Abstract
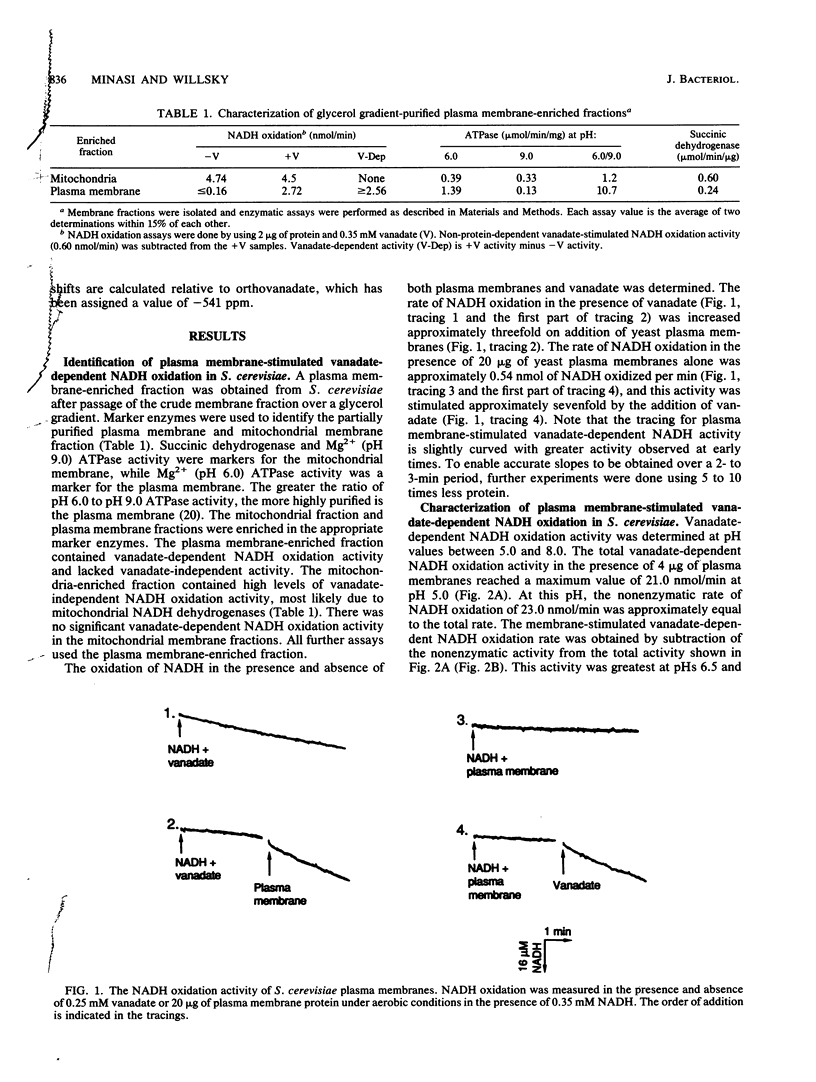
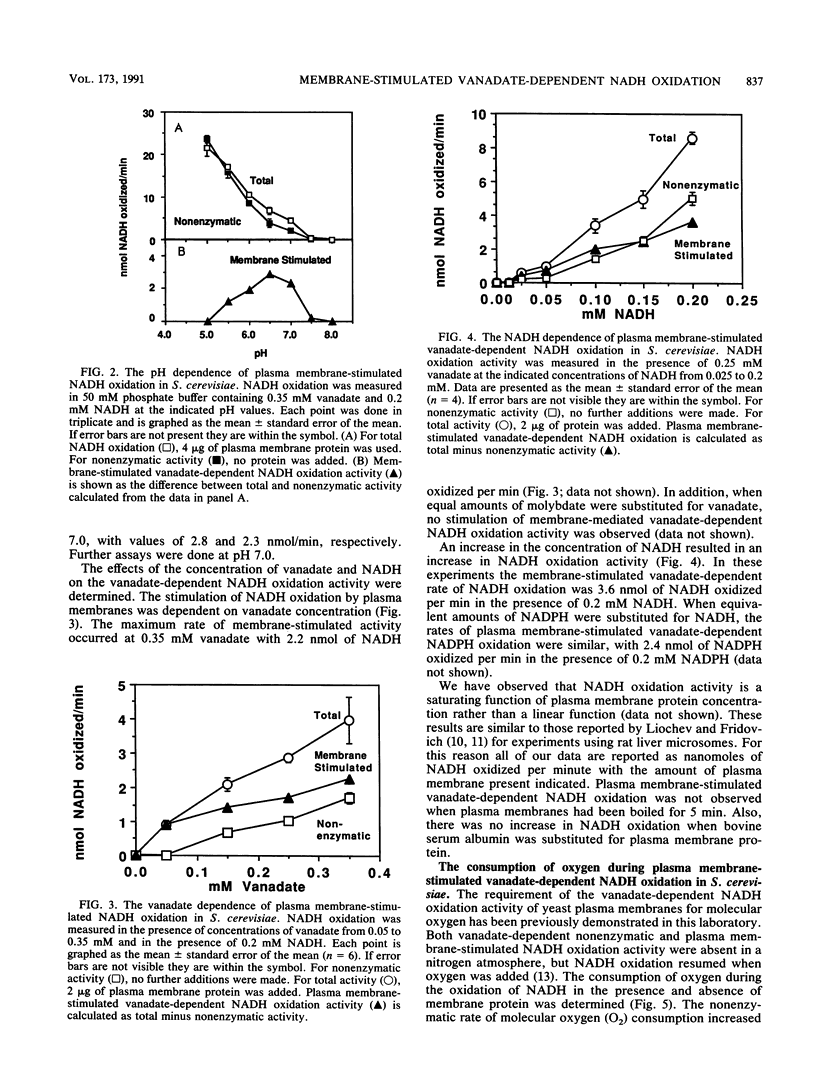
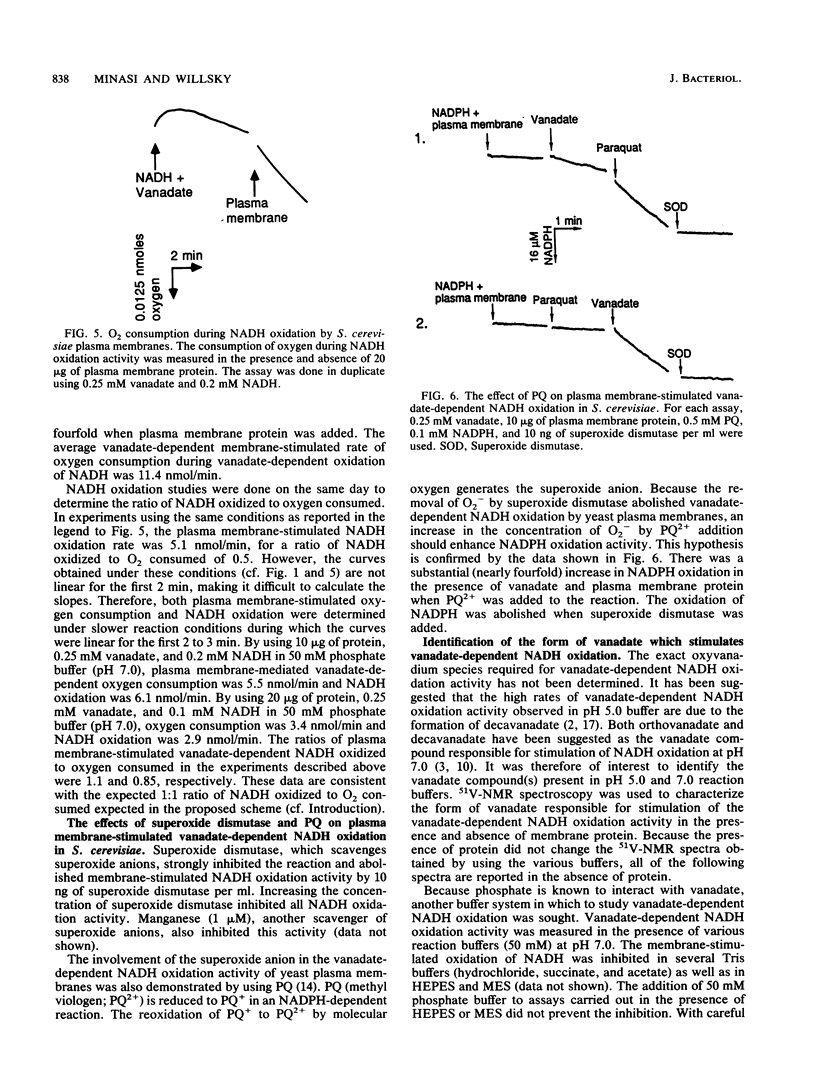
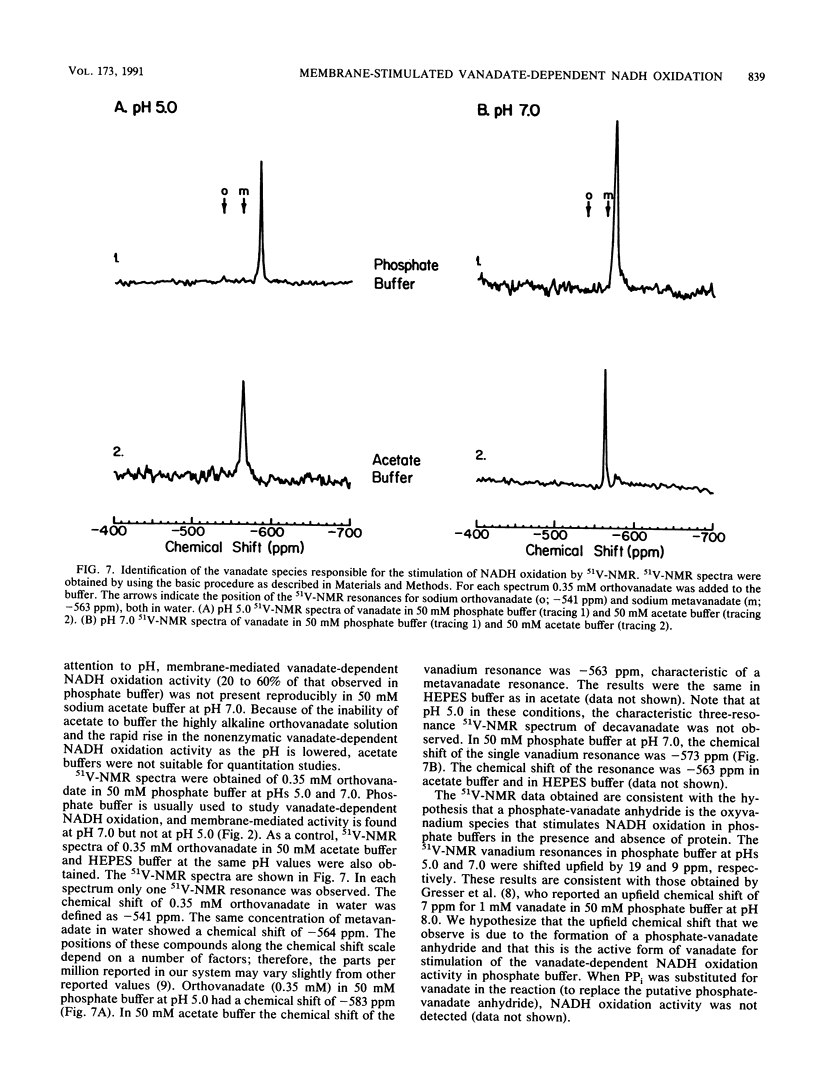
Plasma membrane-stimulated vanadate-dependent NADH oxidation has been characterized in Saccharomyces cerevisiae. This activity is specific for vanadate, because molybdate, a similar metal oxide, did not substitute for vanadate in the reaction. Vanadate-dependent plasma membrane-stimulated NADH oxidation activity was dependent on the concentrations of vanadate, NADH, and NADPH and required functional plasma membranes; no stimulation occurred in the presence of boiled membranes or bovine serum albumin. The dependence of membrane-stimulated vanadate-dependent NADH oxidation was not linearly dependent on added membrane protein. The activity was abolished by the superoxide anion scavenger superoxide dismutase and was stimulated by paraquat and NADPH. These data are consistent with the previously proposed chain reaction for vanadate-dependent NADH oxidation. The role of the plasma membrane appears to be to stimulate superoxide radical formation, which is coupled to NADH oxidation by vanadate. 51V-nuclear magnetic resonance studies are consistent with the hypothesis that a phosphovanadate anhydride is the stimulatory oxyvanadium species in the phosphate buffers used at pHs 5.0 and 7.0. In phosphate buffers, compared with acetate buffers, the single vanadate resonance was shifted upfield at both pH 5.0 and pH 7.0, which is characteristic of the phosphovanadate anhydride. Since the cell contains an excess of phosphate to vanadate, the phosphovanadate anhydride may be involved in membrane-mediated vanadate-dependent NADH oxidation in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Briskin D. P., Thornley W. R., Poole R. J. Vanadate-dependent NADH oxidation in microsomal membranes of sugar beet. Arch Biochem Biophys. 1985 Jan;236(1):228–237. doi: 10.1016/0003-9861(85)90622-8. [DOI] [PubMed] [Google Scholar]
- Coulombe R. A., Jr, Briskin D. P., Keller R. J., Thornley W. R., Sharma R. P. Vanadate-dependent oxidation of pyridine nucleotides in rat liver microsomal membranes. Arch Biochem Biophys. 1987 Jun;255(2):267–273. doi: 10.1016/0003-9861(87)90393-6. [DOI] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- Darr D., Fridovich I. Vanadate and molybdate stimulate the oxidation of NADH by superoxide radical. Arch Biochem Biophys. 1984 Aug 1;232(2):562–565. doi: 10.1016/0003-9861(84)90573-3. [DOI] [PubMed] [Google Scholar]
- Goodno C. C., Taylor E. W. Inhibition of actomyosin ATPase by vanadate. Proc Natl Acad Sci U S A. 1982 Jan;79(1):21–25. doi: 10.1073/pnas.79.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem. 1979 Nov 10;254(21):10846–10852. [PubMed] [Google Scholar]
- Liochev S. I., Fridovich I. Vanadate-stimulated oxidation of NAD(P)H in the presence of biological membranes and other sources of O2-. Arch Biochem Biophys. 1990 May 15;279(1):1–7. doi: 10.1016/0003-9861(90)90454-7. [DOI] [PubMed] [Google Scholar]
- Liochev S. I., Fridovich I. Vanadate-stimulated oxidation of NAD(P)H. Free Radic Biol Med. 1989;6(6):617–622. doi: 10.1016/0891-5849(89)90069-5. [DOI] [PubMed] [Google Scholar]
- Minasi L. A., Chang A., Willsky G. R. Plasma membrane-stimulated vanadate-dependent NADH oxidation is not the primary mediator of vanadate toxicity in Saccharomyces cerevisiae. J Biol Chem. 1990 Sep 5;265(25):14907–14910. [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasarma T., MacKellar W. C., Crane F. L. Vanadate-stimulated NADH oxidation in plasma membrane. Biochim Biophys Acta. 1981 Aug 6;646(1):88–98. doi: 10.1016/0005-2736(81)90275-3. [DOI] [PubMed] [Google Scholar]
- Role of vanadium in biology. Symposium summary. Fed Proc. 1986 Feb;45(2):123–132. [PubMed] [Google Scholar]
- Vijaya S., Crane F. L., Ramasarma T. A vanadate-stimulated NADH oxidase in erythrocyte membrane generates hydrogen peroxide. Mol Cell Biochem. 1984 Jun;62(2):175–185. doi: 10.1007/BF00223308. [DOI] [PubMed] [Google Scholar]
- Willsky G. R. Characterization of the plasma membrane Mg2+-ATPase from the yeast, Saccharomyces cerevisiae. J Biol Chem. 1979 May 10;254(9):3326–3332. [PubMed] [Google Scholar]
- Willsky G. R., White D. A., McCabe B. C. Metabolism of added orthovanadate to vanadyl and high-molecular-weight vanadates by Saccharomyces cerevisiae. J Biol Chem. 1984 Nov 10;259(21):13273–13281. [PubMed] [Google Scholar]


