Abstract
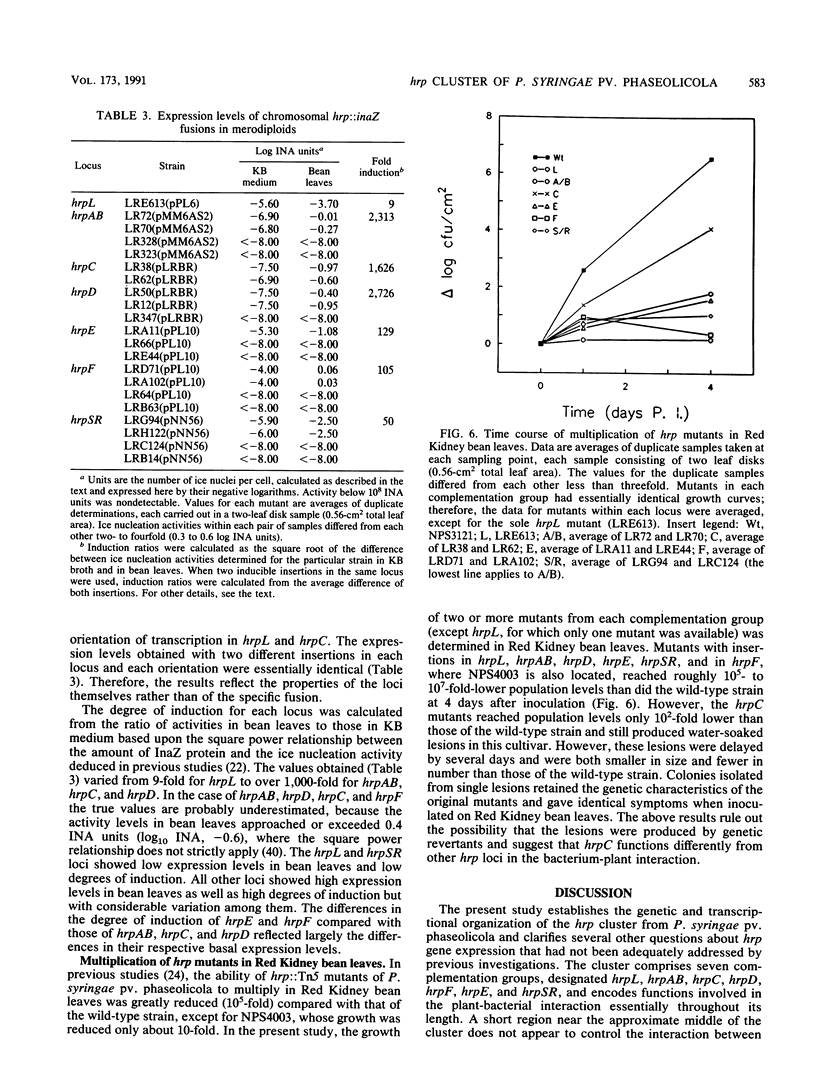
The hrp cluster of Pseudomonas syringae pv. phaseolicola encodes functions that are essential for pathogenicity on bean plants and for the elicitation of the hypersensitive response on resistant plants. The cluster was saturated with insertions of transposon Tn3-spice that served both as a mutagen and as a sensitive reporter of the expression of the target regions. The mutations covered a 17.5-kb segment in strain NPS3121, in which seven hrp::Tn5 insertions had been previously mapped, and regions outside this segment. The cluster is organized into seven distinct complementation groups (hrpL, hrpAB, hrpC, hrpD, hrpE, hrpF, and hrpSR) on the basis of the analysis of over 100 Tn3-spice insertions in plasmids and 43 similar insertions in the chromosome; it spans nearly 22 kb and is chromosomally located. The transcriptional orientation of all genes in the cluster was established by measuring the level of ice nucleation activity of complemented merodiploids carrying chromosomal hrp::inaZ fusions after inoculation in Red Kidney bean leaves. Although all seven loci were actively expressed in Red Kidney bean leaves, none of them was substantially expressed when the bacteria were grown in King B broth medium. Mutations in all loci, except those in hrpC, greatly reduced the ability of the bacteria to multiply in bean leaves. Mutations in the hrpC locus, although preventing the bacteria from eliciting a hypersensitive reaction on tobacco, allowed the bacteria to produce delayed and attenuated symptoms in Red Kidney bean leaves and to multiply to a level 10(2)- to 10(3)-fold lower than that of the wild-type strain. This is the first comprehensive report of the genetic and transcriptional organization of the hrp gene cluster in a phytopathogenic bacterium.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher C. A., Van Gijsegem F., Barberis P. A., Arlat M., Zischek C. Pseudomonas solanacearum genes controlling both pathogenicity on tomato and hypersensitivity on tobacco are clustered. J Bacteriol. 1987 Dec;169(12):5626–5632. doi: 10.1128/jb.169.12.5626-5632.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney R. H., Scott J. R., Vapnek D. Integration of the plasmid prophages P1 and P7 into the chromosome of Escherichia coli. J Mol Biol. 1979 May 15;130(2):161–173. doi: 10.1016/0022-2836(79)90424-8. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppels D. A. Generation and Characterization of Tn5 Insertion Mutations in Pseudomonas syringae pv. tomato. Appl Environ Microbiol. 1986 Feb;51(2):323–327. doi: 10.1128/aem.51.2.323-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C., Panopoulos N. J. The predicted protein product of a pathogenicity locus from Pseudomonas syringae pv. phaseolicola is homologous to a highly conserved domain of several procaryotic regulatory proteins. J Bacteriol. 1989 Sep;171(9):5031–5038. doi: 10.1128/jb.171.9.5031-5038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R., Aricò B., Rappuoli R. Families of bacterial signal-transducing proteins. Mol Microbiol. 1989 Nov;3(11):1661–1667. doi: 10.1111/j.1365-2958.1989.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Huang H. C., Schuurink R., Denny T. P., Atkinson M. M., Baker C. J., Yucel I., Hutcheson S. W., Collmer A. Molecular cloning of a Pseudomonas syringae pv. syringae gene cluster that enables Pseudomonas fluorescens to elicit the hypersensitive response in tobacco plants. J Bacteriol. 1988 Oct;170(10):4748–4756. doi: 10.1128/jb.170.10.4748-4756.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh T. V., Dahlbeck D., Staskawicz B. J. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science. 1989 Sep 22;245(4924):1374–1377. doi: 10.1126/science.2781284. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren P. B., Peet R. C., Panopoulos N. J. Gene cluster of Pseudomonas syringae pv. "phaseolicola" controls pathogenicity of bean plants and hypersensitivity of nonhost plants. J Bacteriol. 1986 Nov;168(2):512–522. doi: 10.1128/jb.168.2.512-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P., Williams J., Mills D. Molecular analysis of a pathogenicity locus in Pseudomonas syringae pv. syringae. J Bacteriol. 1988 Dec;170(12):5479–5488. doi: 10.1128/jb.170.12.5479-5488.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niepold F., Anderson D., Mills D. Cloning determinants of pathogenesis from Pseudomonas syringae pathovar syringae. Proc Natl Acad Sci U S A. 1985 Jan;82(2):406–410. doi: 10.1073/pnas.82.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet R. C., Lindgren P. B., Willis D. K., Panopoulos N. J. Identification and cloning of genes involved in phaseolotoxin production by Pseudomonas syringae pv. "phaseolicola". J Bacteriol. 1986 Jun;166(3):1096–1105. doi: 10.1128/jb.166.3.1096-1105.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salch Y. P., Shaw P. D. Isolation and characterization of pathogenicity genes of Pseudomonas syringae pv. tabaci. J Bacteriol. 1988 Jun;170(6):2584–2591. doi: 10.1128/jb.170.6.2584-2591.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southworth M. W., Wolber P. K., Warren G. J. Nonlinear relationship between concentration and activity of a bacterial ice nucleation protein. J Biol Chem. 1988 Oct 15;263(29):15211–15216. [PubMed] [Google Scholar]
- Stachel S. E., An G., Flores C., Nester E. W. A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 1985 Apr;4(4):891–898. doi: 10.1002/j.1460-2075.1985.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz B., Dahlbeck D., Keen N., Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987 Dec;169(12):5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste J. L., Paulin J. P., Expert D. Bacteriophage Mu as a genetic tool to study Erwinia amylovora pathogenicity and hypersensitive reaction on tobacco. J Bacteriol. 1990 Feb;172(2):932–941. doi: 10.1128/jb.172.2.932-941.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P. L., Leong S., Sequeira L. Molecular cloning of genes that specify virulence in Pseudomonas solanacearum. J Bacteriol. 1988 Feb;170(2):617–622. doi: 10.1128/jb.170.2.617-622.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel I., Xiao Y. X., Hutcheson S. W. Influence of Pseudomonas syringae culture conditions on initiation of the hypersensitive response of culture tobacco cells. Appl Environ Microbiol. 1989 Jul;55(7):1724–1729. doi: 10.1128/aem.55.7.1724-1729.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]