Abstract
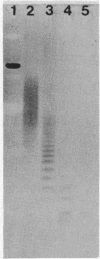
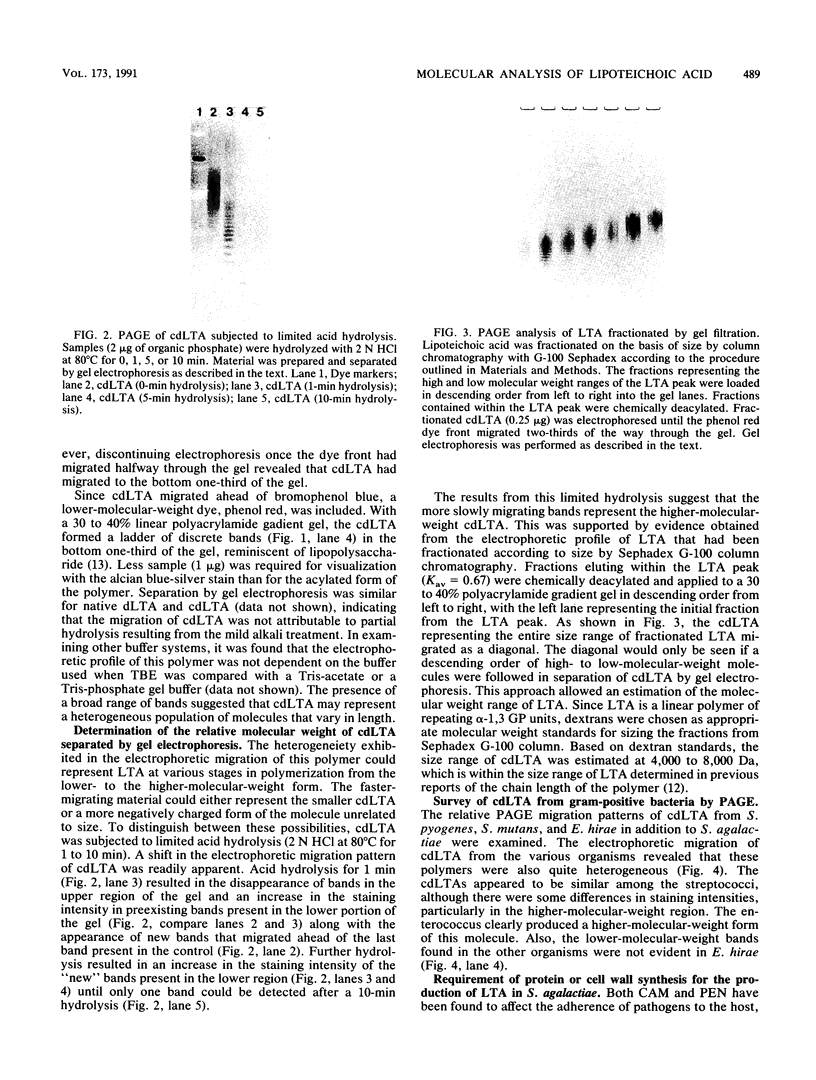
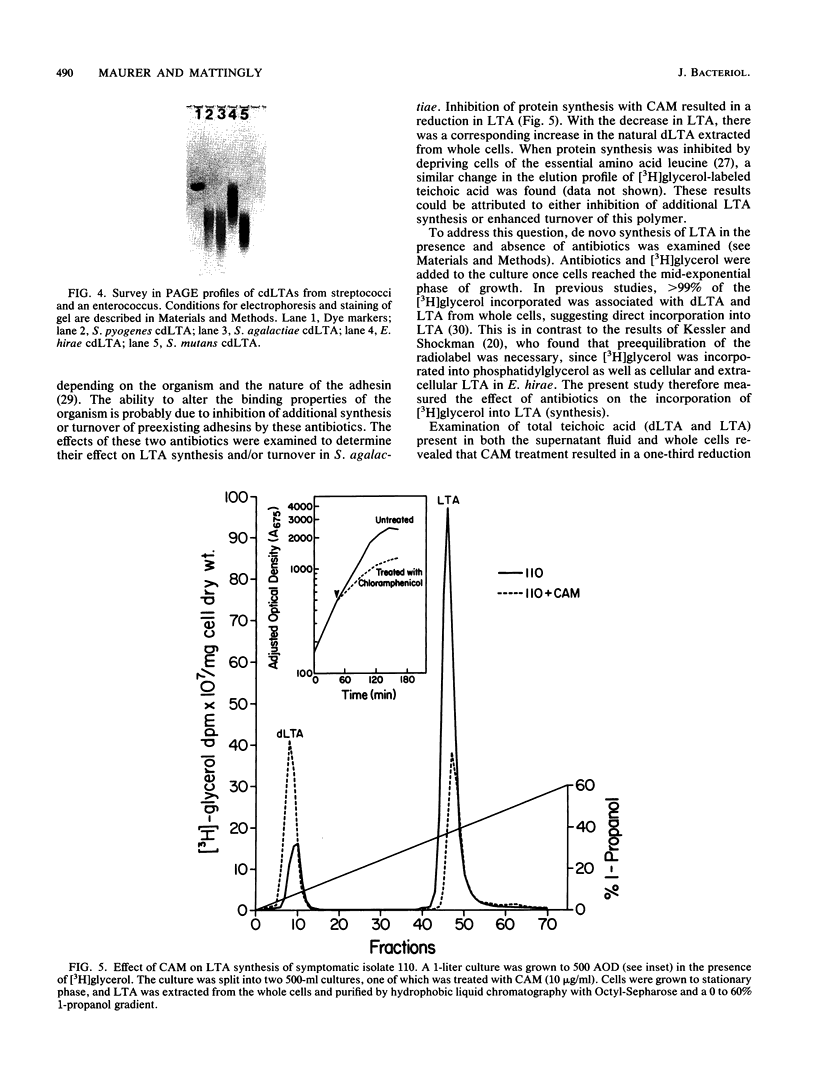
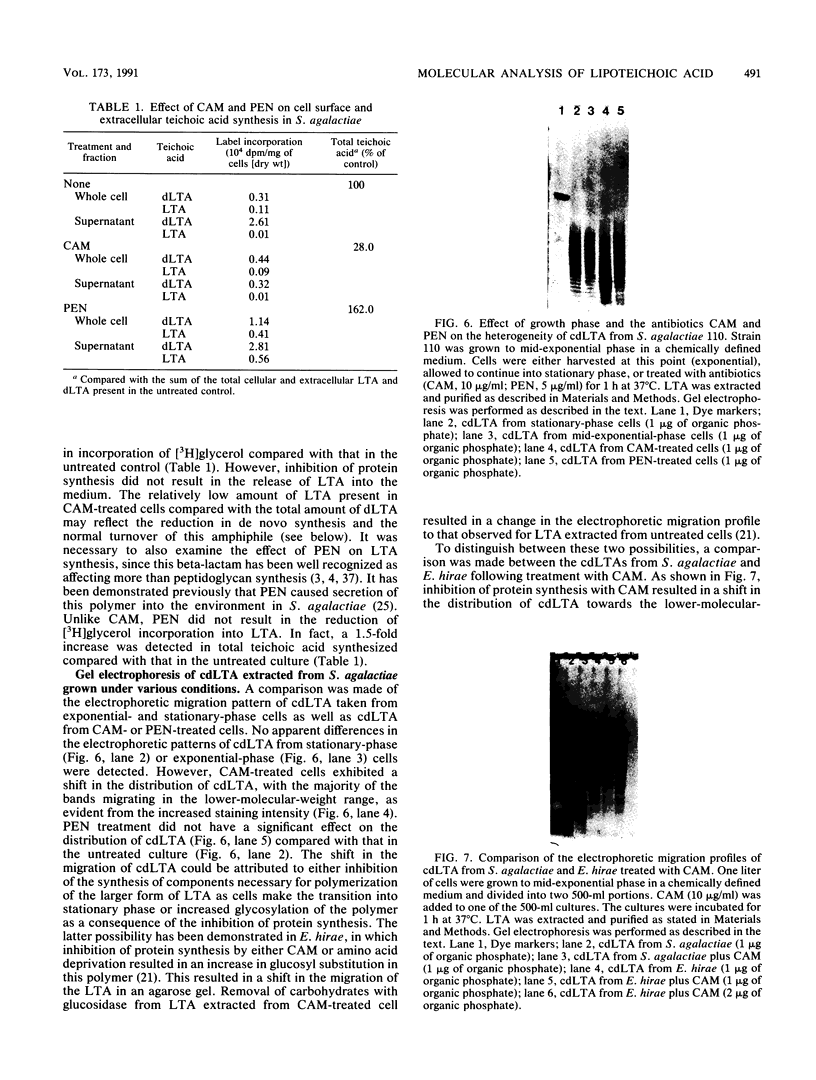
A method for the analysis of lipoteichoic acid (LTA) by polyacrylamide gel electrophoresis (PAGE) is described. Purified LTA from Streptococcus agalactiae tended to smear in the upper two-thirds of a 30 to 40% linear polyacrylamide gel, while the chemically deacylated form (cdLTA) migrated as a ladder of discrete bands, reminiscent of lipopolysaccharides. The deacylated polymer appeared to separate in this system on the basis of size, as evident from results obtained from PAGE analysis of cdLTA subjected to limited acid hydrolysis and LTA that had been fractionated by gel filtration. A survey of cdLTA from other streptococci revealed similarities in molecular weight ranges. The polymer from Enterococcus hirae was of a higher molecular weight. This procedure was used to examine the effect of penicillin and chloramphenicol on the synthesis, turnover, and heterogeneity of LTA in S. agalactiae. Penicillin appeared to enhance LTA synthesis while causing the release of this polymer into the supernatant fluid. In contrast, chloramphenicol inhibited the synthesis of this molecule and resulted in its depletion from the cell surface. Penicillin did not alter the heterogeneity of this polymer, but chloramphenicol caused an apparent shift to a lower-molecular-weight from of the LTA, as determined by PAGE. This shift in the heterogeneity of LTA did not appear to be due to increased carbohydrate substitution, since chloramphenicol did not alter the electrophoretic migration profile of LTA from E. hirae. From a pulse-chase study, it was determined that LTA was released as a consequence of deacylation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M. L., Beachey E. H. Excretion of lipoteichoic acid by group A streptococci. Influence of penicillin on excretion and loss of ability to adhere to human oral mucosal cells. J Clin Invest. 1978 Mar;61(3):671–677. doi: 10.1172/JCI108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Courtney H. S. Bacterial adherence: the attachment of group A streptococci to mucosal surfaces. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S475–S481. doi: 10.1093/clinids/9.supplement_5.s475. [DOI] [PubMed] [Google Scholar]
- Brissette J. L., Pieringer R. A. The effect of penicillin on fatty acid synthesis and excretion in Streptococcus mutans BHT. Lipids. 1985 Mar;20(3):173–179. doi: 10.1007/BF02534250. [DOI] [PubMed] [Google Scholar]
- Brissette J. L., Shockman G. D., Pieringer R. A. Effects of penicillin on synthesis and excretion of lipid and lipoteichoic acid from Streptococcus mutans BHT. J Bacteriol. 1982 Aug;151(2):838–844. doi: 10.1128/jb.151.2.838-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabacungan E., Pieringer R. A. Mode of elongation of the glycerol phosphate polymer of membrane lipoteichoic acid of Streptococcus faecium ATCC 9790. J Bacteriol. 1981 Jul;147(1):75–79. doi: 10.1128/jb.147.1.75-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. C., Reid G., Irvin R. T., Bruce A. W., Costerton J. W. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect Immun. 1985 Jan;47(1):84–89. doi: 10.1128/iai.47.1.84-89.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh T. D., Burns G. J., Shuhaiber H. J., Bahr G. M. Adherence of Staphylococcus epidermidis to fibrin-platelet clots in vitro mediated by lipoteichoic acid. Infect Immun. 1990 Feb;58(2):315–319. doi: 10.1128/iai.58.2.315-319.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham D. L., Mattingly S. J., Doran T. I., Milligan T. W., Straus D. C. Correlation between the production of extracellular substances by type III group B streptococcal strains and virulence in a mouse model. Infect Immun. 1981 Nov;34(2):448–454. doi: 10.1128/iai.34.2.448-454.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdur L., Chiu T. The role of phosphatidylglycerol in the in vitro biosynthesis of teichoic acid and lipoteichoic acid. FEBS Lett. 1975 Jul 15;55(1):216–219. doi: 10.1016/0014-5793(75)80995-1. [DOI] [PubMed] [Google Scholar]
- Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Grossman N., Schmetz M. A., Foulds J., Klima E. N., Jimenez-Lucho V. E., Leive L. L., Joiner K. A., Jiminez V. Lipopolysaccharide size and distribution determine serum resistance in Salmonella montevideo. J Bacteriol. 1987 Feb;169(2):856–863. doi: 10.1128/jb.169.2.856-863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall S., Archibald A. R., Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970 Feb 7;225(5232):519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Release of lipoteichoic acid from Streptococcus sanguis: stimulation of release during penicillin treatment. J Bacteriol. 1979 Mar;137(3):1180–1184. doi: 10.1128/jb.137.3.1180-1184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Vanderwel D., Kusser W. Control of lipopolysaccharide biosynthesis and release by Escherichia coli and Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):328–333. doi: 10.1128/jb.168.1.328-333.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Enzymatic deacylation of lipoteichoic acid by protoplasts of Streptococcus faecium (Streptococcus faecalis ATCC 9790). J Bacteriol. 1979 Mar;137(3):1176–1179. doi: 10.1128/jb.137.3.1176-1179.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Precursor-product relationship of intracellular and extracellular lipoteichoic acids of Streptococcus faecium. J Bacteriol. 1979 Feb;137(2):869–877. doi: 10.1128/jb.137.2.869-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Wicken A. J., Shockman G. D. Increased carbohydrate substitution of lipoteichoic acid during inhibition of protein synthesis. J Bacteriol. 1983 Jul;155(1):138–144. doi: 10.1128/jb.155.1.138-144.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowy F. D., Chang D. S., Neuhaus E. G., Horne D. S., Tomasz A., Steigbigel N. H. Effect of penicillin on the adherence of Streptococcus sanguis in vitro and in the rabbit model of endocarditis. J Clin Invest. 1983 Mar;71(3):668–675. doi: 10.1172/JCI110813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly S. J., Johnston B. P. Comparative analysis of the localization of lipoteichoic acid in Streptococcus agalactiae and Streptococcus pyogenes. Infect Immun. 1987 Oct;55(10):2383–2386. doi: 10.1128/iai.55.10.2383-2386.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan T. W., Baker C. J., Straus D. C., Mattingly S. J. Association of elevated levels of extracellular neuraminidase with clinical isolates of type III group B streptococci. Infect Immun. 1978 Sep;21(3):738–746. doi: 10.1128/iai.21.3.738-746.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan T. W., Doran T. I., Straus D. C., Mattingly S. J. Growth and amino acid requirements of various strains of group B streptococci. J Clin Microbiol. 1978 Jan;7(1):28–33. doi: 10.1128/jcm.7.1.28-33.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H., Cowman M. K. Combined alcian blue and silver staining of glycosaminoglycans in polyacrylamide gels: application to electrophoretic analysis of molecular weight distribution. Anal Biochem. 1986 Jun;155(2):275–285. doi: 10.1016/0003-2697(86)90437-9. [DOI] [PubMed] [Google Scholar]
- Nealon T. J., Beachey E. H., Courtney H. S., Simpson W. A. Release of fibronectin-lipoteichoic acid complexes from group A streptococci with penicillin. Infect Immun. 1986 Feb;51(2):529–535. doi: 10.1128/iai.51.2.529-535.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon T. J., Mattingly S. J. Association of elevated levels of cellular lipoteichoic acids of group B streptococci with human neonatal disease. Infect Immun. 1983 Mar;39(3):1243–1251. doi: 10.1128/iai.39.3.1243-1251.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon T. J., Mattingly S. J. Kinetic and chemical analyses of the biologic significance of lipoteichoic acids in mediating adherence of serotype III group B streptococci. Infect Immun. 1985 Oct;50(1):107–115. doi: 10.1128/iai.50.1.107-115.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon T. J., Mattingly S. J. Role of cellular lipoteichoic acids in mediating adherence of serotype III strains of group B streptococci to human embryonic, fetal, and adult epithelial cells. Infect Immun. 1984 Feb;43(2):523–530. doi: 10.1128/iai.43.2.523-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op den Camp H. J., Oosterhof A., Veerkamp J. H. Interaction of bifidobacterial lipoteichoic acid with human intestinal epithelial cells. Infect Immun. 1985 Jan;47(1):332–334. doi: 10.1128/iai.47.1.332-334.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen S., Häyrinen J., Finne J. Polyacrylamide gel electrophoresis of the capsular polysaccharides of Escherichia coli K1 and other bacteria. J Bacteriol. 1988 Jun;170(6):2646–2653. doi: 10.1128/jb.170.6.2646-2653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A. A., McGroarty E. J. High-molecular-weight components in lipopolysaccharides of Salmonella typhimurium, Salmonella minnesota, and Escherichia coli. J Bacteriol. 1985 May;162(2):738–745. doi: 10.1128/jb.162.2.738-745.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M., Bryan L. E., Hancock R. E., McGroarty E. J. Heterogeneity of lipopolysaccharides from Pseudomonas aeruginosa: analysis of lipopolysaccharide chain length. J Bacteriol. 1988 Feb;170(2):512–521. doi: 10.1128/jb.170.2.512-521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters P. J., Hildebrandt K. M., Dickie J. P., Anderson J. S. Polymer length of teichuronic acid released from cell walls of Micrococcus luteus. J Bacteriol. 1990 Sep;172(9):5154–5159. doi: 10.1128/jb.172.9.5154-5159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M. K., Mattingly S. J. Biosynthetic capacity for type-specific antigen synthesis determines the virulence of serotype III strains of group B streptococci. Infect Immun. 1984 May;44(2):217–221. doi: 10.1128/iai.44.2.217-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]