.W. (1998). Peroxisome biogenesis: involvement of ARF and coatomer. J. Cell Biol. 141, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdue, P.E., and Lazarow, P.B. (2001). Peroxisome biogenesis. Annu. Rev. Cell Dev. Biol. 17, 701–752. [DOI] [PubMed] [Google Scholar]
- Rigaut, G., Shevchenko, A., Rutz, B., Wilm, M., Mann, M., and Seraphin, B. (1999). A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 17, 1030–1032. [DOI] [PubMed] [Google Scholar]
- Rose, M.D., Misra, L.M., and Vogel, J.P. (1989). KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell 57, 1211–1221. [DOI] [PubMed] [Google Scholar]
- Rottensteiner, H., Hartig, A., Hamilton, B., Ruis, H., Erdmann, R., and Gurvitz, A. (2003). Saccharomyces cerevisiae Pip2p-Oaf1p regulates PEX25 transcription through an adenine-less ORE. Eur. J. Biochem. 270, 2013–2022. [DOI] [PubMed] [Google Scholar]
- Rottensteiner, H., Kal, A.J., Hamilton, B., Ruis, H., and Tabak, H.F. (1997). A heterodimer of the Zn2Cys6 transcription factors Pip2p and Oaf1p controls induction of genes encoding peroxisomal proteins in Saccharomyces cerevisiae. Eur. J. Biochem. 247, 776–783. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Schrader, M., Reuber, B.E., Morrell, J.C., Jimenez-Sanchez, G., Obie, C., Stroh, T.A., Valle, D., Schroer, T.A., and Gould, S.J. (1998). Expression of PEX11β mediates peroxisome proliferation in the absence of extracellular stimuli. J. Biol. Chem. 273, 29607–29614. [DOI] [PubMed] [Google Scholar]
- Sikorski, R.S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J.J., Brown, T.W., Eitzen, G.A., and Rachubinski, R.A. (2000). Regulation of peroxisome size and number by fatty acid β-oxidation in the yeast Yarrowia lipolytica. J. Biol. Chem. 275, 20168–20178. [DOI] [PubMed] [Google Scholar]
- Smith, J.J. et al. (2002). Transcriptome profiling to identify genes involved in peroxisome assembly and function. J. Cell Biol. 158, 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, K., Schell-Steven, A., Erdmann, R., and Rottensteiner, H. (2002). Interactions of Pex7p and Pex18p/Pex21p with the peroxisomal docking machinery: implications for the first steps in PTS2 protein import. Mol. Cell. Biol. 22, 6056–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, A., Okumoto, K., and Fujiki, Y. (2003). cDNA cloning and characterization of the third isoform of human peroxin Pex11p. Biochem. Biophys. Res. Commun. 300, 819–823. [DOI] [PubMed] [Google Scholar]
- Titorenko, V.I., and Rachubinski, R.A. (2001). The life cycle of the peroxisome. Nat. Rev. Mol. Cell. Biol. 2, 357–368. [DOI] [PubMed] [Google Scholar]
- van den Bosch, H., Schutgens, R.B., Wanders, R.J., and Tager, J.M. (1992). Biochemistry of peroxisomes. Annu. Rev. Biochem. 61, 157–197. [DOI] [PubMed] [Google Scholar]
- van Roermund, C.W., Tabak, H.F., van Den Berg, M., Wanders, R.J., and Hettema, E.H. (2000). Pex11p plays a primary role in medium-chain fatty acid oxidation, a process that affects peroxisome number and size in Saccharomyces cerevisiae. J. Cell Biol. 150, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhuis, M., Mateblowski, M., Kunau, W.H., and Harder, W. (1987). Proliferation of microbodies in Saccharomyces cerevisiae. Yeast 3, 77–84. [DOI] [PubMed] [Google Scholar]
- Wach, A., Brachat, A., Pohlmann, R., and Philippsen, P. (1994). New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10, 1793–1808. [DOI] [PubMed] [Google Scholar]
- Westermann, B., and Neupert, W. (2000). Mitochondria-targeted green fluorescent proteins: convenient tools for the study of organelle biogenesis in Saccharomyces cerevisiae. Yeast 16, 1421–1427. [DOI] [PubMed] [Google Scholar]
- Yaffe, M.P., and Schatz, G. (1984). Two nuclear mutations that block mitochondrial protein import in yeast. Proc. Natl. Acad. Sci. USA 81, 4819–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]