Abstract
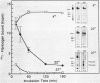
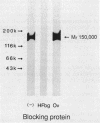
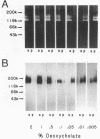
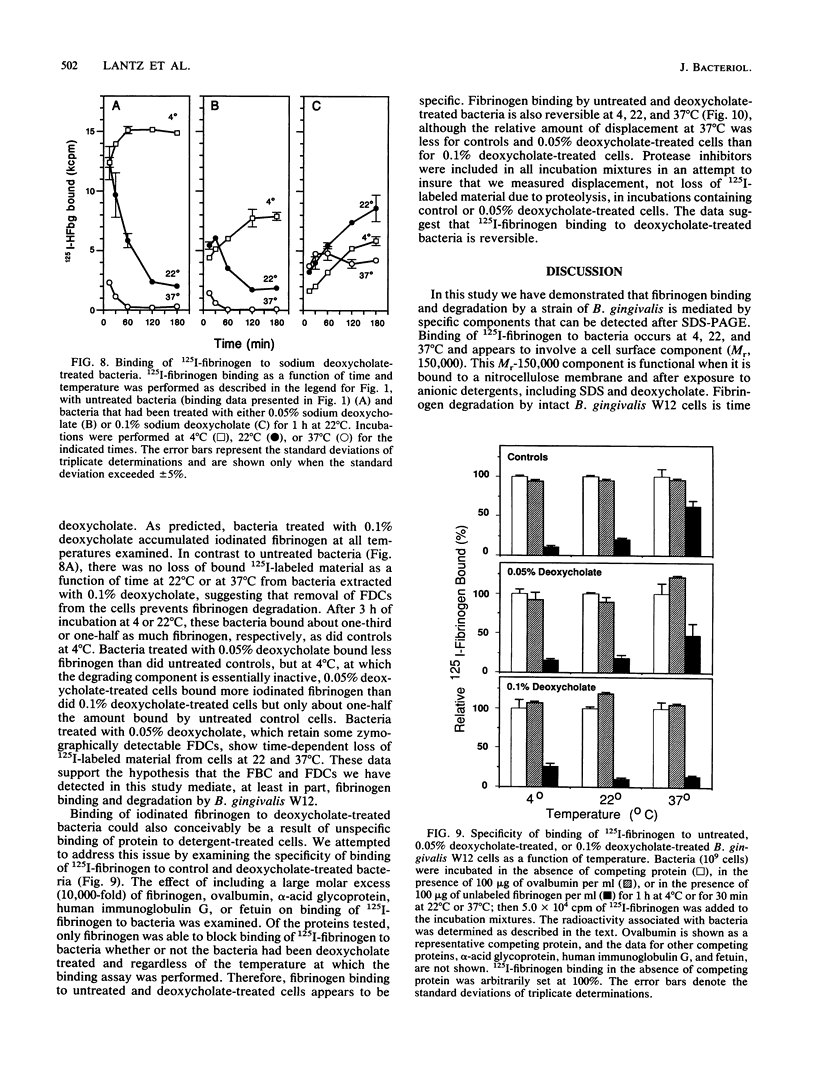
Bacteroides (Porphyromonas) gingivalis, which has been implicated as an etiologic agent in human periodontal diseases, has been shown to bind and degrade human fibrinogen. B. gingivalis strains bind fibrinogen reversibly and with high affinity and bind to a specific region of the fibrinogen molecule that appears to be located between the D and E domains (M. S. Lantz, R. D. Allen, P. Bounelis, L. M. Switalski, and M. Hook, J. Bacteriol. 172:716-726, 1990). We now report that human fibrinogen is bound and then degraded by specific B. gingivalis components that appear to be localized at the cell surface. Fibrinogen binding to bacterial cells occurred at 4, 22, and 37 degrees C. A functional fibrinogen-binding component (Mr, 150,000) was identified when sodium dodecyl sulfate-solubilized bacteria were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and probed with 125I-fibrinogen. Fibrinogen degradation did not occur at 4 degrees C but did occur at 22 and 37 degrees C. When bacteria and iodinated fibrinogen were incubated at 37 degrees C, two major fibrinogen fragments (Mr, 97,000 and 50,000) accumulated in incubation mixture supernatant fractions. Two major fibrinogen-degrading components (Mr, 120,000 and 150,000) have been identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in substrate-containing gels. Fibrinogen degradation by the Mr-120,000 and -150,000 proteases was enhanced by reducing agents, completely inhibited by N-alpha-p-tosyl-L-lysyl chloromethyl ketone, and partially inhibited by n-ethyl maleimide, suggesting that these enzymes are thiol-dependent proteases with trypsinlike substrate specificity. The fibrinogen-binding component could be separated from the fibrinogen-degrading components by selective solubilization of bacteria in sodium deoxycholate.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barua P. K., Neiders M. E., Topolnycky A., Zambon J. J., Birkedal-Hansen H. Purification of an 80,000-Mr glycylprolyl peptidase from Bacteroides gingivalis. Infect Immun. 1989 Aug;57(8):2522–2528. doi: 10.1128/iai.57.8.2522-2528.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Taylor R. E., Zambon J. J., Barwa P. K., Neiders M. E. Characterization of collagenolytic activity from strains of Bacteroides gingivalis. J Periodontal Res. 1988 Jul;23(4):258–264. doi: 10.1111/j.1600-0765.1988.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Boyd J., McBride B. C. Fractionation of hemagglutinating and bacterial binding adhesins of Bacteroides gingivalis. Infect Immun. 1984 Aug;45(2):403–409. doi: 10.1128/iai.45.2.403-409.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- Fujimura S., Nakamura T. Isolation and characterization of a protease from Bacteroides gingivalis. Infect Immun. 1987 Mar;55(3):716–720. doi: 10.1128/iai.55.3.716-720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., McBride B. C. Isolation of a membrane-associated Bacteroides gingivalis glycylprolyl protease. Infect Immun. 1987 Dec;55(12):3131–3136. doi: 10.1128/iai.55.12.3131-3136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Kay H. M., Birss A. J., Smalley J. W. Glycylprolyl dipeptidase activity of Bacteroides gingivalis W50 and the avirulent variant W50/BEI. FEMS Microbiol Lett. 1989 Jan 1;48(1):93–96. doi: 10.1016/0378-1097(89)90153-5. [DOI] [PubMed] [Google Scholar]
- Kinder S. A., Holt S. C. Characterization of coaggregation between Bacteroides gingivalis T22 and Fusobacterium nucleatum T18. Infect Immun. 1989 Nov;57(11):3425–3433. doi: 10.1128/iai.57.11.3425-3433.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annu Rev Microbiol. 1988;42:627–656. doi: 10.1146/annurev.mi.42.100188.003211. [DOI] [PubMed] [Google Scholar]
- Kornman K. S., Loesche W. J. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980 Mar;15(2):111–122. doi: 10.1111/j.1600-0765.1980.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Lantz M. S., Allen R. D., Bounelis P., Switalski L. M., Hook M. Bacteroides gingivalis and Bacteroides intermedius recognize different sites on human fibrinogen. J Bacteriol. 1990 Feb;172(2):716–726. doi: 10.1128/jb.172.2.716-726.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz M. S., Rowland R. W., Switalski L. M., Hök M. Interactions of Bacteroides gingivalis with fibrinogen. Infect Immun. 1986 Dec;54(3):654–658. doi: 10.1128/iai.54.3.654-658.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz M. S., Switalski L. M., Kornman K. S., Hök M. Bacteroides intermedius binds fibrinogen. J Bacteriol. 1985 Aug;163(2):623–628. doi: 10.1128/jb.163.2.623-628.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- Nitzan D., Sperry J. F., Wilkins T. D. Fibrinolytic activity of oral anaerobic bacteria. Arch Oral Biol. 1978;23(6):465–470. doi: 10.1016/0003-9969(78)90078-x. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Endo J., Hinode D., Nagata A., Maehara R., Sato M., Nakamura R. Isolation and characterization of protease from culture supernatant of Bacteroides gingivalis. J Periodontal Res. 1987 Nov;22(6):491–498. doi: 10.1111/j.1600-0765.1987.tb02060.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley J. W., Birss A. J., Shuttleworth C. A. The degradation of type I collagen and human plasma fibronectin by the trypsin-like enzyme and extracellular membrane vesicles of Bacteroides gingivalis W50. Arch Oral Biol. 1988;33(5):323–329. doi: 10.1016/0003-9969(88)90065-9. [DOI] [PubMed] [Google Scholar]
- Smalley J. W., Birss A. J. Trypsin-like enzyme activity of the extracellular membrane vesicles of Bacteroides gingivalis W50. J Gen Microbiol. 1987 Oct;133(10):2883–2894. doi: 10.1099/00221287-133-10-2883. [DOI] [PubMed] [Google Scholar]
- Sorsa T., Uitto V. J., Suomalainen K., Turto H., Lindy S. A trypsin-like protease from Bacteroides gingivalis: partial purification and characterization. J Periodontal Res. 1987 Sep;22(5):375–380. doi: 10.1111/j.1600-0765.1987.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Suido H., Neiders M. E., Barua P. K., Nakamura M., Mashimo P. A., Genco R. J. Characterization of N-CBz-glycyl-glycyl-arginyl peptidase and glycyl-prolyl peptidase of Bacteroides gingivalis. J Periodontal Res. 1987 Sep;22(5):412–418. doi: 10.1111/j.1600-0765.1987.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Sundqvist G., Carlsson J., Hänström L. Collagenolytic activity of black-pigmented Bacteroides species. J Periodontal Res. 1987 Jul;22(4):300–306. doi: 10.1111/j.1600-0765.1987.tb01589.x. [DOI] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Bacteriology of human experimental gingivitis: effect of plaque age. Infect Immun. 1978 Sep;21(3):821–829. doi: 10.1128/iai.21.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda K., Otsuka M., Ishikawa Y., Sato M., Yamamoto Y., Nakamura R. Thiol-dependent collagenolytic activity in culture media of Bacteroides gingivalis. J Periodontal Res. 1984 Jul;19(4):372–381. doi: 10.1111/j.1600-0765.1984.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H., Kinouchi T., Wakano Y., Ohnishi Y. Purification and characterization of a protease from Bacteroides gingivalis 381. Infect Immun. 1987 Feb;55(2):420–427. doi: 10.1128/iai.55.2.420-427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström M. B., Dahlén G., Linde A. Fibrinogenolytic and fibrinolytic activity in oral microorganisms. J Clin Microbiol. 1983 May;17(5):759–767. doi: 10.1128/jcm.17.5.759-767.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]