Abstract
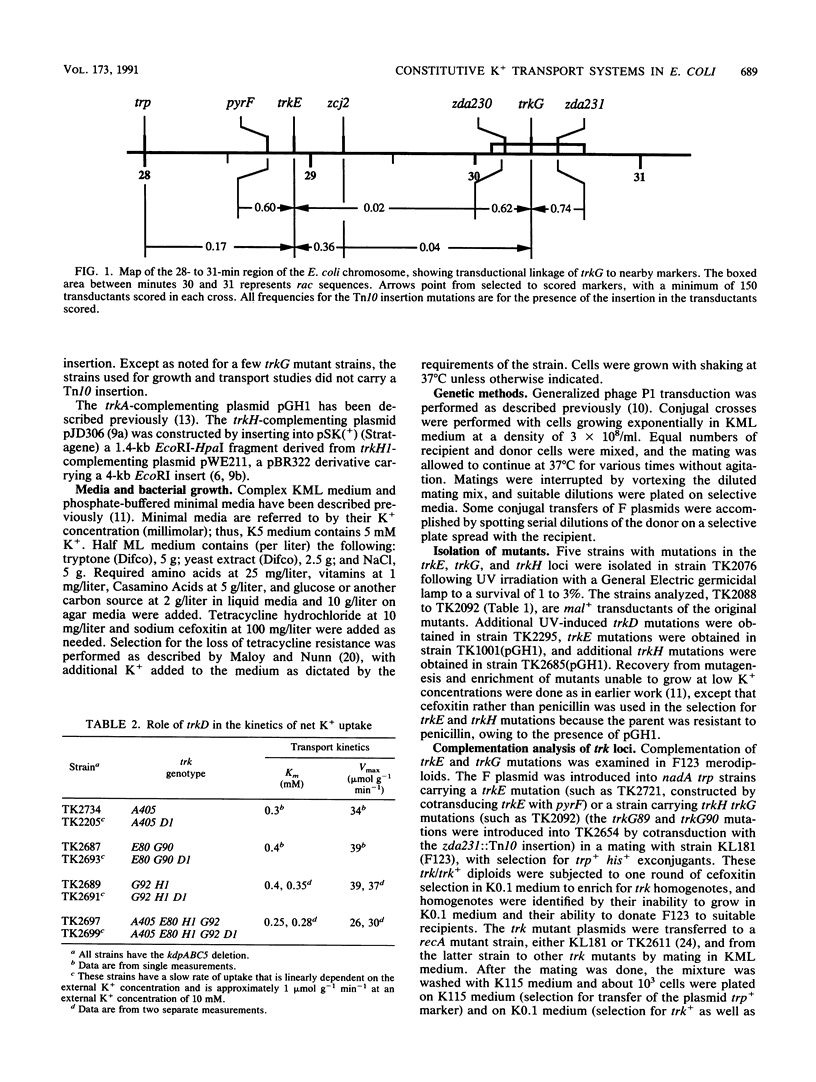
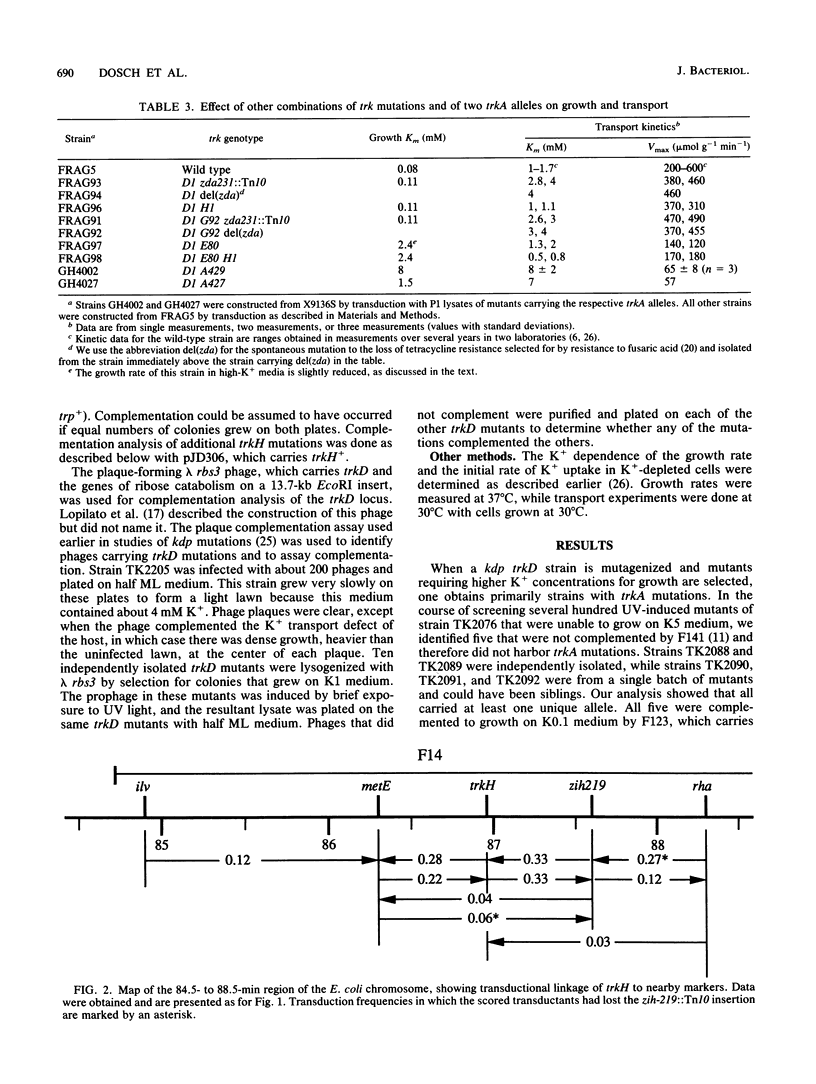
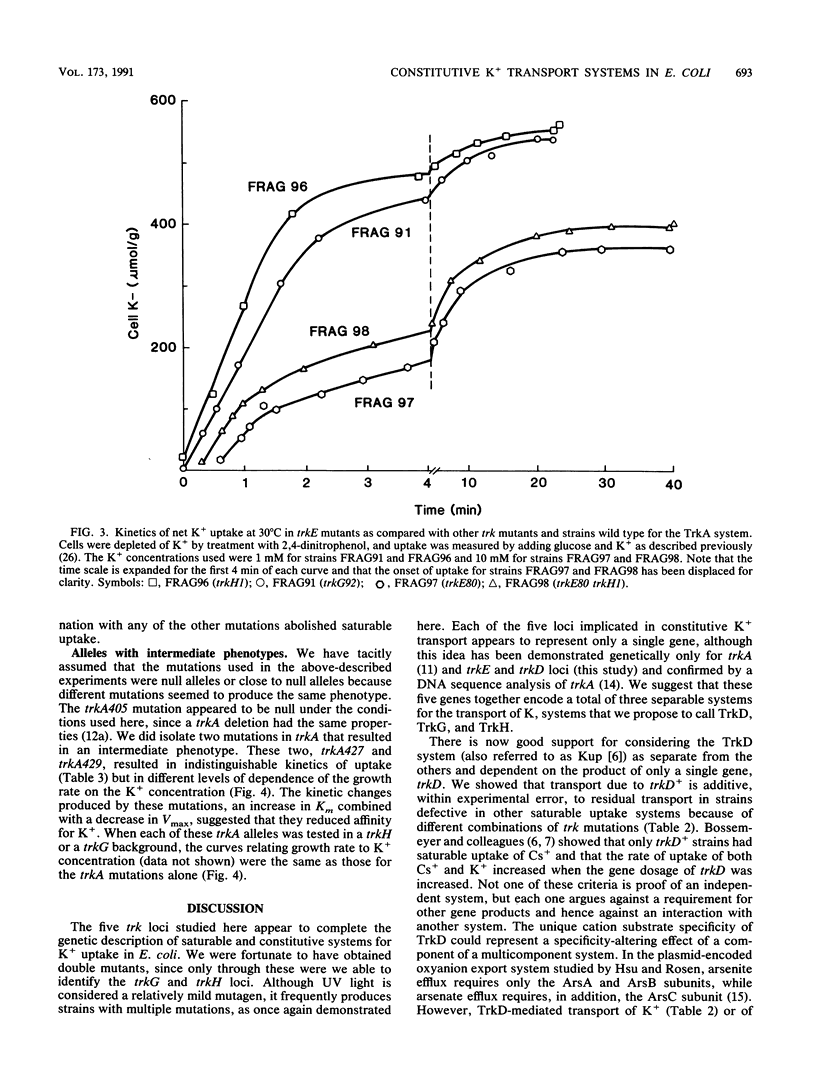
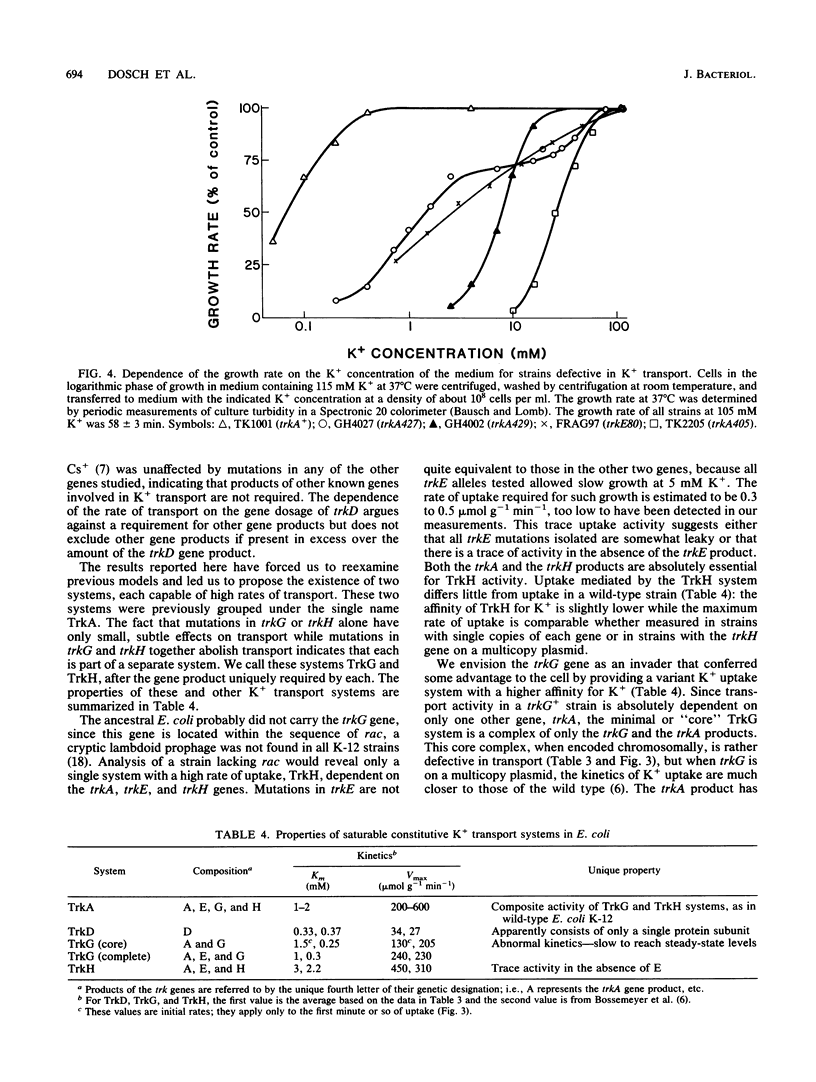
The analysis of mutants of Escherichia coli that require elevated concentrations of K+ for growth has revealed two new genes, trkG, near minute 30 within the cryptic rac prophage, and trkH, near minute 87, the products of which affect constitutive K+ transport. The analysis of these and other trk mutations suggests that high rates of transport, previously considered to represent the activity of a single system, named TrkA, appear to be the sum of two systems, here named TrkG and TrkH. Each of these two is absolutely dependent on the product of the trkA gene, a cytoplasmic protein associated with the inner membrane (D. Bossemeyer, A. Borchard, D. C. Dosch, G. C. Helmer, W. Epstein, I. R. Booth, and E. P. Bakker, J. Biol. Chem. 264:16403-16410, 1989). The TrkH system is also dependent on the products of the trkH and trkE genes, while the TrkG system is also dependent on the product of the trkG gene and partially dependent on the product of the trkE gene. It is suggested that the trkH and trkG products are membrane proteins that form the transmembrane path for the K+ movement of the respective systems. Two mutations altering the trkA product reduce the affinity for K+ of both TrkG and TrkH, indicating that changes in peripheral protein can alter the conformation of the sites at which K+ is bound prior to transport. The TrkD system has a relatively modest rate of transport, is dependent solely on the product of the trkD gene, and is the sole saturable system for Cs+ uptake in this species (D. Bossemeyer, A. Schlösser, and E. P. Bakker, J. Bacteriol. 171:2219-2221, 1989).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spurich E. N. Protein-protein interaction in transport: periplasmic histidine-binding protein J interacts with P protein. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1877–1881. doi: 10.1073/pnas.73.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Structure and mechanism of bacterial periplasmic transport systems. J Bioenerg Biomembr. 1988 Feb;20(1):1–18. doi: 10.1007/BF00762135. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E. P., Booth I. R., Dinnbier U., Epstein W., Gajewska A. Evidence for multiple K+ export systems in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3743–3749. doi: 10.1128/jb.169.8.3743-3749.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binding R., Romansky G., Bitner R., Kuempel P. Isolation and properties of Tn10 insertions in the rac locus of Escherichia coli. Mol Gen Genet. 1981;183(2):333–340. doi: 10.1007/BF00270637. [DOI] [PubMed] [Google Scholar]
- Bossemeyer D., Borchard A., Dosch D. C., Helmer G. C., Epstein W., Booth I. R., Bakker E. P. K+-transport protein TrkA of Escherichia coli is a peripheral membrane protein that requires other trk gene products for attachment to the cytoplasmic membrane. J Biol Chem. 1989 Oct 5;264(28):16403–16410. [PubMed] [Google Scholar]
- Bossemeyer D., Schlösser A., Bakker E. P. Specific cesium transport via the Escherichia coli Kup (TrkD) K+ uptake system. J Bacteriol. 1989 Apr;171(4):2219–2221. doi: 10.1128/jb.171.4.2219-2221.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle A. M., Macnab R. M., Shulman R. G. Coupling between the sodium and proton gradients in respiring Escherichia coli cells measured by 23Na and 31P nuclear magnetic resonance. J Biol Chem. 1986 Jun 15;261(17):7797–7806. [PubMed] [Google Scholar]
- Epstein W., Davies M. Potassium-dependant mutants of Escherichia coli K-12. J Bacteriol. 1970 Mar;101(3):836–843. doi: 10.1128/jb.101.3.836-843.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Kim B. S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann A., Bossemeyer D., Bakker E. P. Physical mapping of the K+ transport trkA gene of Escherichia coli and overproduction of the TrkA protein. J Bacteriol. 1987 Jul;169(7):3138–3145. doi: 10.1128/jb.169.7.3138-3145.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Rhoads D. B., Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Jan;78(1):464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopilato J. E., Garwin J. L., Emr S. D., Silhavy T. J., Beckwith J. R. D-ribose metabolism in Escherichia coli K-12: genetics, regulation, and transport. J Bacteriol. 1984 May;158(2):665–673. doi: 10.1128/jb.158.2.665-673.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Restoration by the rac locus of recombinant forming ability in recB - and recC - merozygotes of Escherichia coli K-12. Mol Gen Genet. 1973 Apr 12;122(2):119–130. doi: 10.1007/BF00435185. [DOI] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meury J., Kepes A. The regulation of potassium fluxes for the adjustment and maintenance of potassium levels in Escherichia coli. Eur J Biochem. 1981 Sep;119(1):165–170. doi: 10.1111/j.1432-1033.1981.tb05589.x. [DOI] [PubMed] [Google Scholar]
- Meury J., Robin A., Monnier-Champeix P. Turgor-controlled K+ fluxes and their pathways in Escherichia coli. Eur J Biochem. 1985 Sep 16;151(3):613–619. doi: 10.1111/j.1432-1033.1985.tb09148.x. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Polarek J. W., Walderhaug M. O., Epstein W. Genetics of Kdp, the K+-transport ATPase of Escherichia coli. Methods Enzymol. 1988;157:655–667. doi: 10.1016/0076-6879(88)57113-6. [DOI] [PubMed] [Google Scholar]
- Rhoads D. B., Laimins L., Epstein W. Functional organization of the kdp genes of Escherichia coli K-12. J Bacteriol. 1978 Aug;135(2):445–452. doi: 10.1128/jb.135.2.445-452.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. B., Waters F. B., Epstein W. Cation transport in Escherichia coli. VIII. Potassium transport mutants. J Gen Physiol. 1976 Mar;67(3):325–341. doi: 10.1085/jgp.67.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey B., Cayley D. S., Mossing M. C., Kolka C., Anderson C. F., Farrar T. C., Record M. T., Jr Variability of the intracellular ionic environment of Escherichia coli. Differences between in vitro and in vivo effects of ion concentrations on protein-DNA interactions and gene expression. J Biol Chem. 1987 May 25;262(15):7157–7164. [PubMed] [Google Scholar]
- Slonczewski J. L., Rosen B. P., Alger J. R., Macnab R. M. pH homeostasis in Escherichia coli: measurement by 31P nuclear magnetic resonance of methylphosphonate and phosphate. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6271–6275. doi: 10.1073/pnas.78.10.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]


