Abstract
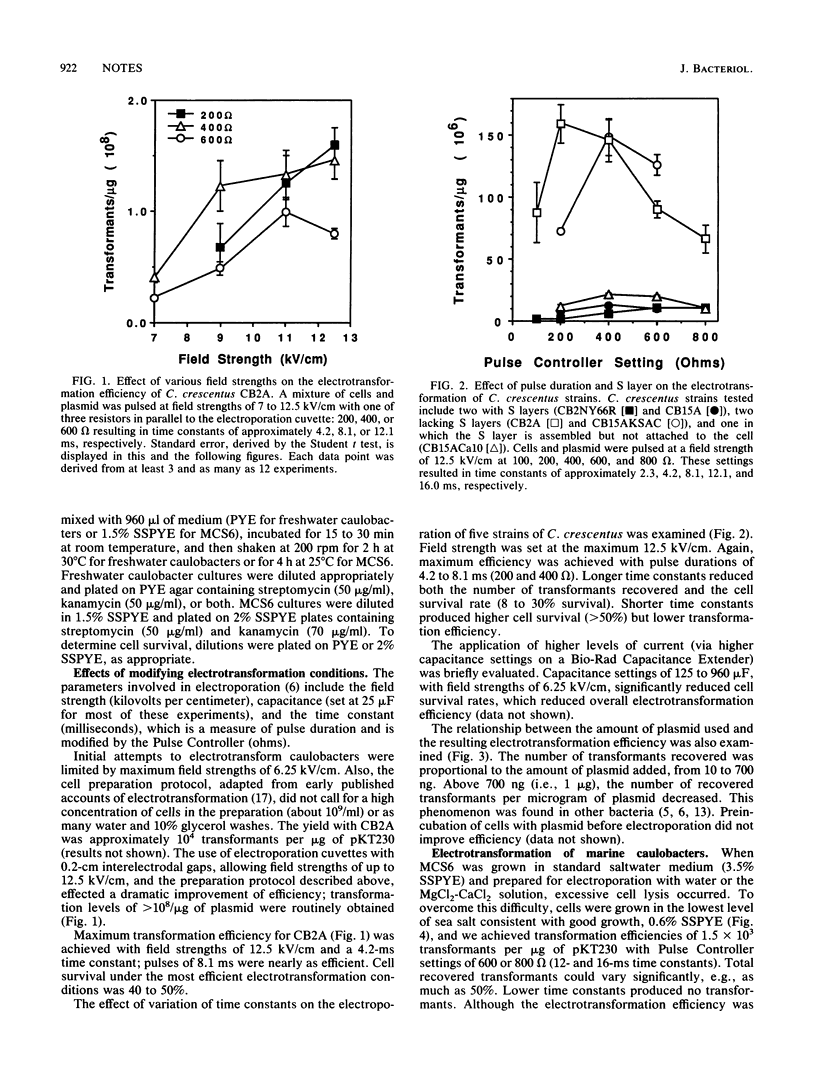
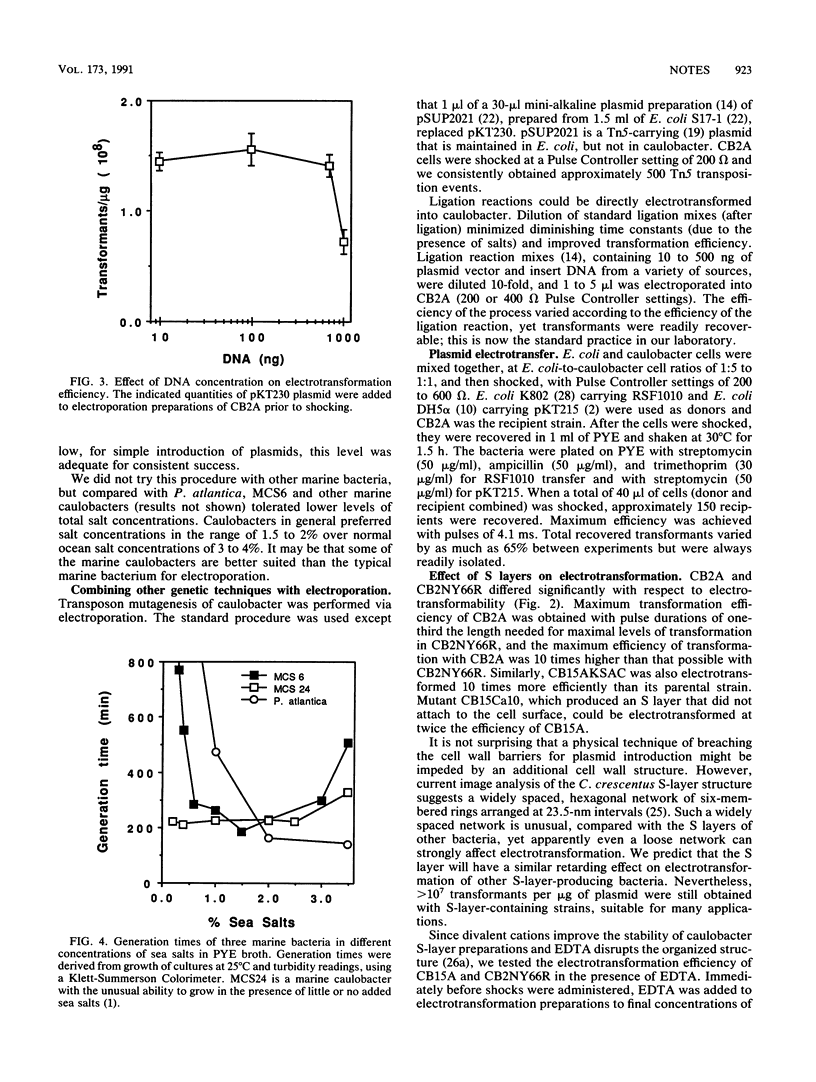
We performed plasmid electrotransformation of Caulobacter crescentus strains and obtained up to 3 x 10(8) transformants per micrograms of pKT230. The presence and integrity of the paracrystalline protein surface (S) layer influenced electroporation; caulobacters lacking the S layer were electrotransformed 10 times more efficiently than caulobacters possessing the S layers. A procedure yielding 1,500 transformants per micrograms of pKT230 was developed for a marine caulobacter. Electroporation was used in combination with several genetic techniques, including introduction of ligation mixtures, suicide transposon mutagenesis, gene replacement, and plasmid electrotransfer from Escherichia coli to caulobacters.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anast Nick, Smit John. Isolation and Characterization of Marine Caulobacters and Assessment of Their Potential for Genetic Experimentation. Appl Environ Microbiol. 1988 Mar;54(3):809–817. doi: 10.1128/aem.54.3.809-817.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Barany F. Single-stranded hexameric linkers: a system for in-phase insertion mutagenesis and protein engineering. Gene. 1985;37(1-3):111–123. doi: 10.1016/0378-1119(85)90263-x. [DOI] [PubMed] [Google Scholar]
- Conchas R. F., Carniel E. A highly efficient electroporation system for transformation of Yersinia. Gene. 1990 Mar 1;87(1):133–137. doi: 10.1016/0378-1119(90)90505-l. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B., Croft R. H. Transposon mutagenesis in Caulobacter crescentus. J Bacteriol. 1982 Feb;149(2):620–625. doi: 10.1128/jb.149.2.620-625.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B., Johnson R. C. Generalized Transduction in CAULOBACTER CRESCENTUS. Genetics. 1977 Nov;87(3):391–399. doi: 10.1093/genetics/87.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinha M. A., Kropinski A. M. High efficiency electroporation of Pseudomonas aeruginosa using frozen cell suspensions. FEMS Microbiol Lett. 1990 Jul;58(2):221–225. doi: 10.1111/j.1574-6968.1990.tb13982.x. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Wnendt S., Stahl U. High-efficiency electro-transformation of Escherichia coli with DNA from ligation mixtures. Nucleic Acids Res. 1990 Mar 25;18(6):1653–1653. doi: 10.1093/nar/18.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl W., Bayerl A., Schein B., Stillner U., Schleifer K. H. High efficiency electroporation of intact Corynebacterium glutamicum cells. FEMS Microbiol Lett. 1989 Dec;53(3):299–303. doi: 10.1016/0378-1097(89)90234-6. [DOI] [PubMed] [Google Scholar]
- Luchansky J. B., Muriana P. M., Klaenhammer T. R. Application of electroporation for transfer of plasmid DNA to Lactobacillus, Lactococcus, Leuconostoc, Listeria, Pediococcus, Bacillus, Staphylococcus, Enterococcus and Propionibacterium. Mol Microbiol. 1988 Sep;2(5):637–646. doi: 10.1111/j.1365-2958.1988.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Marcus H., Ketley J. M., Kaper J. B., Holmes R. K. Effects of DNase production, plasmid size, and restriction barriers on transformation of Vibrio cholerae by electroporation and osmotic shock. FEMS Microbiol Lett. 1990 Mar 1;56(1-2):149–154. doi: 10.1111/j.1574-6968.1990.tb04139.x. [DOI] [PubMed] [Google Scholar]
- Mersereau M., Pazour G. J., Das A. Efficient transformation of Agrobacterium tumefaciens by electroporation. Gene. 1990 May 31;90(1):149–151. doi: 10.1016/0378-1119(90)90452-w. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Dower W. J., Tompkins L. S. High-voltage electroporation of bacteria: genetic transformation of Campylobacter jejuni with plasmid DNA. Proc Natl Acad Sci U S A. 1988 Feb;85(3):856–860. doi: 10.1073/pnas.85.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D., Smit J. Identification of genes affecting production of the adhesion organelle of Caulobacter crescentus CB2. J Bacteriol. 1990 Sep;172(9):5425–5431. doi: 10.1128/jb.172.9.5425-5431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill E. A., Kiely G. M., Bender R. A. Transposon Tn5 encodes streptomycin resistance in nonenteric bacteria. J Bacteriol. 1984 Jul;159(1):388–389. doi: 10.1128/jb.159.1.388-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. T., Rood J. I. Electroporation-mediated transformation of lysostaphin-treated Clostridium perfringens. Gene. 1989 Oct 30;82(2):327–333. doi: 10.1016/0378-1119(89)90059-0. [DOI] [PubMed] [Google Scholar]
- Smit J., Agabian N. Cloning of the major protein of the Caulobacter crescentus periodic surface layer: detection and characterization of the cloned peptide by protein expression assays. J Bacteriol. 1984 Dec;160(3):1137–1145. doi: 10.1128/jb.160.3.1137-1145.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Grano D. A., Glaeser R. M., Agabian N. Periodic surface array in Caulobacter crescentus: fine structure and chemical analysis. J Bacteriol. 1981 Jun;146(3):1135–1150. doi: 10.1128/jb.146.3.1135-1150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. K., Withers H. L. Electrotransfer: direct transfer of bacterial plasmid DNA by electroporation. Nucleic Acids Res. 1990 Apr 25;18(8):2192–2192. doi: 10.1093/nar/18.8.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth R., Friesenegger A., Fiedler S. Transformation of various species of gram-negative bacteria belonging to 11 different genera by electroporation. Mol Gen Genet. 1989 Mar;216(1):175–177. doi: 10.1007/BF00332248. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]


