Abstract
Patients with defects in phagocytic function are predisposed to intracellular microorganisms and typically have early dissemination of the infection. Recognition of the underlying disorder and aggressive antimicrobial therapy has been beneficial for the patients. Improved understanding of the pathophysiology has also affected patient management by allowing specific, targeted immunomodulatory intervention. The disorders described in this review are not common but have had a significant impact on our understanding of the role of phagocytic cells in host defense. Conversely, understanding the role of the neutrophil and macrophage in infection has benefited not just the patients described in this review but also other patients with similar disease processes.
INTRODUCTION
A characteristic of infections in patients with disorders of phagocytic cells is the frequency of infections at epithelial surfaces and the frequency of dissemination. The skin and the gastrointestinal tract harbor large numbers of potentially pathogenic organisms. Host defense at these sites relies primarily on innate immunity because the constant exposure to microorganisms requires a very rapid response. Phagocytic cells are the cornerstone of the innate immune system. This review describes the pathophysiology of the primary disorders of phagocytic cell function. Primary defects in macrophage function and neutrophil function are not common in the general population but require very aggressive and specific management and lifelong prophylaxis in some cases. Recognition of the characteristic infections occurring in both types of disorders is important both from the perspective of diagnosis and for treatment. Studies of these disorders also provide important insights into the role of phagocytic cells in host defense.
THE CELLS OF THE INNATE IMMUNE SYSTEM CONSTITUTE THE FIRST LINE OF DEFENSE
The cells of the innate immune system are monocytes/macrophages, neutrophils, and natural killer (NK) cells. NK cells are not phagocytic and are not discussed here. In some sense, the epithelial surfaces themselves contribute to host defense and could be considered a component of the innate immune system. The physical characteristics of epithelial surfaces assist in the defense against infection, and they typically produce soluble substances with antibacterial effects. Lysozyme, defensins, cryptidins, and surfactants are examples of antimicrobial substances produced by epithelial surfaces. There are data to support the concept that defects in these soluble mediators significantly compromise host defense (224, 225, 231); however, this review focuses on primary disorders of macrophage and neutrophil phagocytic function.
Pattern Recognition Receptors
The cells of the innate immune system utilize evolutionarily ancient pattern recognition receptors to distinguish among pathogens. These receptors are highly conserved among all multicellular eukaryotic species. They recognize a relatively small number of highly conserved structures which are common to large numbers of microorganisms. Microbial structural motifs are sometimes referred to as pathogen-associated molecular patterns, and the receptors which recognize them are called pattern recognition receptors (181). The advantages of this type of innate system are its heritability and the immediacy of the response. The overall goal of the innate immune system is the recognition of pathogens, recruitment of the requisite cells, and eradication of the pathogens. Pattern recognition receptors are involved in the initial stages.
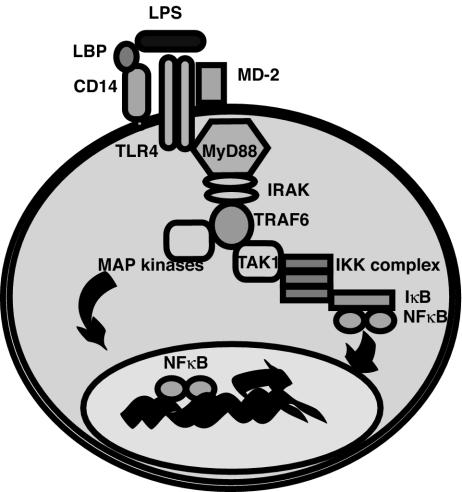
There are three functional categories of pattern recognition receptors. Secreted pattern recognition molecules typically function as opsonins, and the best known examples of this category are the proteins of the alternative complement pathway, the lectin activation pathway of the complement cascade, surfactants, C-reactive protein, serum amyloid protein, and lipopolysaccharide (LPS) binding protein (95, 125, 250, 296). Through their actions as opsonins, they can influence the uptake of antigen by phagocytic cells (56, 230). The second category of pattern recognition receptors is the endocytic category. Their main function is to mediate the uptake of pathogens; examples of this category include the macrophage mannose receptor and the macrophage scavenger receptor. Defects in these molecules have not been described in humans, but their importance has been demonstrated in mice. Mice deficient in type I/II macrophage scavenger receptors show defective killing of Listeria monocytogenes, clearance of malaria, and regulation of responses to endotoxin (134, 146, 203). The last category, and the one most relevant to the disorders discussed in this review, are the signaling receptors. The best characterized set of receptors that perform a direct signaling function are the toll-like receptors (TLRs). These are extremely highly conserved throughout evolution and were originally identified in Drosophila (115). There are 10 TLRs in humans; they recognize different pathogen-associated molecular patterns (182). All share a conserved leucine-rich extracellular domain and a cytoplasmic domain with homology to the interleukin-1 (IL-1) receptor. The TLRs may not recognize pathogens directly. In the case of bacterial lipopolysaccharide (LPS), at least three additional proteins appear to be required for receptor engagement (Fig. 1) (229, 256). Recognition of peptidoglycan and gram-negative bacteria by toll in Drosophila also requires a soluble cofactor, suggesting that this may be a common feature of this class of receptors (165, 166, 183).
FIG. 1.
Signaling through TLR4. TLRs are dimers which signal Myd88 and TIRAP (not shown) to induce the MAP kinase cascade and activation of NF-κB. These molecules induce the transcription of a variety of inflammatory mediators. Certain TLRs are known to require soluble cofactors for recognition, such as LPS binding protein (LBP), CD14, and MD-2.
The TLRs all have a common signaling pathway involving NF-κB. As shown in Fig. 1, phosphorylation of IκB by IKK and release of active NF-κB is a final common pathway for all known TLRs (281). This is significant not only for the generation of inflammatory mediators such as IL-12 but also for the induction of expression of T-cell costimulatory receptors. The importance of this pathway in activating NF-κB is shown by the profound immunodeficiency that occurs in people with defective IKK, the kinase that phosphorylates IκB (discussed below). The TLRs also activate mitogen-activated protein (MAP) kinases, and this group of signaling molecules is important in the activation of macrophages and phagocytosis (168).
A second category of signaling receptors has recently been described. These receptors are unusual in that they are completely intracellular. They also activate NF-κB and the MAP kinases but are thought to participate primarily in the regulation of inflammatory responses. The best known examples are the members of the nucleotide-binding oligomerization domain (NOD) family of caspase recruitment domain. proteins and the double-stranded RNA-activated protein kinase (35, 132). NOD mutations have been implicated in Crohn's disease and infantile-onset sarcoidosis.
Opsonization
Opsonization refers to the “tagging” of a pathogen by serum proteins such that it is more likely to be phagocytosed. Many proteins participate in this process, and many are activated as part of the acute-phase response. Mannose-binding lectin, C-reactive protein, C3, and antibody are all potent opsonins which act to facilitate the uptake of pathogens by phagocytic cells. Receptors for each opsonin are present on the macrophage surface. Fc receptors on neutrophils are the primary receptors for opsonins present.
Mechanisms of Neutrophil-Mediated Killing
Neutrophils are capable of recognizing bacteria directly, often via TLRs. In addition, the neutrophil integrins, CD11b/CD18 (Mac-1) and CD11c/CD18 (p150), recognize and bind Leishmania spp., Bordetella spp., Candida spp., and Histoplasma capsulatum directly (48). Most frequently, microbes are coated with antibody or complement. Receptors recognize complement activation products and facilitate engulfment, as do the immunoglobulin Fc receptors. Cross-linking of these surface receptors activates the respiratory burst that is unique to myeloid cells and activates the phagocytic pathway (51). On phagocytosis of the microbe, reactive oxygen species are released into the primary phagosome. A charge differential, generated during the respiratory burst, leads to neutrophil granules releasing their contents into the phagosome, enhancing killing (44, 112, 235, 254). Table 1 shows the main microbicidal substances released into the phagosome to promote killing.
TABLE 1.
Neutrophil-mediated microbial killing
| Microbicidal substance | Action |
|---|---|
| Defensins | Cause gram-negative bacterial permeability |
| Proteinase 3 | Amplifies inflammation |
| Elastase | Degrades outer membrane protein of gram-negative bacteria |
| PLA2 | Has potent bactericidal activity against S. aureus |
| Myeloperoxidase | Converts H2O2 to HOCI |
| Metalloproteinases | Gelatinase and collagenase may activate chemokines in addition to cleaving matrix proteins |
| Reactive oxygen species | Cause oxidation of bacterial proteins and nucleic acids |
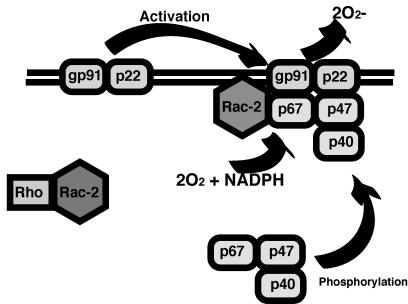
The respiratory burst is critical, and patients with defective NADPH oxidase (chronic granulomatous disease [CGD]) have frequent infections with Staphylococcus aureus and other catalase-positive organisms (295). The NADPH oxidase system is part of a larger biochemical pathway regulating the flow of electrons to various substrates. There are five subunits comprising NADPH oxidase proper (Fig. 2) and a regulatory subunit termed Rac-2. gp91 phox and p22 phox (cytochrome b558) are embedded primarily in the membrane of specific granules, with scattered complexes in the plasma membrane and in secretory granules. These two membrane-bound subunits are constitutively associated (214, 251). Cell surface signaling events lead to GTP binding on Rac-2 in the cytoplasm (2). Rac-2 then binds and stabilizes the cytoplasmic protein p67 phox, which is phosphorylated by protein kinase C. Phosphorylation of p47 phox leads to its interaction with the cytoskeleton and translocation to the membrane (197). The entire complex is required for the production of superoxide, O2−.
FIG. 2.
NADPH oxidase. The NADPH oxidase complex consists of five structural components and Rac-2, which serves to regulate activation of the NADPH oxidase complex. Superoxide (O2−) is produced by the complex and released extracellularly or into the primary phagosome.
Superoxide itself is a weakly microbicidal agent. Metabolism to H2O2 by superoxide dismutase results in a more microbicidal compound, and H2O2 can be converted to HOCl by myeloperoxidase. Both H2O2 and HOCl are strongly microbicidal. Additional biochemical reactions produce peroxynitrite anion and nitryl chloride, which contribute additional microbicidal activities (49).
Mechanisms of Macrophage-Mediated Killing
Macrophages are important antigen-presenting cells and thus participate both directly in killing microorganisms and indirectly by cueing of the adaptive lymphocyte responses. Macrophage activation and granuloma formation depend on both tumor necrosis factor alpha (TNF-α) and gamma interferon (53, 93, 145, 171, 184, 237), and these two cytokines influence each other's expression (199). In humans with gamma interferon receptor 1 deficiency, one of the first immunologic defects identified was a lack of gamma interferon enhancement of macrophage production of TNF-α. Nearly all other tests of immunologic function were normal (162). The patients had no detectable granulomas or poorly formed granulomas, depending on the severity of the mutation.
The mechanism by which gamma interferon elicits the killing of intracellular organisms in mice is well defined. Gamma interferon induces superoxide and nitric oxide synthase. TNF-α then triggers NO production. Mice deficient for inducible nitric oxide synthase or components of NADPH oxidase are uniformly more susceptible to infection with intracellular organisms such as Mycobacterium spp., Listeria spp., Salmonella spp., Aspergillus spp., and Toxoplasma gondii (41). Human macrophages, in contrast, produce little NO, and inducible nitric oxide synthase is induced by IL-4 and cross-linking of the immunoglobulin E (IgE) receptor on macrophages (85, 217, 278, 279). The role of TNF-α and gamma interferon in humans may be to augment this pathway.
Interfacing with Adaptive Immunity
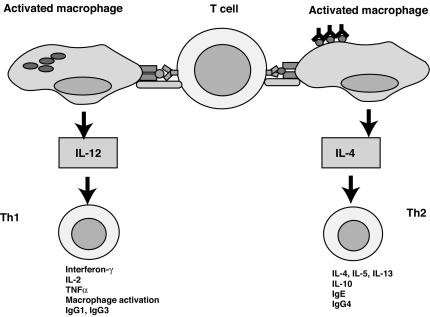
The innate immune system interfaces with and directs the subsequent responses of the adaptive immune system. There is ample evidence for the concept that exposures of mice and humans to microbes or microbial products directs a Th1-skewed immune system (22, 126). A common model for this phenomenon is that stimulation through TLRs leads to the production of IL-12, which is a Th1-polarizing cytokine (Fig. 3). Engagement of TLRs also leads to the expression of costimulatory molecules, which would allow the T cells to support an antimicrobial response. There is also evidence to support the concept that regulatory T cells are affected by exposures to pathogens (7, 301, 302).
FIG. 3.
T cells become polarized toward Th1 by macrophages activated by microbes. T cells generally can be categorized as Th1 or Th2 depending on the cytokines produced by the individual cell. Th1 cells predominantly activate macrophages to enhance killing through the release of gamma interferon and TNF-α. In mice they can stimulate B cells to make opsonizing antibodies of the IgG1 and IgG3 subclasses. Th2 cells predominantly activate B cells through the secretion of IL-4 and IL-5. They can also activate eosinophils and are important in defense against parasites. The infected macrophage acts to determine the fate of the naive T cell by producing IL-12, which is produced in response to engagement of TLRs by microbes. In the absence of IL-12, IL-4 from other cells can direct a Th2 response. The response then ultimately directs effector functions listed at the bottom of the figure.
INHERITED NEUTROPHIL DISORDERS
Many of the inherited neutrophil disorders are associated with neutropenia. This review does not focus on pure congenital neutropenias because they have recently been reviewed elsewhere (17). Instead, it focuses on disorders of phagocytic function. Neutrophils are cells with a complex functional program that is executed in response to an array of cues from the environment. The final common process is usually phagocytosis, but neutrophils can infiltrate and release inflammatory mediators in the absence of phagocytosis.
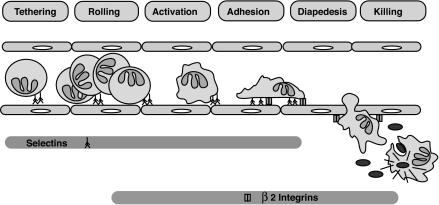
Neutrophils are derived from pluripotent progenitors in the bone marrow. Production is increased in response to granulocyte-monocyte colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) (116, 119). As neutrophils mature, they proceed through the myeloblast, promyelocyte, and myelocyte stages as actively dividing cells. Metamyelocytes and fully differentiated neutrophils are nondividing cells. Once released from the bone marrow, neutrophils have a short half-life and circulate for 8 to 24 h before undergoing constitutive apoptosis (284). The life span is extended in inflammatory conditions and is shortened by active phagocytosis (297, 298). While circulating, the cells are attracted to sites of inflammation by complement component C5a, various chemokines, leukotrienes, and bacterial peptides. Travel from the circulation to the issue requires a coordinated interaction of adhesion molecules (Fig. 4).
FIG. 4.
Neutrophil responses to infection. Initial interactions are mediated by P-selectin, which is released from Weibel-Palade bodies in endothelial cells on exposure to C5a, leukotriene B4, TNF-α, or LPS. E-selectin is expressed subsequently, and together they bind fucosylated glycoproteins on the tips of neutrophil surface villi. This initial interaction is often termed rolling. IL-8 and other chemokines cause a conformational change in neutrophil surface integrins and allow binding to ICAM-1 and ICAM-2, which are induced on inflamed endothelial cells. Extravasation requires the β2-integrins and PECAM (CD31). Migration toward C5a, bacterial peptides, or a chemokine gradient is mediated by interactions with cell surface receptors and cytoskeletal changes. Resident tissue macrophages produce chemokines in response to pathogen recognition receptor engagement. Phagocytosis is activated by the binding of complement receptors, immunoglobulin receptors, or TLRs. Once engulfed, bacteria are killed by the combined actions of granule contents and reactive oxygen species. Rolling is defective in LAD 2, tight adhesion is defective in LAD 1, and chemotaxis is defective in Chédiak-Higashi syndrome, specific granule deficiency, Schwachman syndrome, myelokathexis, and glycogen storage disease type Ib. Phagocytosis is defective in LAD 1, and intracellular killing is defective in CGD, specific granule deficiency, Chédiak-Higashi syndrome, myeloperoxidase deficiency, myelokathexis, and glycogen storage disease type Ib.
Once at the site of inflammation, neutrophils bind to the pathogen by means of pathogen recognition receptors or receptors for opsonins. Following phagocytosis, the respiratory burst is activated as described above and the granule contents are released into the primary phagosome. In this review we broadly considers defects in phagocytic function to be defects that impair chemotaxis, adhesion, and/or killing. Certain of the primary neutrophil disorders are associated with a mild to moderate neutropenia due to impaired survival or production. These are considered separately from the others.
Neutrophil Disorders Associated with Neutropenia
After infancy, neutrophil counts lower than 1,500 × 109/liter are considered to be abnormal and neutrophil counts lower than 500 × 109/liter are associated with significant risk of serious infection (289). The causes of neutropenia encompass drug effects, infection, autoimmune processes, congenital disorders, and malignancy. In some cases, the neutropenia is indicative of an underlying defect in neutrophil function. Many metabolic disorders are associated with neutropenia on the basis of accumulation of toxins detrimental to neutrophil survival (Table 2). Generally, these resolve as the metabolic derangement is addressed. This section reviews primary neutrophil disorders associated with neutropenia.
TABLE 2.
Inborn errors of metabolism associated with neutropenia
| Disorder | Clinical features | Infections and causes |
|---|---|---|
| α-Mannosidosis | Dysostosis multiplex, hepatomegaly, facial coarsening, developmental delay | Recurrent bacterial infections due to neutrophil chemotaxis defect |
| Glycogen storage disease 1b/c | Hepatomegaly, hypoglycemia, seizures | Recurrent mucous membrane ulcers, recurrent infections due to neutropenia and neutrophil dysfunction |
| Transcobalamin II deficiency | FTT,a vomiting, megaloblastic anemia, increasing neurologic dysfunction | Oral ulcers, frequent infections; pancytopenia and hypogammaglobulinemia |
| Branched-chain organic acidurias | 16 types with variable clinical features; typically include acidosis, neurologic signs, and cardiomyopathy; may present with a Reye's-like syndrome | Accumulation of toxic metabolites diminishes leukocyte function; 3-methylglutaconic aciduria type II (Barth syndrome) is associated with neutropenia |
FTT, failure to thrive.
Chédiak-Higashi syndrome.
Chédiak-Higashi syndrome is diagnosed clinically in patients with mild neutropenia, peripheral nerve conduction defects, pigmentary dilution with partial oculocutaneous albinism, easy bruising, and frequent infections (25, 144, 220). The syndrome is due to mutations in LYST, which encodes a cytoplasmic protein involved in vacuole formation and transport of proteins (26, 192, 219). While Chédiak-Higashi syndrome is usually considered an immunodeficiency, all cells with lysosomes are affected. Giant inclusions due to fusion of cytoplasmic granules are seen in hematopoietic cells, renal tubular cells, neurons, Schwann cells, melanocytes, and fibroblasts (290, 293). A common diagnostic strategy is to observe the giant inclusions in neutrophils or hair under high power. There is a spectrum of severity including a few adults who present with isolated peripheral neuropathy; however, the most common presentation involves recurrent infections due to the defective neutrophil function (144). The neutrophil inclusions are due to fusion of azurophilic and specific granules (233, 291). These giant granules fuse poorly with the primary phagosome, and intracellular killing is delayed on this basis (241). In addition, chemotaxis is inefficient due to impaired assembly of microtubules (99). For reasons that remain to be elucidated, the cells are also deficient in cathepsin G and elastase (102). Diminished elastase amounts may contribute to the neutropenia since elastase mutations have been found to underlie most cases of congenital neutropenia and cyclic neutropenia (17).
Most of the infectious manifestations are due to the neutrophil defect. The severe form of Chédiak-Higashi syndrome frequently presents with recurrent infections in infancy. Infections usually involve the skin and respiratory system and include cellulitis, abscess, otitis media, pneumonias, pyoderma, and periodontal disease. Skin abscesses can be particularly problematic, requiring frequent surgical intervention (24). Infections with S. aureus are common. Other bacterial pathogens include beta-hemolytic Streptococcus spp. and aerobic gram-negative rods (39). Periodontal disease can be severe, leading to alveolar bone loss and tooth exfoliation (72).
While bacterial infections are the most common feature of the immunodeficiency, the most dangerous feature is a hemophagocytic process that arises as a consequence of NK cell dysfunction in response to a viral infection (25, 61, 133, 241). These viruses are typically members of the Herpesviridae family, although other types of viruses have been recovered, and in one case Rickettsia was thought to be the trigger. This hemophagocytic process is sometimes termed the accelerated phase and is characterized by fever, hepatosplenomegaly, adenopathy, and pancytopenia (39, 232). The age of onset of the hemophagocytic process is variable, but onset is usually seen before adulthood and is ultimately seen in 85% of patients with Chédiak-Higashi syndrome.
Miscellaneous.
Glycogen storage disease type Ib, myelokathexis, and Schwachman syndrome are all associated with defects in neutrophil function and neutropenia. The neutropenia in glycogen storage disease type Ib ranges from mild to severe, and patients experience very frequent infections as a consequence of the combined effects of the neutropenia, impaired expression of adhesion molecules, impaired mobilization from the bone marrow, impaired chemotaxis, diminished calcium mobilization, and dysfunction of microbial killing (77, 105, 275, 276, 287). Many of these defects are reversed by G-CSF administration (50). The typical clinical manifestations include colitis, abscesses, gingivitis, and frequent skin infections (276). There is a spectrum of severity, but most patients present in infancy with hypoglycemia. The defect is in one member of the glucose-6-phosphatase system, which functions to maintain glucose homeostasis (58).
Myelokathexis is a rare disorder which occurs as an isolated defect or as part of complex with hypogammaglobulinemia and warts, sometimes called the WHIM syndrome (warts, hypogammaglobulinemia, infection, and myelokathexis) (20, 42, 127). The relationship between the two disorders is not understood, and the gene defect(s) is not known. Defects in the chemokine receptor CXCR4 have been found in some patients with WHIM. Both disorders appear to be inherited in an autosomal dominant fashion. Affected individuals have neutrophils that are hypersegmented with condensed nuclear lobes, which may be due to accelerated apoptosis (16). There may also be defective release from the bone marrow (210). Many defects in neutrophil function have been described, including defective chemotaxis, superoxide production, and Fc receptor expression (226, 288). The neutropenia and infections improve with administration of G-CSF; however, the defects in cell morphology are not altered (288).
Schwachman syndrome (or Schwachman-Diamond syndrome) is another poorly defined disorder. It is the second most common cause of pancreatic insufficiency in children. The manifestations are dysostosis multiplex, pancreatic insufficiency, and neutropenia with defective chemotaxis (60, 236, 246). Patients have an increased risk of leukemia, and there have been reports of increased chromosome breakage. G-CSF is not usually required, and its use must be balanced against the theoretical possibility of increasing the risk of leukemia (272).
Functional Neutrophil Disorders
The functional disorders of neutrophils are all characterized by recurrent infections of surfaces. In some cases the predisposition to infection is mild, while in others the infections may be life-threatening. Most of the disorders considered in this section are pure disorders of neutrophils. Only Gaucher's disease and α-mannosidosis have organ involvement unrelated to neutrophil dysfunction.
Chronic granulomatous disease.
CGD is the prototypic functional neutrophil disorder for most immunologists. It has a frequency of 1:100,000 to 1:200,000 people. Patients generally are normal in all other respects, although there may be mildly diminished T-cell numbers (120). The NADPH oxidase complex is expressed at its highest levels in neutrophils, although it is also seen in monocytes, B cells, and fibroblasts. Monocyte defects in NADPH oxidase may predispose to mycobacterial disease which is found with increased frequency in patients with CGD; however, the majority of infections are thought to be due to defective production of superoxide by neutrophils. In CGD, all other aspects of neutrophil function are normal (66, 67). As described above, there are five structural components in NADPH oxidase and a regulatory subunit called Rac-2 (Fig. 2) (253). Defects in all of the subunits except p40 phox have been found to cause CGD (295). Approximately two-thirds of patients with CGD have defects in the X-linked gene, encoding gp91 phox. Most of the mutations in the X-linked form are associated with a complete null phenotype, although there are reports of dysfunctional proteins produced in normal or near normal levels (295). Approximately 5% of patients with CGD have defects in the gene encoding the p22 phox subunit. These are also generally associated with a complete absence of superoxide production. Similarly, defects in the gene encoding p67 phox are also found in approximately 5% of patients, and nearly all patients with this gene defect have absent production of superoxide. In contrast to the diverse mutations seen in the other genes, the defects in p47 phox are often due to a 2-bp deletion causing a premature stop codon. Defects in p47 phox are seen in approximately 20% of patients with CGD (62, 295).
All patients with CGD, regardless of the genotype, suffer from recurrent infections with catalase-positive organisms. Catalase-negative organisms are not often medically significant in patients with CGD because these organisms provide their own H2O2, which is converted by the neutrophils to HOCl and other potent microbicidal reactive oxygen species. In essence, the microbes carry their own killing mechanism. In contrast, catalase-positive organisms eliminate their own H2O2, and in patients with CGD, there is no eukaryotic production of H2O2, and the pathogens survive and multiply. While all catalase-positive organisms have the potential to cause serious infection, there are a few pathogens which dominate the infection pattern. This is probably due to the increased exposure to certain organisms (S. aureus) and to the differential effects of microbicidal peptides or NO (Aspergillus nidulans). For example, Pseudomonas aeruginosa is a catalase-positive organism not frequently seen in patients with CGD whereas Burkholderia cepacia causes much morbidity and mortality in these individuals (152). Neutrophils from patients with X-linked CGD show reduced in vitro killing of Burkholderia cepacia, while Pseudomonas aeruginosa killing is intact (262). These studies suggest that other virulence factors or aspects of neutrophil killing contribute to the infection pattern in CGD.
(i) Infections in patients with CGD.
A national registry for patients with CGD in the United States was established in 1992 (295). The most common organisms, in order of frequency, are Staphylococcus spp., Aspergillus spp., Serratia spp., Nocardia spp., Burkholderia spp., Klebsiella spp., and Candida spp. A comparison with a previous report from 1989 shows an increasing frequency of recovering Aspergillus spp. (187). Other trends identified by the review of the national registry include an increasing frequency of Burkholderia cepacia and Nocardia infections and a decreasing frequency of Salmonella infections.
Pneumonia is the most frequent type of infection in CGD patients, affecting approximately 80% of patients (Table 3). Aspergillus spp. (41%) are the microorganisms most frequently recovered, followed by the bacteria Staphylococcus spp. (12%), B. cepacia (8%), and Nocardia spp. (8%). Abscesses are the second most frequent type of infection in patients with CGD, and the location of the abscess may suggest the microorganism responsible for the infection. Table 4 summarizes the pathogens most frequently causing infections in CGD patients by location in the body (295).
TABLE 3.
Clinical features in patients with CGDa
| Feature | % of patients affected |
|---|---|
| Penumonia | 79 |
| Liver abscess | 27 |
| Lung abscess | 16 |
| Perirectal abscess | 15 |
| Brain abscess | 3 |
| Adenitis | 53 |
| Osteomyelitis | 25 |
| Inflammatory bowel disease | 17 |
| Idiopathic thrombocytopenia purpura | 1 |
| Lupus-like syndrome | 3 |
Adapted from reference 294.
TABLE 4.
Organisms causing infection in patients with CGD
| Location or infection type | Organisms (listed in order of frequency) |
|---|---|
| Subcutaneous | Staphylococcus spp., Serratia spp., Aspergillus spp., Klebsiella spp. |
| Liver | Staphylococcus spp. Serratia spp., Steptococcus spp., Nocardia spp. |
| Lung | Aspergillus spp., Nocardia spp., Staphylococcus spp., Burkholderia spp. |
| Brain | Aspergillus spp., Staphylococcus spp. |
| Osteomyelitis | Serratia spp., Aspergillus spp., Paecilomyces spp., Staphylococcus spp. |
| Adenitis | Staphylococcus spp., Serratia spp., Candida spp., Klebsiella spp. |
| Perirectal abscesses | Staphylococcus spp., Klebsiella spp., Escherichia spp. |
| Bloodstream | Salmonella spp., Burholderia spp., Candida spp., Staphylococcus spp. |
Adapted from reference 295.
Osteomyelitis has been specifically examined in two separate studies. In the first study, Aspergillus spp. were the causative organisms in 38% of the cases and Serratia spp. were the causative organisms in 30% (263). The remainder of the cases were due to Nocardia spp., Mycobacterium spp., or Staphylococcus spp. In the second study, Serratia spp. and Aspergillus spp. each caused approximately 30% of the cases of osteomyelitis, with the remainder due to Paecilomyces spp., Staphylococcus spp., Nocardia spp., B. cepacia, or other gram-negative bacteria (295).
Nocardia species, including N. asteroides, N. farcinica, N. nova, and N. otitidiscaviarum, have frequently been identified as causing infections in patients with CGD (138, 255). A retrospective review of 28 episodes of Nocardia infections in CGD patients noted that all episodes involved pulmonary infection with dissemination occurring in one fourth of the episodes. Dissemination was less likely in patients receiving prophylaxis with gamma interferon or a sulfonamide (84).
Liver abscesses caused by Staphylococcus spp. or Serratia spp., as well as osteomyelitis caused by Serratia spp., are very suggestive of CGD, since these infections rarely occur in patients without an underlying immunodeficiency. Unusual infections such as these are suggestive of an underlying immunodeficiency and warrant an evaluation when encountered. Other unusual pathogens that have been identified infrequently in CGD patients include Chromobacterium violaceum, Mycobacterium spp., and Legionella spp. (59, 172, 173, 207, 218, 261).
A. fumigatus and A. nidulans are the most common fungi in patients with CGD at any site except the meninges and lymph nodes, where Candida spp. predominate (295). Candida spp. also frequently cause fungemia in patients with CGD. A. fumigatus and A. nidulans occur with similar frequency; however, A. nidulans is more virulent (252). The reason behind the increased virulence of A. nidulans in this population is unknown. In surveys of non-CGD patients with invasive Aspergillus infection, A. fumigatus is the most common and A. flavus is the next most common (73). A. nidulans is an uncommon pathogen outside of the CGD population. Unusual fungi have been occasionally reported in patients with CGD; these include A. sydowi, Penicillium chrysogenum, Paecilomyces varioti, Wangiella dermatitidis, Sarcinosporon inkin, Acremonium strictum, Trichoderma spp., Pseudoallescheria boydii, and Mucormycosis spp. (186, 295).
Two studies have recently shown that the most common cause of death in CGD patients is fungal infection. Advances in antifungal treatments may lead to improvement; however, at this time, fungal disease remains life-threatening. Deaths due to Aspergillus spp. comprised approximately 35% of the deaths in two separate studies (164, 295). Candida spp./Torulopsis spp. were a less common cause of death (6%). While other infections may be nearly as common, fungal infections require more sustained treatment, and recurrence and relapse are distressingly frequent. The limited armamentarium and the development of resistance to multiple antifungal drugs have limited the ability to effectively treat serious fungal disease in patients with CGD (283).
Patients with CGD would be expected to develop viral and parasitic infections at the same rate as the general population. There is no reason to conclude that viral infections would be any more severe in patients with CGD or other pure neutrophil disorders. The chance of bacterial superinfection is greater, but viral processes should be cleared normally. Nevertheless, significant infections with respiratory syncytial virus and adenovirus have been reported (295). This could reflect the diminished T-cell numbers seen in patients with CGD (120).
Leukocyte adhesion deficiency.
Leukocyte adhesion deficiency (LAD) refers to a number of defects in adhesion molecule expression and function (Table 5). The nomenclature for the LADs has not always been straightforward, but there is a trend toward more consistency. Traditionally, LAD I is due to mutations in the gene encoding CD18 (ITGB2). This is the common β subunit for all β2-integrins. Thus, expression of all β2-integrins is deficient. There are four members in this family of adhesion molecules: LFA-1 (CD11a/CD18), Mac-1 (CD11b/CD18, CR3), p150 (CD11c/CD18, CR4), and CD11d/CD18 (131, 260). All four molecules participate in the tight adhesion of neutrophils to endothelial cells, although the functions of LFA-1 and Mac-1 are the best characterized. In the absence of this tight adhesion step, transendothelial migration to the site of inflammation is markedly deficient (14). The disorder is characterized by a high resting neutrophil count and recurrent infections with frequent dissemination and sepsis. The infections are necrotic rather than pustular because neutrophils are unable to migrate to the site of infection. The mutations within the gene encoding CD18 are diverse, and there is substantial heterogeneity in the clinical presentation. Patients with 1 to 10% residual expression have a milder course than those who have completely absent expression of β2-integrins (13). Heterozygous carriers have no clinical manifestations, suggesting that a modest level of expression is sufficient for full function.
TABLE 5.
Features of LAD
| Clinical and laboratory feature | LAD I | LAD I variant | LAD II |
|---|---|---|---|
| Diagnosis | Diminished CD11b/CD18 expression | Functional analyses | Bombay blood type, absent Lewis sialyl X |
| Typical white cell count (μl−1) | 20,000-100,000 | 35,000-96,000 | 20,000-70,000 |
| T-cell function | Diminished delayed-type hypersensitivity | Normal except diminished proliferation to CD2 | Absent cutaneous lymphocyte antigen, and diminished delayed-type hypersensitivity |
| Binding defect | Fails to bind ICAMs and complement opsonized particles | Normal binding, failure to signal | Fails to bind endothelial selectins |
| Types of infections | Necrotic skin infections, cellulitis, periodontal disease, pneumonia, spontaneous peritonitis, frequent sepsis | Recurrent skin infections, periodontitis, otitis media, pneumonia | Pneumonia early in life, periodontitis in later childhood, severe recurrent sepsis early in life in one patient Pneumonia early in life periodontitis in later childhood, severe recurrent sepsis early in life in patient |
| Types of bacteria | S. aureus, Psuedomonas spp., Enterococcus spp., E. coli, Klebsiella spp. many mixed bacterial infections | S. aureus, Psuedomonas spp., Streptococcus spp., Enterococcus spp., E. coli, Bacteroides spp. | Not reported |
| Other infections | Candida spp., Aspergillus spp., two deaths from viral infections | P. carinii | Not reported |
| Other features | Abnormal NK cell function; colitis seen in 50% of severely affected individuals; 87% of severely affected individuals have delayed separation of the umbilical cord | Myelodysplasia in one case, hypogammaglobulinemia in one case, and diminished platelet activation in two cases | Developmental delay (in 5 of 5 patients), overlapping toes (in 2 of 5), response to oral fucose (in 1 of 5), no response to oral fucose (in 2 of 5) |
“LAD 1 variant” refers to small number of patients with normal or near normal expression of β2-integrins on the neutrophil surface, but clinical features consistent with LAD 1 and dysfunction of β2-integrins when tested in adhesion or signaling assays (114, 122, 150). The three patients identified with this variant have been consistent in having high circulating neutrophil counts and poor extravasation of neutrophils into sites of infections. Two of the patients developed a bleeding diathesis that may be due to impaired signaling through β3-integrins on platelets.
LAD 2 is due to a defect in fucosylation and leads to a more complicated syndrome (170). It is part of a family of glycosylation defects and is also termed carbohydrate-deficient glycoprotein IIc disease. Six patients are known, and they have all demonstrated significant infections early in life and developmental delay (90, 91, 121, 177). Other features include growth delay, leukocytosis, and the Bombay blood phenotype. In two patients, the infection pattern improved with age (90), and in another two patients, biochemical and clinical improvement was noted after fucose supplementation (121, 169).
Initial interactions between neutrophils and endothelium are mediated by membrane glycoproteins called selectins (Fig. 4). They are induced on the endothelium by TNF-α and other inflammatory mediators and bind to fucosylated oligosaccharides on leukocytes (98). LAD 2 patients cannot initiate adhesion via this process, although in situations where blood flow is reduced, the cells are able to engage integrins. Tight adhesion is mediated by intercellular cell adhesion molecules (ICAMs) on the endothelium, binding to members of the integrin family on neutrophils (76). LFA-1 binds ICAM-1 and Mac-1 binds ICAM-2. Binding is enhanced by induction of a conformational change in the integrins in response to chemokines and upregulation of ICAM expression in response to inflammatory mediators (55). Following integrin binding, the neutrophil arrests and undergoes a morphologic change such that it orients toward the endothelium. Diapedesis requires PECAM in addition to the molecules involved in tight adhesion (57). Loss of the common β2-integrin chain renders the neutrophils unable to form tight adhesion to endothelium because all three members of the β2-integrins participate in this process. This is one of the mechanisms underlying the chronically elevated neutrophil counts. Mac-1 and p150 are also found on monocytes and NK cells, and there is evidence for in vitro dysfunction of NK cells, although there has been little clinical evidence of NK cell dysfunction, suggesting that it may not be medically significant (148, 245).
Clinical manifestations of LAD include delayed umbilical cord separation, leukocytosis, poor wound healing, and recurrent infection with pyogenic bacteria (271). In severely affected individuals, infections can appear shortly after birth with omphalitis and delayed cord separation (38, 118). Necrotizing and ulcerative soft tissue infections, often requiring surgical drainage, are common. Pyoderma gangrenosum has been found in patients with LAD 1 (29). Periodontal disease may be severe, leading to bone erosion requiring dental extractions. Other sites of infection include the meninges, peritoneal cavity, pericardium; in an older individual with a milder form of LAD, bronchiectasis was found (178).
(i) Infections in patients with LAD.
The most common bacterial pathogens include S. aureus and Streptococcus spp. Other pathogens reported to cause infections include Escherichia coli, Proteus mirabilis, Enterococcus spp., and P. aeruginosa (14, 193, 238). The most common sites of infection are the skin, gingiva, oral mucosa, respiratory tract, and sepsis. Spontaneous peritonitis and sepsis are relatively frequent due to an inability to contain even mild infections.
Candida spp. are the primary fungi isolated from patients with LAD 1. Candidal skin infections were seen in approximately 16% of patients. Candida esophagitis has also been frequently described (14). LAD 1 variant patients have a broad spectrum of severity. One of the three reported patients had Candida skin and pulmonary infections.
Six patients with LAD 2 have been identified to date, and recurrent episodes of pneumonia, periodontal disease, otitis media, and localized cellulitis have been observed (89, 90, 121). One of the identified patients suffered several sepsis-like episodes and required prophylaxis with antibiotics to prevent further episodes (177). The initial patients described with LAD 2 showed less severe and less frequent infections as they matured, suggesting that compensation can occur (90).
Patients with LAD have not had serious viral infections or parasitic infections in general. This is surprising, given the defects in T cells and NK cells which have been demonstrated in vitro (148). Nevertheless, a few serious viral infections have been noted. Significant herpesvirus and picornavirus infections and aseptic meningitis due to presumed viruses have all been reported (13). The most severely affected LAD 1 variant patient was hospitalized for respiratory syncytial virus, parainfluenza virus, and Pneumocystis carinii infections (114).
Myeloperoxidase deficiency.
Congenital myeloperoxidase deficiency is seen in 1:4,000 individuals (216). Acquired forms are even more common. Most patients have a missense mutation in the MPO gene which leads to failure to incorporate heme into the mature molecule (198). Neutrophils deficient in myeloperoxidase produce superoxide and H2O2 properly but are unable to convert H2O2 to HOCl. Myeloperoxidase is a constituent of the azurophilic granule and is responsible for the greenish tinge seen in dense neutrophilic infiltrates. As a consequence of the deficient myeloperoxidase, neutrophil killing of some organisms is diminished early but is normal late in killing assays (64). HOCl is a potent microbicidal compound, but H2O2 and granule contents may be primarily responsible for neutrophil killing in standard laboratory assays. In contrast, neutrophil-mediated killing of Candida spp. and Aspergillus spp. is significantly impaired in neutrophils from myeloperoxidase-deficient individuals (216). This is consistent with the clinical picture. Patients are generally asymptomatic; however, those who have symptoms generally experience Candida spp. infections (156). Myeloperoxidase deficiency is associated with a mild predisposition to infection, and its effects are usually seen when the patients develop another defect in host defense. For example, Candida infections in patients with diabetes are more frequently seen in patients with concomitant myeloperoxidase deficiency (154, 156, 196).
Specific granule deficiency.
Specific granule deficiency is a rare disorder of neutrophils, although an acquired form is seen in a number of preleukemic states (101). The disorder is characterized by a nuclear morphologic alteration called the Pelger-Huët anomaly, which is characterized by a bilobed nucleus instead of the normal trilobed neutrophil nucleus. The neutrophils appear to have a ground-glass appearance on Giemsa stain due to an absence of specific granules. Interestingly, mRNAs for the constituents of the specific granules are absent, which was the first suggestion that the defect is developmental (139). The azurophilic granules are relatively normal, although they lack defensins, and eosinophils and platelets have abnormal granules (102, 242). Functional studies of neutrophils from patients with specific granule deficiency have demonstrated impaired chemotaxis, impaired disaggregation, reduced respiratory burst, and deficient bactericidal activity (100, 101, 107). The gene defect was identified as C/EBP epsilon, and a knockout mouse has been produced (106, 157, 159). C/EBP epsilon is a transcription factor with both inhibitory and activation roles. In mice, the transcription factor is relevant for TNF-α downregulation and the induction of expression of several adhesion molecules (157). Therefore, the phenotype of recurrent infections may be due to multiple biochemical defects.
Patients with specific granule deficiency present in infancy with recurrent deep and superficial skin infections. Respiratory infections, otitis, and mastoiditis are common, and abscesses frequently require surgical drainage. Skin lesions are often indolent, requiring months to heal. S. aureus is responsible for most infections, and P. aeruginosa is occasionally identified in cases of mastoiditis (12, 101, 149, 265). Candida infections have been seen, but invasive fungal disease has not (46, 101, 149, 215).
Miscellaneous.
Papillon-Lefèvre syndrome is due to cathepsin C deficiency (155). Patients develop hyperkeratosis and juvenile periodontitis. With very aggressive dental care, the secondary teeth may be preserved, but it is more typical to have complete loss of the teeth by adulthood (23, 270). Generally, the infections are limited to gingivitis; however, there are several reports of recurrent skin infections and deep abscesses in patients with Papillon-Lefèvre syndrome, which is consistent with the role of cathepsin C (34, 45, 113, 176). Phagocytosis and chemotaxis have been demonstrated to be aberrant (104, 167), although the current understanding of the role of cathepsin C is that it cleaves granzymes and serine proteases (3, 37). The typical gingivitis organisms are Actinobacillus actinomycetemcomitans, Capnocytophaga spp., Streptococcus constellatus, S. oralis, and S. sanguis. Neisseria spp., Bacillus spp. and Prevotella spp. have also been recovered (239, 273).
The phagocytic defects in α-mannosidosis and Gaucher's disease have not been well characterized, and the predisposition to infection appears to be mild (4, 75). Patients with both disorders appear to have an increased predisposition to infection, but there have been no comprehensive studies of prevalence.
Hyper-IgE syndrome is a poorly understood immunodeficiency which is thought by many to be due to abnormal neutrophil function. The basis of the disease is not known, and the affected cell type is often disputed, with various studies suggesting abnormal macrophage function, others documenting abnormal neutrophil chemotaxis, and still others describing defects in T cells. Recent evidence suggests that the defect may occur very early in the recognition of microbes. Cytokine and chemokine release after stimulation with microbial products is aberrant (43, 54). The infections are characteristic of neutrophil disorders, with recurrent staphylococcal abscesses being a prominent feature (160). An unusual feature of the abscesses is that they engender little pain and warmth. Often, the patient has little or no fever, suggesting that the ability to incite the early inflammatory changes is lacking in this syndrome. Other clinical features include abnormal dentition, scoliosis, pneumatoceles, mild facial dysmorphia, and osteopenia (109). The serum IgE level is usually extremely high, although it can fall with age (109). Other laboratory examinations are usually normal, although abnormal neutrophil chemotaxis is seen in a majority of patients. The infections are not exclusively due to S. aureus, but the vast majority of abscesses are due to that organism. There seems to be a mildly increased risk of fungal disease and infections with other bacteria.
PATHOPHYSIOLOGY OF INHERITED DISORDERS OF MACROPHAGE FUNCTION
The primary defects in macrophage function are all defects of intracellular killing. They all have the common phenotype of increased susceptibility to infections with intracellular organisms. They are sometimes collectively referred to as disorders with Mendelian susceptibility to mycobacteria because of the frequency with which mycobacterial disease occurs (Tables 6 and 7).
TABLE 6.
Diagnostic algorithm for the inherited susceptibility to mycobacteriaa
| Diagnostic evaluation | Type of patient |
|---|---|
| High priority for diagnostic evaluation | Patients with disseminated or recurrent infections with poorly pathogenic mycobacteria |
| Patients with infection with poorly pathogenic mycobacteria and a positive family history for either disseminated non-typhi Salmonella or nontuberculous mycobacteria | |
| Patients with persistent or recurrent infection with non-typhi Salmonella | |
| Consider a diagnostic evaluation | Patients with extraintestinal Salmonella enterica serovor Typhi or Paratyphii |
| Patients with M. tuberculosis who have persistence or recurrence in spite of adequate therapy | |
| Patients with systemic symptoms compatible with mycobacterial disease and a history of either Salmonella or severe herpesvirus infections | |
| Patients with atypical histiocytosis X |
Adapted from reference 153.
TABLE 7.
Infections in patients with macrophage activation disorders
| Disorder | Mycobacterial infections | Other types of infections | References |
|---|---|---|---|
| EDA-ID | M. avium, M. kansasii, M. chelonae, M. tuberculosis, Mycobacterium spp. | C. parapsilosis, P. carinii, S. enterica serovar Enteriditis, S. pneumoniae, S. aureus, E. faecalis, P. aeruginosa | 18, 47, 79, 87, 175 |
| IFN-γR1 deficiency | M. bovis BCG, M. avium, M. kansasii, M. chelonae, M. fortuitum, M. smegmatis, M. peregrinum, M. szulgai, M. gordonae, M. margaritense, M. tuberculosis | S. enterica serovar Enteriditis, Salmonella spp., L. monocytogenes, Legionella spp., M. pneumoniae, H. capsulatum, respiratory syncytial virus, herpesvirus, parainfluenza virus | 6, 9, 65, 83, 123, 140, 143, 201, 223, 240, 243 |
| IFN-γR2 deficiency | M. bovis BCG, M. avium, M. fortuitum, M. abscessus | 78, 81, 124 | |
| STAT-1 deficiency | M. bovis BCG, M. avium | 86 | |
| IL-12Rβ1 | M. bovis BCG, M. avium, M. fortuitum | Salmonella spp. | 5, 8, 11, 71, 88, 247, 274 |
| IL-12p40 | M. bovis BCG, M. tuberculosis, M. chelonae | Salmonella spp. varicella-zoster virus Candida, N. asteroides | 10, 88, 221 |
In spite of multiple distinct genetic defects, primary functional macrophage defects all have a common presentation: all are associated with an increased susceptibility to mycobacterial disease (Tables 6 and 7). In this section, we review the pathophysiologic basis for the susceptibility to mycobacterial disease. The disorders discussed include anhidrotic ectodermal dysplasia with immunodeficiency (EDA-ID), defects in the gamma interferon receptor complex, defects in STAT1, and defects in IL-12 and its receptor. These disorders all result in mycobacterial infection and other types of intracellular infections. All the genes involved encode proteins involved in a circuit which culminates in intracellular killing of bacteria (Fig. 5). These disorders have only recently been described, and the true prevalence is not known, nor is the phenotypic spectrum fully appreciated.
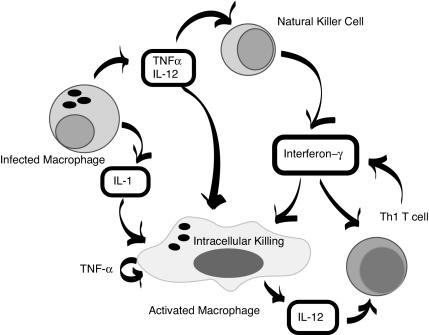
FIG. 5.
Macrophage killing of intracellular pathogens. An infected macrophage produces cytokines which stimulate NK cells (TNF-α and IL-12) and which activate macrophages (IL-1, TNF-α, and IL-12). Gamma interferon produced by the NK cell further activates the macrophage and acts to amplify the T-cell response.
Mycobacterium spp. are recognized and killed via a complex multicellular pathway. Once the macrophage is activated, the molecules which actually participate in the killing are not completely understood. NO may play a role, as may H2O2; however, there are probably mechanisms yet to be defined which play a dominant role. What is clear is that Mycobacterium spp. and Salmonella spp. are killed in a fashion distinct from that used to kill T. gondii and L. monocytogenes. Macrophage killing of mycobacteria and Salmonella spp. appears to be completely due to gamma interferon, while the intracellular killing of L. monocytogenes and T. gondii in human cells is mediated equally by gamma interferon and TNF-α (137). This is consistent with what is seen in patients with defects in the gamma interferon receptor complex, who have markedly increased susceptibility to Mycobacterium spp. and Salmonella spp. but not to L. monocytogenes and T. gondii. In fact, several patients with IFNGR1 mutations have had confirmed exposures to T. gondii without experiencing overt disease (137).
Initially, Mycobacterium spp., Salmonella spp., L. monocytogenes, and T. gondii are bound by TLRs. For mycobacteria, these TLRs appear to be primarily TLR2 and TLR4. Once the mycobacteria are taken up by macrophages, the NF-κB and MAP kinase pathways are activated. This leads to upregulation of costimulatory molecules on the macrophage, which in turn activates T cells. TNF-α, gamma interferon, and IL-12 are released, which further enhances containment and killing (195). Gamma interferon leads to improved antigen presentation, increased expression of TLRs, and further activation of the macrophage. Activation of macrophages leads to increased production of lytic enzymes, chemokines, reactive oxygen species, and an increased metabolic rate. At the extreme, macrophages become epithelioid giant cells comprising granulomas. The final activation of the macrophage elicits the production of NO and other reactive oxygen species. NO has been directly demonstrated to be required for mycobacterial killing in murine cells (41), while killing of mycobacteria appears to be NO independent in human monocytes and alveolar macrophages (267). This, coupled with the finding that patients with CGD develop mycobacterial disease only infrequently, has led to speculation that the killing mechanism may be less dependent on reactive oxygen species in human cells than in mice. The recent identification of intracellular molecules with pattern recognition potential, such as NOD2, may allow further definition of the final pathway responsible for killing mycobacteria in humans (130, 205, 206).
Immunity to mycobacteria represents a concerted effort by a number of cells. The role of the T cell has long been recognized, and T cells play a significant role in the containment of mycobacteria within granulomas (211). It is thought that cytotoxic T cells contribute to mycobacterial stasis through the secretion of perforin and granulysin (264).
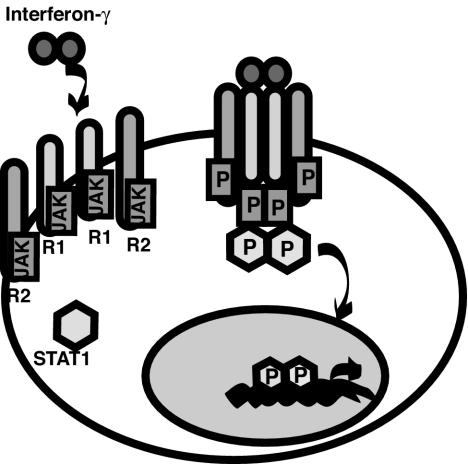
The circuit shown in Fig. 5 relies heavily on the coordinated interactions of cells and cytokines. The three main cytokines involved in this circuit are gamma interferon, TNF-α, and IL-12. Gamma interferon is secreted as a homodimer primarily by Th1 T cells and NK cells (Fig. 6) (40). The receptor is composed of two gamma interferon receptor 1 chains and two gamma interferon receptor 2 chains. These are already bound to inactive JAK1 and JAK2, respectively. On ligand binding, the receptor subunits approximate and JAK1 and JAK2 are activated by phosphorylation. This act leads to recruitment from the cytoplasm of STAT1 monomers, which bind to the complex and are themselves phosphorylated (40). Phosphorylation is a prerequisite for homodimerization of STAT1 and translocation to the nucleus, where a broad range of STAT1-inducible genes are upregulated.
FIG. 6.
Gamma interferon binds its receptor and activates STAT1. It acts as a dimer and binds to the receptor chains. Once the complex is formed, STAT1 is recruited to the receptor complex, where it is phosphorylated (P) and dimerizes. The STAT1 dimer is a potent transcription factor which activates transcription of an array of inflammatory mediators.
The production of gamma interferon is regulated by IL-12 (52, 103, 174, 249). This cytokine is produced primarily by antigen-presenting cells and consists of a heterodimer of 40- and 35-kDa subunits. IL-23 shares the p40 subunit from IL-12 (208). IL-12 binds a receptor which is also a heterodimer of IL-12Rβ1 and IL-12Rβ2. The β1 subunit is also shared by the receptor for IL-23 (213). The fact that no patients with Mendelian inherited susceptibility to mycobacteria have had IL-12 p35 or IL-12Rβ2 deficiencies perhaps suggests that IL-23 plays an important role in mycobacterial killing.
TNF-α is critical for granuloma formation, and its relevance in mycobacterial killing has been demonstrated in patients who receive therapeutic TNF-α inhibitors. Approximately 300,000 people have received these drugs, and 8 cases of nontuberculous mycobacterial disease, >100 cases of M. tuberculosis infection, 9 to 16 cases of H. capsulatum infection, 12 to 17 cases of L. monoctogenes infection, 8 to 11 cases of Aspergillus infection, and 17 to 21 cases of P. carinii infection have occurred (http://www.fda.gov/ohrms/dockets/ac/01/briefing/3779b2.htm). The patients who receive TNF-α inhibitors are often also receiving other immunosuppressive drugs, which could magnify the effect; however, mycobacterial disease is infrequently seen in immunocompromised patients outside of the transplant setting, suggesting that these infections are due primarily to the inhibition of TNF-α.
Pathogens Found in Patients with Disorders of Macrophage Function
Mycobacteria.
Mycobacteria are the most common infectious agents in patients with disorders of macrophage activation. Nearly all of the patients with the macrophage activation disorders described above presented with mycobacterial disease (Table 7). There may be some selection bias toward evaluating patients with unusual mycobacterial infections as opposed to M. tuberculosis. Nevertheless, nontuberculous mycobacteria are the most common causes of infection seen in patients with inherited defects of macrophage function. Rapidly growing nonpigmented pathogenic species in the M. fortuitum complex are frequently seen (M. fortuitum, M. peregrinum, M. chelonae, M. abscessus, and M. mucogenicum). M. smegmatis may be either pigmented or nonpigmented and has been seen infrequently. The rapidly growing pigmented species have not caused infection in this unique patient population to date. The slowly growing M. avium complex (M. avium and M. intracellulare) is another group which is frequently found in patients with defective macrophage activation. The remainder of the mycobacterial species are fairly evenly divided between the photochromagen species and the scotochromagen species. The frequency of M. avium complex infections in patients with human immunodeficiency virus (HIV) and CD4 counts of <100 cells/mm3 suggests that defense against this subgroup may be subtly different from that against other mycobacteria (202).
The mycobacterial disease is nearly always disseminated, and positive cultures have been obtained from draining fistulae, lymph nodes, and liver biopsy specimens. Patients from Europe with M. bovis BCG infections often report that the inoculation site never healed and the process evolved into a generalized lymphadenopathy with continued drainage from the inoculation site (212). Many patients have had unusual presentations requiring multiple biopsies before the diagnosis was established. Fever and adenopathy were occasionally confused with proliferative processes. A diagnostic algorithm may be used as a guide (Table 6); however, the limited number of patients known makes it likely that other presentations are possible.
Other intracellular bacteria.
Salmonella spp. are typically ingested through contaminated food or water, and they must pass through the acid barrier of the stomach. The organisms interact with M cells that overlie the Peyer's patches, where they are rapidly internalized (147) and transported into the submucosal lymphoid tissue, from where they can enter the general circulation. The Salmonella-containing primary phagosome fuses rapidly with the lysosomal compartment in both macrophages and neutrophils (286). Just as for mycobacteria, a complex interplay of T cells, macrophages, and cytokines is required to eradicate Salmonella spp. Humoral immunity may offer protection from initial infection by acting as an opsonin and encouraging uptake by neutrophils (163). It is not surprising, based on the mechanism of host defense, that patients with macrophage activation disorders experience a high frequency of Salmonella infections. These infections are seen in 34% of patients with IL-12 or IL-12 receptor defects and in 7% of patients with defects in the gamma interferon receptor complex (80).
L. monocytogenes has been found in a single individual with complete gamma interferon receptor 1 deficiency (240). The frequency of infection may be low because exposures are infrequent. L. monocytogenes is a small, facultatively anaerobic, nonsporulating, catalase-positive, oxidase-negative, gram-positive bacillus. It typically enters the gastrointestinal tract and may be aided by concomitant infection with other organisms. Once it is in the bloodstream, hematogenous dissemination occurs. Both T cells and macrophages are important in the host defense against L. monocytogenes (258). Thus, defects in macrophage activation would be expected to increases susceptibility to L. monocytogenes.
Legionella spp. have been found in a single patient with a partial gamma interferon receptor 1 defect (141). This may have been a coincidental finding, but our understanding of the mechanisms of host defense against Legionella spp. suggests that there may be an increased susceptibility in patients with defects in macrophage activation. Organisms enter and replicate within respiratory epithelial cells. Alveolar macrophages phagocytose Legionella spp. avidly in the presence of antibody (194). The primary phagosome does not fuse with lysosomes, and the organisms proliferate until the cell ruptures. Macrophages activated with gamma interferon inhibit the proliferation of Legionella spp. (36). This suggests that patients with defects in macrophage activation could be at increased risk for Legionella infections even though only a single infection has been noted clinically.
Other causes of infection.
Histoplasmosis was seen in a single patient with a partial defect in gamma interferon receptor 1 (212). H. capsulatum is present at various densities in soil around the world. It is not known if the low frequency in patients with macrophage activation defects is due to lack of exposure or lack of increased susceptibility. Infection begins with aspiration of conidia and uptake by neutrophils and macrophages via CD11/CD18 receptors. The conidia transform to yeast forms, which proliferate in macrophages (180, 200). T cells and macrophages provide active defense, and neutrophil antimicrobial peptides can inhibit the yeast forms. As with mycobacteria, granuloma formation is thought to be critical for containment. Therefore, it is likely that patients with inherited defects in macrophage activation are at increased risk of H. capsulatum infection.
Herpesvirus, parainfluenza virus, and respiratory syncytial virus infections have been found in single patients with complete gamma interferon receptor 1 defects (212). Varicella was found in a single patient with IL-12p40 deficiency. One could hypothesize that lack of an effect of gamma interferon on T cells led to poorer Th1 responses and impaired responses to viruses. It could also be hypothesized that lack of an effect of gamma interferon on NK cells could lead to increased susceptibility to herpesviruses. While all of these are theoretical reasons for these patients to have an increased risk of severe viral disease, it is striking how few patients have had severe viral infections, given the high rate of exposure.
Disorders Associated with Defective Responses to Gamma Interferon
The first genetically defined primary immunodeficiencies associated with Mendelian inherited susceptibility to mycobacteria were defects in the gamma interferon receptor complex. Defects in the gamma interferon receptor 1 chain were the first described (Fig. 6). In 1996, four children from Malta were found to have multiple mycobacterial infections (201). All four children carried homozygous mutations leading to a truncated protein. Simultaneously, another child in France was reported to have fatal disseminated M. bovis BCG infection (140). She also had a mutation in the gamma interferon receptor 1 chain. Since the initial descriptions, over 50 patients with mutations in the gamma interferon receptor 1 chain have been described (6, 9, 65, 83, 123, 143, 223, 240, 243). Gamma interferon and TNF-α are the final common mediators of granuloma formation and intracellular killing. Thus, defects at this distal point are difficult to overcome therapeutically. This is borne out clinically, since nearly 20% of the patients with gamma inteferferon receptor 1 defects have died prematurely from infection. Demonstrating the importance of the macrophage specifically, the patients had detectable anti-mycobacterial antibodies and mycobacterially responsive T cells (140). In patients with gamma interferon receptor defects, M. avium was the most common infectious agent, occurring in 55% of the patients. Disseminated M. bovis BCG was seen in 43% of the patients, and M. fortuitum, M. tuberculosis, and M. chelonae were each seen in two patients (3% each). The remainder of the mycobacterial species were seen in single patients (212).
There is a spectrum of severity, and the severity of the biochemical defect correlates well with clinical severity. Both autosomal recessive (43%) and autosomal dominant (57%) forms exist (212). The autosomal dominant forms are generally somewhat leaky, and those patients have some ability to form granulomas. In addition, certain of the autosomal recessive mutations are associated with residual function. Patients with some residual receptor function do better clinically. In fact, in patients with residual function, treatment with gamma interferon is possible and can be beneficial (162). There is a mutational hot spot which typically leads to the production of one chain which is truncated in the cytoplasmic domain. While the mutant chain is unable to bind JAK1, the wild-type chain is competent. Over half of the children with gamma interferon receptor 1 mutations have this leaky autosomal dominant mutation with an attenuated phenotype, and few of these patients have died. In contrast, nearly 50% of those with complete absence of receptor function have died. Interestingly, the severity of the defect did not seem to affect the type of mycobacteria infecting the patients.
Only three patients with gamma interferon receptor 2 mutations have been described, and they all suffered from multiple mycobacterial infections (78, 81, 124). Two mutations led to premature stop codons, and the third was a partial defect due to a single amino acid substitution. All three patients had autosomal recessive patterns of inheritance.
STAT 1 is the primary effector of gamma interferon signaling (Fig. 6). The STAT1-deficient patients resembled those deficient in the gamma interferon receptor. The two patients both had recurrent infections with mycobacteria: one with M. bovis BCG and one with M. avium (86).
The clinical phenotypes of the human disorders were initially surprising. The gamma interferon and the gamma interferon receptor knockout mice had been studied for some years. These mice were known to have an increased susceptibility to viruses, intracellular and extracellular bacteria, and certain parasites (69, 129). The explanation for the more limited phenotype seen in humans may have to do with the type and size of inoculum typically used in murine experiments. There is also evidence that the effect of the mutations in humans is more limited with respect to Th1 T-cell differentiation (69, 140).
Disorders Associated with Defective Production of Gamma Interferon
Nearly 40 patients with defects in either IL-12 or the IL-12 receptor have been described. Interestingly, all cases have been due to defects in the subunit that is shared with IL-23. All of the IL-12 defects have been in the IL-12p40 subunit, and all of the receptor defects have been in the IL-12Rβ1 subunit. This has suggested that the effects of IL-23 are at least as important in the defense against mycobacteria as are those of IL-12. IL-12 and IL-23 have many overlapping functions, although IL-23 induces a profound systemic inflammatory disorder and appears to have a more potent inducing effect on dendritic cells (30, 292). IL-23 and IL-12 both act on T cells, but IL-23 directs the proliferation of memory T cells while IL-12 acts predominantly on naive T cells to polarize their subsequent cytokine production (Fig. 3) (97). How these actions could be relevant to host defense against mycobacteria is not known. It may be that the actions of IL-23 on T cells are significant in granuloma formation.
The IL-12p40-deficient individuals have thus far had complete defects inherited in an autosomal recessive fashion (10, 88, 221). Similarly, all but one of the cases of IL-12Rβ1-deficient individuals have had complete deficiencies, which have been inherited in an autosomal recessive fashion (5, 8, 11, 71, 88, 247, 274). One leaky mutation in the IL-12Rβ1 gene has been described, and the only manifestation in this patient was recurrent M. bovis BCG infections (212). It is of interest that deaths are more frequent in the IL-12-deficient individuals (44%) than in the IL-12 receptor-deficient individuals (19%) (212). It is also noteworthy that Salmonella infections are more common in the patients with defects in IL-12 and the IL-12 receptor (34%) than in the patients with gamma interferon receptor defects (7%) (212). Both of these clinical observations may have to do with the fact that IL-12 can act to promote intracellular killing independently of gamma interferon (190). In addition, IL-12 plays an important role in the induction of chemokine expression, which serves to regulate the migration of immunologically competent cells (266, 282). Patients with defects in IL-12 or the IL-12 receptor have a similar distribution of mycobacterial species, and the infections are nearly always disseminated. M. bovis BCG has been seen in 68% of these patients, and M. avium has been seen in 16%. Other mycobacterial species were seen in single patients (212).
It is thought that the clinical similarity of the gamma interferon receptor defects and the IL-12 and IL-12 receptor defects in humans is because they all lead to impaired production of intracellular moieties which kill pathogens. In this model, the main effect of IL-12 and IL-12 receptor deficiencies may be the impaired stimulation of gamma interferon production. A few patients have been treated with gamma interferon in an effort to bypass the defect, with limited success (10, 153), providing support for the concept that it is the integrity of the circuit which is critical for the defense against intracellular organisms.
EDA-ID is a disorder of males which is allelic with the female disorder incontinentia pigmenti (18, 175). The clinical features of incontinentia pigmenti are a streaky vesicular rash in early infancy, which resolves with hyperpigmentation. Patients also have abnormal retinal vessels, missing and deformed teeth, and neurologic symptoms. Incontinentia pigmenti is generally lethal in males and is caused by defects in the regulatory subunit of IKK, which is encoded on the X chromosome (259). The regulatory subunit is referred to as IKK-γ or NF-κB essential modulator. Most patients with incontinentia pigmenti bear a frameshift mutation leading to premature termination and failure to produce active protein (19). Recently, four male patients with dysgammaglobulinemia, frequent infections that did not improve after administration intravenous gammaglobulin, abnormal dentition, and ectodermal dysplasia were described (300). These patients had mutations in IKK-γ which allowed the production of dysfunctional protein. The mutations identified to date have been diverse but have been consistent in allowing the production of partially functional protein.
The phenotype (Table 8) is easily explainable on the basis of impaired activation of NF-κB (151). The dysgammaglobulinemia is probably due in part to impaired signaling through CD40, which directs isotype switching via an NF-κB-dependent pathway (33). Two patients have had osteopetrosis due to defective osteoclast differentiation which is NF-κB dependent, and this mechanism is probably the basis for the abnormal dentition (94, 128).
TABLE 8.
Phenotypic features in EDA-ID patientsa
| Phenotypic feature | No. of patients affected/total no. (%) |
|---|---|
| Ectodermal dysplasia | 24/24 (100) |
| Osteopetrosis | 6/24 (25) |
| Lymphedema | 3/15 (20) |
| Bronchiectasis | 2/2 (100) |
| Mycobacterial infections | 8/24 (33) |
| Severe bacterial infections | 23/24 (96) |
| Severe viral infections | 1/14 (7) |
| Severe fungal infections | 2/22 (9) |
| Hypogammaglobulinemia | 7/9 (78) |
| Elevated IgM Levels | 4/7 (57) |
| Elevated IgA Levels | 5/9 (56) |
| Poor antibody responses to polysaccharide antigens | 6/7 (86) |
| Increased B-cell counts | 2/5 (40) |
| Decreased | 13/24 (54) |
Compiled from references 1, 18, 19, 79, 136, 175, 210, and 300.
The immunodeficiency is more pleomorphic in this disorder than in the other macrophage function defects being described. While mycobacterial infections are a prominent feature, other infections and bronchiectasis are common findings. The macrophage dysfunction is probably due to impaired activation of NF-κB; however, it appears to be stimulus specific. In one study, IκBα was normally degraded in monocytes of EDA-1D patients after LPS stimulation and was abnormal after CD40L stimulation (136). This suggests that monocyte responses to T cells are critically affected in EDA-ID patients. Monocytes typically produce IL-12 and gamma interferon after CD40L stimulation, and these cytokines were not seen in supernatants of cultures of patient cells (136). Cytokine production after stimulation through TLRs was preserved. NF-κB is also required for responses to IL-1, IL-18, and TNF-α, which may contribute to the severity of the immunodeficiency and the inability to compensate with intravenous gamma globulin (79). B-cell defects have been demonstrated, with a failure to signal through CD40 and a picture similar to X-linked hyper-IgM syndrome, with elevated IgM levels and normal or depressed IgG levels (136). T-cell function appears to be largely normal in patients with EDA-ID, although NK cell function is impaired (209). This population does not have many severe viral infections, but prolonged or recurrent herpesvirus infections have been seen.
The patients with EDA-ID have a marked predisposition to bacterial infections. Specific bacteria described in this population are S. pneumoniae, S. aureus, Enterococcus faecalis, P. aeruginosa, and H. influenzae (1, 18, 79, 136, 175, 209, 300). Some of the infections are probably due to the compromise in forming antibody to polysaccharide antigens. In most (but not all) cases where it was examined, the patients either failed to develop isohemagglutinins or antibodies to polysaccharide pneumococcal antigens. This doubtless contributes to the high frequency of penumococcal disease in this population. The mycobacterial infections are similar to what is seen in patients with the other macrophage defects, with a preponderance of atypical mycobacteria.
MANAGEMENT ISSUES
Neutrophil Disorders
Management of neutrophil disorders is complex. Diagnosis of the immunodeficiency provides important insights into the types of infections to which the patient is susceptible. It also defines a therapeutic strategy in some cases. For example, patients with Chédiak-Higashi syndrome usually receive a stem cell transplant near the time of diagnosis in an effort to prevent the accelerated phase, which has a high mortality rate (25, 74). Similarly, patients with the severe form of LAD, in which there is no detectable expression of the β2-integrins, should receive a stem cell transplant soon after the diagnosis is established (13). Supportive and conservative measures for these patients are insufficient, and the risk of stem cell transplantation is low compared to the risk of death in the absence of that intervention.
Patients with neutropenia have an improved quality of life when treated with G-CSF to stimulate neutrophil production and survival. Patients with myelokathexis and glycogen storage disease type Ib have benefited from G-CSF treatment (50, 127). In addition to improved neutrophil counts, the G-CSF improves function.
Initial therapy.
The initial therapy for any infection in patients with a neutrophil disorder should be tailored to the specific organism and its susceptibility pattern. The possible infecting organisms are legion, and culture of significant infections is essential. Complicating the management is the fact that infections in patients with neutrophil defects often present in atypical ways. In CGD patients, fever is often delayed and symptoms may be nonspecific. Fungal infections can be indolent, and in some cases a rising erythrocyte sedimentation rate and mild discomfort are the only indication. In patients with LAD, the infections may progress very rapidly from an innocuous skin papule to a large necrotic ulcer with dissemination. A general strategy is to treat infections in patients with neutrophil defects aggressively and for a longer period than what would be typical for a host without these defects. For example, fungal infections are usually treated for at least a month after radiologic evidence of cure and the erythrocyte sedimentation rate and C-reactive protein have normalized or plateaued at a low level. The more severe neutrophil defects such as Chédiak-Higashi syndrome, glycogen storage disease type Ib, myelokathexis, CGD, LAD, and specific granule deficiency nearly always require prolonged antibiotic therapy for infections. White cell transfusions have a history of success in CGD and LAD patients and would be expected to be beneficial in serious infections arising in any patient with a neutrophil disorder (277, 299). Several of the neutrophil disorders benefit from the use of immunomodulatory agents, and two of the disorders, LAD and Chédiak-Higashi syndrome, are usually treated by stem cell transplantation soon after the diagnosis of the immunodeficiency is established.
Nearly all of the neutrophil disorders are associated with gingivitis, and this can be the major management issue faced by patients with mild LAD and Papillon-Lefèvre syndrome. There is little consensus; however, aggressive dental scaling and hygiene are thought to be beneficial for the gingivitis as well as for the prevention of disseminated disease from the gingiva. Here too, culture can be instructive. A. actinomycetemcomitans is often an important pathogen. Treatment with systemic amoxicillin and metronidazole or amoxicillin and a quinolone is usually effective, but in infections with multiple organisms, different combinations may be required (23, 270). Treatment is prolonged and may be lifelong.
Patients with neutrophil disorders are often predisposed to deep abscesses, which require surgical treatment in addition to prolonged antibiotics (189, 228). In the absence of adequate neutrophil killing, antibiotics are insufficient to kill large accumulations of infection. Female carriers of X-linked CGD with as few as 10% normal neutrophils have no increase in infections, suggesting that granulocyte transfusions, although imperfect, can provide a significant increase in microbicidal activity and clinical benefit. For serious infections, granulocyte transfusions are appropriate. Concern about side effects and alloimmunization in a population potentially treated by stem cell transplantation limits the use of granulocyte transfusions to seriously ill patients.
Chédiak-Higashi syndrome occasionally presents in the accelerated phase. There is no evidence that treatment of potential viral triggers modifies the process. In fact, the mainstay of treatment is immunosuppression. Most of the current protocols involve the use of steroids, cyclosporin A, and VP-16 in combination. Intrathecal therapy is required if there is central nervous system involvement. Once remission is established, a stem cell transplant should be arranged. Transplants undertaken during the accelerated phase are associated with particularly high risk.
Empiric therapy.
Empiric therapy in patients with neutrophil defects is unsatisfying because of the broad range of potential infecting organisms. Nevertheless, it is sometimes unavoidable because of the tenuous clinical condition of the patient or the inaccessible nature of the infection. Biopsies in CGD patients will occasionally be unrevealing in spite of ample evidence of infection, perhaps because the exuberant granulomas encase the infection. In LAD patients, cultures of surface infections often contain multiple organisms, and colonization versus infection can be difficult to distinguish. Fortunately, the majority of infections in patients with LAD and CGD are limited to a few organisms, and it is often possible to devise a strategy that addresses many of the potential organisms. The five most common pathogens in CGD patients are S. aureus, B. cepacia, S. marcescens, Nocardia spp., and Aspergillus spp. (253). Most physicians would not undertake empiric therapy for fungal infections unless there was strong supportive evidence. Therefore, initial empiric therapy is usually directed at S. aureus and gram-negative organisms. CGD patients with suspected fungal disease are often initially given amphotericin or voriconazole. The unpredictable absorption of itraconazole makes it a poor choice for treatment of an acute infection. Patients with LAD most often suffer from infections with S. aureus, P. aeruginosa, and enteric gram-negative organisms. The potential range of infections is broader than what is seen in CGD patients, and there have been no systematic studies of the organisms causing infection at different sites. Dissemination, sepsis, and progressive necrosis are terrible consequences of infections that often begin innocuously. Therefore, empiric therapy is often extremely broad spectrum. Fungal infections are less common than what is seen in CGD patients, and therefore empiric therapy for fungi is needed less frequently. Candida spp. are the major fungal pathogens.
The less severe neutrophil disorders are associated primarily with S. aureus and Pseudomonas infections. Gingivitis again requires special consideration. Many institutions do not offer the special cultures required to define the infecting species. Empiric therapy with tetracyclines, amoxicillin plus metronidazole, metronidazole, clindamycin, or quinolones may be attempted if culture is not available (280).
Immunomodulatory therapy.
Stem cell transplantation is the most radical immunomodulatory intervention. Most caregivers would agree that it is clearly indicated for severe LAD and Chédiak-Higashi syndrome (110, 268). It is, in principle, an effective therapy for all of the neutrophil disorders. The risk associated with stem cell transplants limits their use to patients at high risk of morbidity or mortality. It has also been performed for CGD and Schwachman syndrome, although there is no consensus about patient selection (21, 27, 161).
Immunomodulatory therapy more typically refers to cytokine therapy. Gamma interferon has been used to both treat and prevent infections in patients with CGD. There is controversy about its mechanism of action, but a large multicenter study showed a 70% reduction in infections (132a). The usual dose is 50 μg/m2 subcutaneously three times a week. Side effects seem to be less of an issue in children than in adults and consist of flu-like symptoms and fever. Premedication with acetaminophen is beneficial. There has been concern that one long-term consequence of the use of gamma interferon would be an increase in inflammatory disorders. This has not been noted thus far in patients (31). Gamma interferon has also been used acutely in patients with active infection. It has also been infused via catheter topically to treat liver abscesses (111, 158).
G-CSF has a long history of use for congenital neutropenia. It improves neutrophil counts in patients with disorders associated with neutropenia and improves function in patients with myelokathexis and glycogen storage disease type Ib (50, 127, 287). It is used cautiously in patients with Schwachman syndrome and significant morbidity from infections. Myelodysplasia and malignancy have been seen in patients receiving G-CSF; however, patients with congenital neutropenia and Schwachman syndrome are known to have an increased predisposition to these disorders. It is not known whether G-CSF increases the risk. The side effects of G-CSF are generally manageable. Acutely, it is associated with musculoskeletal pain. Splenomegaly occurs with sustained use, and hypersplenism has led to splenectomy in some cases. Bone density should be monitored in patients receiving significant doses.
GM-CSF has been used topically for patients with nonhealing ulcers. This is primarily a problem in LAD patients; however, it has been used to treat other conditions with success (227, 257). The GM-CSF promotes healing, and its role in infection per se is not known. It is administered as a sterile 5-μg/ml solution in water. The ulcer dressing is soaked with GM-CSF four times a day.
Prophylaxis.
Most patients with one of the severe neutrophil disorders require antimicrobial prophylaxis to prevent infections. Antimicrobial prophylaxis plays an important role; however, the risk of microbial resistance is a growing threat. Some centers use a rotating series of antibiotics for prophylaxis in an effort to prevent the emergence of resistance. There is no evidence that this is beneficial on an individual patient basis; however, it decreases population resistance in an intensive care setting (234). There have been only a few studies evaluating the role of antimicrobial prophylaxis for immunodeficient patients. The use of co-trimoxozole is standard practice for patients with CGD, and the prophylactic use of itraconazole is often advocated (188, 285). In addition, most CGD patients receive gamma interferon as a prophylactic agent because of its ability to reduce the frequency of hospitalizations (132a). CGD patients often benefit from education regarding high-risk exposures. Marijuana contains fungal spores that could be problematic, and stagnant water could also potentially lead to infection. Other settings where exposure to mold or fungus is likely should be avoided.
Prophylaxis for LAD is usually required and is often based on the assumption that S. aureus is the dominant pathogen for the skin. This does not address periodontitis, but some patients can tolerate local therapy for the gingiva. Prophylaxis for LAD often uses co-trimoxazole or dicloxacillin. Patients with significant infections by gram negative bacteria could benefit from metronidazole prophylaxis. No controlled trials have been performed to elucidate the role of prophylaxis for this patient population. Antifungal prophylaxis has not been widely used in this patient population.
The other neutrophil disorders often require prophylaxis with co-trimoxazole or another antibiotic with a restricted spectrum of activity. The risk of bacterial resistance must be weighed against the benefit to the patient of minimizing infections.
Aberrant inflammatory responses.
Two of the neutrophil disorders demonstrate aberrant healing or aberrant inflammatory responses which can cause significant morbidity. One of the major chronic issues for patients with CGD are the granulomas (28, 63, 244). These can cause obstruction of the gastrointestinal tract, urinary tract, or airways. It is sometimes difficult to determine whether there is an underlying infectious component. Generally, a short course of steroids does not exacerbate genuine infection and can provide significant relief from obstruction. Two exceptions are diffuse pulmonary granulomas and Crohn's-like colitis. Pulmonary disease may require very prolonged steroid therapy. Crohn's-like colitis also often requires a prolonged course or even administration of a second immunosuppressive agent. The colitis causes significant morbidity and occurs in approximately 30% of patients with CGD. One case of Crohn's-like colitis responded to G-CSF administration (191). For patients who are not receiving antimicrobial prophylaxis, immunosuppression warrants the administration of both co-trimoxazole and itraconazole. Gamma interferon may drive the formation of granulomas, and management of granulomatous disease in patients receiving gamma interferon may require dose modification if the granulomas persist after a course of steroids.
CGD patients and mothers of CGD patients with the X-linked form have a markedly increased risk of discoid lupus erythematosus and systemic lupus erythematosus (295). The mechanism underlying this association is not known. It is another example of aberrant inflammation, and the lesions often respond to topical approaches rather than systemic immunosuppression.
Patients with LAD experience a type of ulcerative colitis which is distinct from that seen in CGD patients (68, 238). This colitis has generally responded to intensive antibiotic therapy and white cell transfusions. Immunosuppression has not been used. The basis of this complication is not understood but may relate to the abnormal healing seen in this disorder. Cutaneous ulcers often heal slowly, poorly, and with an atrophic scar which is susceptible to further infection (13). Topical administration of GM-CSF can be of benefit, as described above.
Macrophage Disorders
Initial therapy.
Accurate diagnosis of mycobacterial infection through biopsy and culture is essential. The antimycobacterial agents may be best selected on the basis of in vitro sensitivities. There are imperfections in the current analyses being used for determining antimycobacterial sensitivities, and certain in vitro sensitivities do not translate to clinical efficacy. With that caveat, in vitro sensitivities can be used to help guide therapeutic options for the less well known mycobacteria. Mycobacteria from the M. fortuitum group are often sensitive to amikacin, cefoxitin, ciprofloxacin, clarithromycin, doxycycline, sulfonamides, or imipenem (179). M. abscessus and M. chelonae are often sensitive only to amikacin, cefoxitin, imipenem, and clarithromycin or only amikacin, imipenem, tobramycin, and clarithromycin. Treatment of M. avium involves a multidrug regimen including clarithromycin and ethambutol (70, 108). Rifabutin does not appear to enhance eradication of the mycobacteria but does protect against the development of clarithromycin resistance (32). Rifamycin may play a role, but this requires additional study.
The case reports detail many difficulties in diagnosing the initial mycobacterial infections. For patients who present with disseminated M. bovis BCG infections, there is usually persistent drainage from the inoculation site. Even those cases have occasionally required multiple biopsies before the diagnosis was established (140, 162, 223). Two patients were initially diagnosed with histiocytosis X, and initial staining did not reveal mycobacteria (142). Other patients have had biopsies which showed chronic inflammatory changes but no evidence of mycobacteria. Two patients with complete gamma interferon receptor 1 deficiency were treated with immunosuppressive drugs for presumed autoimmune disease and on subsequent biopsy were found to have acid-fast bacilli within the chronic inflammatory infiltrates (162, 223). In acknowledgement of the difficulty in establishing a diagnosis of the mycobacteria and the underlying immunodeficiency, a diagnostic algorithm was recently developed in an effort to assist in the prioritization of patient evaluations (Table 4). The diagnostic studies are not widely available and are not performed in a standard fashion at present. It is therefore critical to define the patient population most likely to benefit from this labor-intensive analysis. On the other hand, timely diagnosis of the underlying immunodeficiency is important to deliver the appropriate aggressive care required in this patient population.
Once the diagnosis of an inherited susceptibility to mycobacteria has been established and the infecting mycobacteria have been identified, it becomes important to determine whether a biological agent could augment therapy. Gamma interferon is unquestionably beneficial, although patients with complete deficiencies of gamma interferon receptor 1 or gamma interferon receptor 2 would not be expected to respond (153). Treatment with antimicrobials with or without gamma interferon is usually tailored to the individual, but long courses of therapy (up to 2 years) are common (142). Relapses are frequent, and there may be a role for lifelong prophylaxis in patients particularly at risk. High-risk patients would include those with any type of complete deficiency or those with a history of recurrent disease.
Salmonella infection is the other common type of infection in patients with inherited defects in macrophage function. Approximately 25% of patients develop an infection with a Salmonella sp. (153). The infections are more frequently either disseminated or extraintestinal than in the general population. As with mycobacterial disease, recurrences and relapses are common. Cultures of abscesses, blood, intestinal secretions, and bone marrow should be considered to establish the diagnosis. A prolonged course of therapy should be considered.
Antimicrobial resistance among human nontyphoidal Salmonella isolates is increasing worldwide due to the widespread use of antimicrobial agents in humans and domesticated animals. High rates of resistance to chloramphenicol, trimethoprim-sulfamethoxazole, and ampicillin have been reported in Africa, Asia, and South America and are seen with increasing frequency in North America and Europe (269). Some nontyphoidal Salmonella infections are resistant to third-generation cephalosporins. Therefore, culture is essential to define sensitivities. A treatment regimen developed for patients with AIDS is to initiate treatment with 1 to 2 weeks of intravenous antimicrobial therapy followed by 4 weeks of oral quinolone therapy (135). AIDS patients who relapse after 6 weeks of quinolone therapy are usually treated with long-term suppressive therapy with a quinolone or trimethoprim-sulfamethoxazole. This strategy could be used as a starting point for treating Salmonella infections in patients with a functional macrophage defect. Particular care seems warranted for focal Salmonella infections which seem to require both drainage and very aggressive antimicrobial therapy. Persistent carriage is a significant risk in patients with impaired immunity, and patients may benefit from treatment of long-term carriage to prevent recurrences or relapses (96, 204, 248). Amoxicillin, trimethoprim-sulfamethoxazole, ciprofloxacin, and norfloxacin are effective in eradication of long-term carriage. The high concentrations of amoxicillin and quinolones in bile are theoretical advantages.
A few cases of significant herpesvirus infections have been found in patients with gamma interferon receptor 1 deficiency (82). The mechanism underlying this predisposition is not known. The observation that it was limited to a very small number of patients with gamma interferon receptor 1 deficiency, while many others had normal courses of varicella or measles, suggests that it may have to do with the influence of background genes or concomitant infection which may influence an abnormal immune response. For patients with a severe herpesvirus infection, antiviral therapy should be considered. For patients with EDA-ID, standard antiviral therapy plus IL-2 has been successful in treating one case of chronic cytomegalovirus infection (209).
Empiric therapy.
Empiric therapy for mycobacterial infections is undesirable, and it is worthwhile to obtain additional samples for culture rather than to attempt an empiric treatment regimen. This is largely because the mycobacterial species vary so drastically in their sensitivities. Broad-based empiric therapy is undesirable because each of the drugs is associated with significant side effects. If necessary, empiric therapy in patients who received M. bovis BCG should include rifampicin, isoniazid, ethambutol, and clofazimine. Patients with suspected mycobacterial disease who have not been vaccinated with M. bovis BCG and have no known exposures to M. tuberculosis should receive a combination including rifampicin or rifabutin, clarithromycin or azithromycin, and a quinolone. Empiric therapy for Salmonella spp. is not usually required because these bacteria are easily cultured even when associated with extraintestinal disease.
Immunomodulatory therapy.
Therapy with gamma interferon is currently the only available immunomodulatory therapy. It has been used successfully for acute therapy (3 to 50 μg/m2 initially, with stepwise increases if necessary) but could be considered for prophylaxis in patients at risk of recurrence or relapse of mycobacterial disease (10, 81, 140). It would not be expected to benefit patients with complete gamma interferon receptor 1 defects because that receptor is necessary to transduce signals from gamma interferon. IL-12 therapy has not been attempted.
IL-2 therapy for chronic cytomegalovirus infection has been attempted in a single patient with EDA-ID (209). IL-2 has been used to treat selected patients with primary immunodeficiencies and defective NK cell function. It is thought to induce NK cell activity.
Prophylaxis.
Preventive strategies for L. monocytogenes and Salmonella infections should be instituted. Immunization with the inactivated Typhoid Vi vaccine could be considered in areas where typhoid is endemic. The numbers of M. avium infections can be reduced by the use of prophylactic antimicrobials in the HIV-positive population. Rifabutin (300 mg daily), clarithromycin (500 mg twice daily), and azithromycin (1,200 mg weekly), either alone or in combination with rifabutin, have all been shown in controlled trials of HIV-positive adults to be effective as prophylactic agents for the prevention of M. avium infections (117, 185, 222). While unproven in this population with an inherited susceptibility to mycobacteria, it is likely that rifabutin would have similar clinical efficacy. For patients with a clinical response to gamma interferon, it could be considered a prophylactic agent. In patients with CGD, long-term prophylaxis with gamma interferon has been well tolerated (31).
CONCLUDING REMARKS
Many of the disorders described in this review are uncommon. Nevertheless, some generalizations can be drawn from this diverse set of uncommon disorders. Treatment of infections in patients with these primary immunodeficiencies requires sustained and aggressive antimicrobial therapy. Often, adjunctive treatment with cytokines or white cell transfusions is required. Another generalization is that many of the disorders increase the susceptibility of the host to a very limited number of organisms. For example, the macrophage disorders, with the exception of EDA-ID, all lead to increased susceptibility to Mycobacterium spp. and Salmonella spp. Only a few other infections are problematic. This allows the caregiver to focus on the diagnosis and therapy of a relatively small number of types of infections. The list is slightly longer for patients with CGD; however, the number of organisms most likely to cause infection at given site is still very limited.
The recent identification of the gene defects responsible for the macrophage function disorders has led to a new appreciation of the role of the innate immune system in the eradication of intracellular bacteria. Study of these disorders has also illuminated new cytokine-based treatment strategies which could be exploited to enhance the eradication of intracellular bacterial in competent hosts in the future.
REFERENCES
- 1.Abinun, M., G. Spickett, A. L. Appleton, T. Flood, and A. J. Cant. 1996. Anhidrotic ectodermal dysplasia associated with specific antibody deficiency. Eur. J. Pediatr. 155:146-147. [DOI] [PubMed] [Google Scholar]
- 2.Abo, A., M. R. Webb, A. Grogan, and A. W. Segal. 1994. Activation of NADPH oxidase involves the dissociation of p21rac from its inhibitory GDP/GTP exchange protein (rhoGDI) followed by its translocation to the plasma membrane. Biochem. J. 298:585-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adkison, A. M., S. Z. Raptis, D. G. Kelley, and C. T. Pham. 2002. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J. Clin. Investig. 109:363-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aker, M., A. Zimran, A. Abrahamov, M. Horowitz, and Y. Matzner. 1993. Abnormal neutrophil chemotaxis in Gaucher disease. Br. J. Haematol. 83:187-191. [DOI] [PubMed] [Google Scholar]
- 5.Aksu, G., C. Tirpan, C. Cavusoglu, S. Soydan, F. Altare, J. L. Casanova, and N. Kutukculer. 2001. Mycobacterium fortuitum-chelonae complex infection in a child with complete interleukin-12 receptor beta 1 deficiency. Pediatr. Infect. Dis. J. 20:551-553. [DOI] [PubMed] [Google Scholar]
- 6.Allende, L. M., A. Lopez-Goyanes, E. Paz-Artal, A. Corell, M. A. Garcia-Perez, P. Varela, A. Scarpellini, S. Negreira, E. Palenque, and A. Arnaiz-Villena. 2001. A point mutation in a domain of gamma interferon receptor 1 provokes severe immunodeficiency. Clin. Diagn. Lab. Immunol. 8:133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.al-Sabbagh, A., A. Miller, L. M. Santos, and H. L. Weiner. 1994. Antigen-driven tissue-specific suppression following oral tolerance: orally administered myelin basic protein suppresses proteolipid protein-induced experimental autoimmune encephalomyelitis in the SJL mouse. Eur. J. Immunol. 24:2104-2109. [DOI] [PubMed] [Google Scholar]
- 8.Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432-1435. [DOI] [PubMed] [Google Scholar]
- 9.Altare, F., E. Jouanguy, S. Lamhamedi-Cherradi, M. C. Fondaneche, C. Fizame, F. Ribierre, G. Merlin, Z. Dembic, R. Schreiber, B. Lisowska-Grospierre, A. Fischer, E. Seboun, and J. L. Casanova. 1998. A causative relationship between mutant IFNgR1 alleles and impaired cellular response to IFN gamma in a compound heterozygous child. Am. J. Hum. Genet. 62:723-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altare, F., D. Lammas, P. Revy, E. Jouanguy, R. Doffinger, S. Lamhamedi, P. Drysdale, D. Scheel-Toellner, J. Girdlestone, P. Darbyshire, M. Wadhwa, H. Dockrell, M. Salmon, A. Fischer, A. Durandy, J. L. Casanova, and D. S. Kumararatne. 1998. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guerin and Salmonella enteritidis disseminated infection. J. Clin. Investig. 102:2035-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altare, F., A. Ensser, A. Breiman, J. Reichenbach, J. E. Baghdadi, A. Fischer, J. F. Emile, J. L. Gaillard, E. Meinl, and J. L. Casanova. 2001. Interleukin-12 receptor beta1 deficiency in a patient with abdominal tuberculosis. J. Infect. Dis. 184:231-236. [DOI] [PubMed] [Google Scholar]
- 12.Ambruso, D. R., M. Sasada, H. Nishiyama, A. Kubo, A. Komiyama, and R. H. Allen. 1984. Defective bactericidal activity and absence of specific granules in neutrophils from a patient with recurrent bacterial infections. J. Clin. Immunol. 4:23-30. [DOI] [PubMed] [Google Scholar]
- 13.Anderson, D. C., F. C. Schmalsteig, M. J. Finegold, B. J. Hughes, R. Rothlein, L. J. Miller, S. Kohl, M. F. Tosi, R. L. Jacobs, and T. C. Waldrop. 1985. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J. Infect. Dis. 152:668-689. [DOI] [PubMed] [Google Scholar]
- 14.Anderson, D. C., and T. A. Springer. 1987. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu. Rev. Med. 38:175-194. [DOI] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Aprikyan, A. A., W. C. Liles, J. R. Park, M. Jonas, E. Y. Chi, and D. C. Dale. 2000. Myelokathexis, a congenital disorder of severe neutropenia characterized by accelerated apoptosis and defective expression of bcl-x in neutrophil precursors. Blood 95:320-327. [PubMed] [Google Scholar]
- 17.Aprikyan, A. A., and D. C. Dale. 2001. Mutations in the neutrophil elastase gene in cyclic and congenital neutropenia. Curr. Opin. Immunol. 13:535-538. [DOI] [PubMed] [Google Scholar]
- 18.Aradhya, S., G. Courtois, A. Rajkovic, R. A. Lewis, M. Levy, A. Israel, and D. L. Nelson. 2001. Atypical forms of incontinentia pigmenti in male individuals result from mutations of a cytosine tract in exon 10 of NEMO (IKK-gamma). Am. J. Hum. Genet. 68:765-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aradhya, S., H. Woffendin, T. Jakins, T. Bardaro, T. Esposito, A. Smahi, C. Shaw, M. Levy, A. Munnich, M. D'Urso, R. A. Lewis, S. Kenwrick, and D. L. Nelson. 2001. A recurrent deletion in the ubiquitously expressed NEMO (IKK-gamma) gene accounts for the vast majority of incontinentia pigmenti mutations. Hum. Mol. Genet. 10:2171-2179. [DOI] [PubMed] [Google Scholar]
- 20.Arai, J., H. Wakiguchi, H. Hisakawa, H. Kubota, and T. Kurashige. 2000. A variant of myelokathexis with hypogammaglobulinemia: lymphocytes as well as neutrophils may reverse in response to infections. Pediatr. Hematol. Oncol. 17:171-176. [DOI] [PubMed] [Google Scholar]
- 21.Arseniev, L., H. Diedrich, and H. Link. 1996. Allogeneic bone marrow transplantation in a patient with Shwachman-Diamond syndrome. Ann. Hematol. 72:83-84. [DOI] [PubMed] [Google Scholar]
- 22.Bach, J. F. 2002. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347:911-920. [DOI] [PubMed] [Google Scholar]
- 23.Baer, P. N. 1989. Preventing loss of teeth in patients with Papillon-Lefevre syndrome. J. Pedodont. 13:182-183. [PubMed] [Google Scholar]
- 24.Baldus, M., V. Zunftmeister, G. Geibel-Werle, B. Claus, D. Mewes, M. Uppenkamp, and T. Nebe. 1999. Chediak-Higashi-Steinbrinck syndrome (CHS) in a 27-year-old woman—effects of G-CSF treatment. Ann. Hematol. 78:321-327. [DOI] [PubMed] [Google Scholar]
- 25.Barak, Y., and E. Nir. 1987. Chediak-Higashi syndrome. Am. J. Pediatr. Hematol. Oncol. 9:42-55. [DOI] [PubMed] [Google Scholar]
- 26.Barbosa, M. D., Q. A. Nguyen, V. T. Tchernev, J. A. Ashley, J. C. Detter, S. M. Blaydes, S. J. Brandt, D. Chotai, C. Hodgman, R. C. Solari, M. Lovett, and S. F. Kingsmore. 1996. Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature 382:262-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrios, N., D. Kirkpatrick, O. Regueira, B. Wuttke, J. McNeil, and J. Humbert. 1991. Bone marrow transplant in Shwachman-Diamond syndrome. Br. J. Haematol. 79:337-338. [DOI] [PubMed] [Google Scholar]
- 28.Bauer, S. B., and S. J. Kogan. 1991. Vesical manifestations of chronic granulomatous disease in children. Its relation to eosinophilic cystitis. Urology 37:463-466. [DOI] [PubMed] [Google Scholar]
- 29.Bedlow, A. J., E. G. Davies, A. L. Moss, N. Rebuck, A. Finn, and R. A. Marsden. 1998. Pyoderma gangrenosum in a child with congenital partial deficiency of leucocyte adherence glycoproteins. Br. J. Dermatol. 139:1064-1067. [DOI] [PubMed] [Google Scholar]
- 30.Belladonna, M. L., J. C. Renauld, R. Bianchi, C. Vacca, F. Fallarino, C. Orabona, M. C. Fioretti, U. Grohmann, and P. Puccetti. 2002. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J. Immunol. 168:5448-5454. [DOI] [PubMed] [Google Scholar]
- 31.Bemiller, L. S., D. H. Roberts, K. M. Starko, and J. T. Curnutte. 1995. Safety and effectiveness of long-term interferon gamma therapy in patients with chronic granulomatous disease. Blood Cells Mol. Dis. 21:239-247. [DOI] [PubMed] [Google Scholar]
- 32.Benson, C. A., P. L. Williams, D. L. Cohn, S. Becker, P. Hojczyk, T. Nevin, J. A. Korvick, L. Heifets, C. C. Child, M. M. Lederman, R. C. Reichman, W. G. Powderly, G. F. Notario, B. A. Wynne, R. Hafner, and The AIDS Clinical Trials Group 196/Terry Beirn Community Programs for Clinical Research on AIDS 009 Protocol Team. 2000. Clarithromycin or rifabutin alone or in combination for primary prophylaxis of Mycobacterium avium complex disease in patients with AIDS: a randomized, double-blind, placebo-controlled trial. J. Infect. Dis. 181:1289-1297. [DOI] [PubMed] [Google Scholar]
- 33.Berberich, I., G. L. Shu, and E. A. Clark. 1994. Cross-linking CD40 on B cells rapidly activates nuclear factor-kappa B. J. Immunol. 153:4357-4366. [PubMed] [Google Scholar]
- 34.Bergman, R., and R. Friedman-Birnbaum. 1988. Papillon-Lefevre syndrome: a study of the long-term clinical course of recurrent pyogenic infections and the effects of etretinate treatment. Br. J. Dermatol. 119:731-736. [DOI] [PubMed] [Google Scholar]
- 35.Bertin, J., W. J. Nir, C. M. Fischer, O. V. Tayber, P. R. Errada, J. R. Grant, J. J. Keilty, M. L. Gosselin, K. E. Robison, G. H. Wong, M. A. Glucksmann, and P. S. DiStefano. 1999. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-kappaB. J. Biol. Chem. 274:12955-12958. [DOI] [PubMed] [Google Scholar]
- 36.Bhardwaj, N., T. W. Nash, and M. A. Horwitz. 1986. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J. Immunol. 137:2662-2669. [PubMed] [Google Scholar]
- 37.Bidere, N., M. Briet, A. Durrbach, C. Dumont, J. Feldmann, B. Charpentier, G. de Saint-Basile, and A. Senik. 2002. Selective inhibition of dipeptidyl peptidase I, not caspases, prevents the partial processing of procaspase-3 in CD3-activated human CD8(+) T lymphocytes. J. Biol. Chem. 277:32339-32347. [DOI] [PubMed] [Google Scholar]
- 38.Bissenden, J. G., M. R. Haeney, M. J. Tarlow, and R. A. Thompson. 1981. Delayed separation of the umbilical cord, severe widespread infections, and immunodeficiency. Arch. Dis. Child. 56:397-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blume, R. S., and S. M. Wolff. 1972. The Chediak-Higashi syndrome: studies in four patients and a review of the literature. Medicine 51:247-280. [PubMed] [Google Scholar]
- 40.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 41.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12:64-76. [DOI] [PubMed] [Google Scholar]
- 42.Bohinjec, J. 1981. Myelokathexis: chronic neutropenia with hyperplastic bone marrow and hypersegmented neutrophils in two siblings. Blut 42:191-196. [DOI] [PubMed] [Google Scholar]
- 43.Borges, W. G., N. H. Augustine, and H. R. Hill. 2000. Defective interleukin-12/interferon-gamma pathway in patients with hyperimmunoglobulinemia E syndrome. J. Pediatr. 136:176-180. [DOI] [PubMed] [Google Scholar]
- 44.Borregaard, N., and J. B. Cowland. 1997. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89:3503-3521. [PubMed] [Google Scholar]
- 45.Borroni, G., A. Pagani, A. Carcaterra, R. Pericoli, P. Gabba, and M. Marconi. 1985. Immunological alterations in a case of Papillon-Lefevre syndrome with recurrent cutaneous infections. Dermatologica 170:27-30. [DOI] [PubMed] [Google Scholar]
- 46.Breton-Gorius, J., D. Y. Mason, D. Buriot, J. L. Vilde, and C. Griscelli. 1980. Lactoferrin deficiency as a consequence of a lack of specific granules in neutrophils from a patient with recurrent infections. Detection by immunoperoxidase staining for lactoferrin and cytochemical electron microscopy. Am. J. Pathol. 99:413-428. [PMC free article] [PubMed] [Google Scholar]
- 47.Brooks, E. G., G. R. Klimpel, S. E. Vaidya, S. E. Keeney, S. Raimer, A. S. Goldman, and F. C. Schmalstieg. 1994. Thymic hypoplasia and T-cell deficiency in ectodermal dysplasia: case report and review of the literature. Clin. Immunol. Immunopathol. 71:44-52. [DOI] [PubMed] [Google Scholar]
- 48.Bullock, W. E. 1993. Interactions between human phagocytic cells and Histoplasma capsulatum. Arch. Med. Res. 24:219-223. [PubMed] [Google Scholar]
- 49.Burg, N. D., and M. H. Pillinger. 2001. The neutrophil: function and regulation in innate and humoral immunity. Clin. Immunol. 99:7-17. [DOI] [PubMed] [Google Scholar]
- 50.Calderwood, S., L. Kilpatrick, S. D. Douglas, M. Freedman, K. Smith-Whitley, M. Rolland, and J. Kurtzberg. 2001. Recombinant human granulocyte colony-stimulating factor therapy for patients with neutropenia and/or neutrophil dysfunction secondary to glycogen storage disease type 1b. Blood 97:376-382. [DOI] [PubMed] [Google Scholar]
- 51.Caron, E., and A. Hall. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282:1717-1721. [DOI] [PubMed] [Google Scholar]
- 52.Caspi, R. R. 1998. IL-12 in autoimmunity. Clin. Immunol. Immunopathol. 88:4-13. [DOI] [PubMed] [Google Scholar]
- 53.Cheever, A. W., Y. H. Xu, A. Sher, and J. G. Macedonia. 1991. Analysis of egg granuloma formation in Schistosoma japonicum-infected mice treated with antibodies to interleukin-5 and gamma interferon. Infect. Immun. 59:4071-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chehimi, J., M. Elder, J. Greene, L. Noroski, E. R. Stiehm, J. A. Winkelstein, and K. E. Sullivan. 2001. Cytokine and chemokine dysregulation in hyper-IgE syndrome. Clin. Immunol. 100:49-56. [DOI] [PubMed] [Google Scholar]
- 55.Chen, P., C. Melchior, N. H. Brons, N. Schlegel, J. Caen, and N. Kieffer. 2001. Probing conformational changes in the I-like domain and the cysteine-rich repeat of human beta 3 integrins following disulfide bond disruption by cysteine mutations: identification of cysteine 598 involved in alphaIIbbeta3 activation. J. Biol. Chem. 276:38628-38635. [DOI] [PubMed] [Google Scholar]
- 56.Chen, Z., S. B. Koralov, M. Gendelman, M. C. Carroll, and G. Kelsoe. 2000. Humoral immune responses in Cr2−/− mice: enhanced affinity maturation but impaired antibody persistence. J. Immunol. 164:4522-4532. [DOI] [PubMed] [Google Scholar]
- 57.Chosay, J. G., M. A. Fisher, A. Farhood, K. A. Ready, C. J. Dunn, and H. Jaeschke. 1998. Role of PECAM-1 (CD31) in neutrophil transmigration in murine models of liver and peritoneal inflammation. Am. J. Physiol. 274:G776-G782. [DOI] [PubMed] [Google Scholar]
- 58.Chou, J. Y. 2001. The molecular basis of type 1 glycogen storage diseases. Curr. Mol. Med. 1:25-44. [DOI] [PubMed] [Google Scholar]
- 59.Chusid, M. J., J. E. Parrillo, and A. S. Fauci. 1975. Chronic granulomatous disease. Diagnosis in a 27-year-old man with Mycobacterium fortuitum. JAMA 233:1295-1296. [DOI] [PubMed] [Google Scholar]
- 60.Cipolli, M., C. D'Orazio, A. Delmarco, C. Marchesini, A. Miano, and G. Mastella. 1999. Shwachman's syndrome: pathomorphosis and long-term outcome. J. Pediatr. Gastroenterol. Nutr. 29:265-272. [DOI] [PubMed] [Google Scholar]
- 61.Clark, R. A., and H. R. Kimball. 1971. Defective granulocyte chemotaxis in the Chediak-Higashi syndrome. J. Clin. Investig. 50:2645-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark, R. A., H. L. Malech, J. I. Gallin, H. Nunoi, B. D. Volpp, D. W. Pearson, W. M. Nauseef, and J. T. Curnutte. 1989. Genetic variants of chronic granulomatous disease: prevalence of deficiencies of two cytosolic components of the NADPH oxidase system. N. Engl. J. Med. 321:647-652. [DOI] [PubMed] [Google Scholar]
- 63.Collman, R. J., and J. D. Dickerman. 1990. Corticosteroids in the management of cystitis secondary to chronic granulomatous disease. Pediatrics 85:219-221. [PubMed] [Google Scholar]
- 64.Cramer, R., M. R. Soranzo, P. Dri, G. D. Rottini, M. Bramezza, S. Cirielli, and P. Patriarca. 1982. Incidence of myeloperoxidase deficiency in an area of northern Italy: histochemical, biochemical and functional studies. Br. J. Haematol. 51:81-87. [DOI] [PubMed] [Google Scholar]
- 65.Cunningham, J. A., J. D. Kellner, P. J. Bridge, C. L. Trevenen, D. R. McLeod, and H. D. Davies. 2000. Disseminated bacille Calmette-Guerin infection in an infant with a novel deletion in the interferon-gamma receptor gene. Int. J. Tuberc. Lung Dis. 4:791-794. [PubMed] [Google Scholar]
- 66.Curnutte, J. T., and B. M. Babior. 1987. Chronic granulomatous disease. Adv. Hum. Genet. 16:229-297. [DOI] [PubMed] [Google Scholar]
- 67.Curnutte, J. T. 1993. Chronic granulomatous disease: the solving of a clinical riddle at the molecular level. Clin. Immunol. Immunopathol. 67:S2-S15. [DOI] [PubMed] [Google Scholar]
- 68.D'Agata, I. D., K. Paradis, Z. Chad, Y. Bonny, and E. Seidman. 1996. Leucocyte adhesion deficiency presenting as a chronic ileocolitis. Gut 39:605-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 70.Dautzenberg, B., C. Truffot-Pernot, J. Hazebroucq, S. Legris, C. Guerin, C. Begelman, G. Guermonprez, M. H. Fievet, C. Chastang, and J. Grosset. 1997. A randomized comparison of two clarithromycin doses for treatment of Mycobacterium avium complex infections. Infection 25:16-21. [DOI] [PubMed] [Google Scholar]
- 71.de Jong, R., F. Altare, I. A. Haagen, D. G. Elferink, T. Boer, P. J. van Breda Vriesman, P. J. Kabel, J. M. Draaisma, J. T. van Dissel, F. P. Kroon, J. L. Casanova, and T. H. Ottenhoff. 1998. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280:1435-1438. [DOI] [PubMed] [Google Scholar]
- 72.Delcourt-Debruyne, E. M., H. R. Boutigny, and H. F. Hildebrand. 2000. Features of severe periodontal disease in a teenager with Chediak-Higashi syndrome. J. Periodontol. 71:816-824. [DOI] [PubMed] [Google Scholar]
- 73.Deming, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-805. [DOI] [PubMed] [Google Scholar]
- 74.de Saint-Basile, G. 2002. Chediak-Higashi and Griscelli syndromes. Immunol. Allergy Clin. North Am. 22:301-317. [Google Scholar]
- 75.Desnick, R. J., H. L. Sharp, G. A. Grabowski, R. D. Brunning, P. G. Quie, J. H. Sung, R. J. Gorlin, and J. U. Ikonne. 1976. Mannosidosis: clinical, morphologic, immunologic, and biochemical studies. Pediatr. Res. 10:985-996. [DOI] [PubMed] [Google Scholar]
- 76.Diacovo, T. G., S. J. Roth, J. M. Buccola, D. F. Bainton, and T. A. Springer. 1996. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood 88:146-157. [PubMed] [Google Scholar]
- 77.Di Rocco, M., C. Borrone, F. Dallegri, G. Frumento, and F. Patrone. 1984. Neutropenia and impaired neutrophil function in glycogenosis type Ib. J. Inherited Metab. Dis. 7:151-154. [DOI] [PubMed] [Google Scholar]
- 78.Doffinger, R., E. Jouanguy, S. Dupuis, M. C. Fondaneche, J. L. Stephan, J. F. Emile, S. Lamhamedi-Cherradi, F. Altare, A. Pallier, G. Barcenas-Morales, E. Meinl, C. Krause, S. Pestka, R. D. Schreiber, F. Novelli, and J. L. Casanova. 2000. Partial interferon-gamma receptor signaling chain deficiency in a patient with bacille Calmette-Guerin and Mycobacterium abscessus infection. J. Infect. Dis. 181:379-384. [DOI] [PubMed] [Google Scholar]
- 79.Doffinger, R., A. Smahi, C. Bessia, F. Geissmann, J. Feinberg, A. Durandy, C. Bodemer, S. Kenwrick, S. Dupuis-Girod, S. Blanche, P. Wood, S. H. Rabia, D. J. Headon, P. A. Overbeek, F. Le Deist, S. M. Holland, K. Belani, D. S. Kumararatne, A. Fischer, R. Shapiro, M. E. Conley, E. Reimund, H. Kalhoff, M. Abinun, A. Munnich, A. Israel, G. Courtois, and J. L. Casanova. 2001. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat. Genet. 27:277-285. [DOI] [PubMed] [Google Scholar]
- 80.Doffinger, R., S. Dupuis, C. Picard, C. Fieschi, J. Feinberg, G. Barcenas-Morales, and J. L. Casanova. 2002. Inherited disorders of IL-12- and IFNgamma-mediated immunity: a molecular genetics update. Mol. Immunol. 38:903-909. [DOI] [PubMed] [Google Scholar]
- 81.Dorman, S. E., and S. M. Holland. 1998. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J. Clin. Investig. 101:2364-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dorman, S. E., G. Uzel, J. Roesler, J. S. Bradley, J. Bastian, G. Billman, S. King, A. Filie, J. Schermerhorn, and S. M. Holland. 1999. Viral infections in interferon-gamma receptor deficiency. J. Pediatr. 135:640-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dorman, S. E., and S. M. Holland. 2000. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 11:321-333. [DOI] [PubMed] [Google Scholar]
- 84.Dorman, S. E., S. V. Guide, P. S. Conville, E. S. DeCarlo, H. L. Malech, J. I. Gallin, F. G. Witebsky, and S. M. Holland. 2002. Nocardia infection in chronic granulomatous disease. Clin. Infect. Dis. 35:390-394. [DOI] [PubMed] [Google Scholar]
- 85.Dugas, B., M. D. Mossalayi, C. Damais, and J. P. Kolb. 1995. Nitric oxide production by human monocytes: evidence for a role of CD23. Immunol. Today 16:574-580. [DOI] [PubMed] [Google Scholar]
- 86.Dupuis, S., C. Dargemont, C. Fieschi, N. Thomassin, S. Rosenzweig, J. Harris, S. M. Holland, R. D. Schreiber, and J. L. Casanova. 2001. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science 293:300-303. [DOI] [PubMed] [Google Scholar]
- 87.Dupuis-Girod, S., N. Corradini, S. Hadj-Rabia, J. C. Fournet, L. Faivre, F. Le Deist, P. Durand, R. Doffinger, A. Smahi, A. Israel, G. Courtois, N. Brousse, S. Blanche, A. Munnich, A. Fischer, J. L. Casanova, and C. Bodemer. 2002. Osteopetrosis, lymphedema, anhidrotic ectodermal dysplasia, and immunodeficiency in a boy and incontinentia pigmenti in his mother. Pediatrics 109:e97. [DOI] [PubMed] [Google Scholar]
- 88.Elloumi-Zghal, H., M. R. Barbouche, J. Chemli, M. Bejaoui, A. Harbi, N. Snoussi, S. Abdelhak, and K. Dellagi. 2002. Clinical and genetic heterogeneity of inherited autosomal recessive susceptibility to disseminated Mycobacterium bovis bacille calmette-guerin infection. J. Infect. Dis. 185:1468-1475. [DOI] [PubMed] [Google Scholar]
- 89.Etzioni, A., M. Frydman, S. Pollack, I. Avidor, M. L. Phillips, J. C. Paulson, and R. Gershoni-Baruch. 1992. Brief report: recurrent severe infections caused by a novel leukocyte adhesion deficiency. N. Engl. J. Med. 327:1789-1792. [DOI] [PubMed] [Google Scholar]
- 90.Etzioni, A., R. Gershoni-Baruch, S. Pollack, and N. Shehadeh. 1998. Leukocyte adhesion deficiency type II: long-term follow-up. J. Allergy Clin. Immunol. 102:323-324. [DOI] [PubMed] [Google Scholar]
- 91.Etzioni, A., L. Sturla, A. Antonellis, E. D. Green, R. Gershoni-Baruch, P. M. Berninsone, C. B. Hirschberg, and M. Tonetti. 2002. Leukocyte adhesion deficiency (LAD) type II/carbohydrate deficient glycoprotein (CDG) IIc founder effect and genotype/phenotype correlation. Am. J. Med. Genet. 110:131-135. [DOI] [PubMed] [Google Scholar]
- 92.Reference deleted.
- 93.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 94.Franzoso, G., L. Carlson, L. Xing, L. Poljak, E. W. Shores, K. D. Brown, A. Leonardi, T. Tran, B. F. Boyce, and U. Siebenlist. 1997. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 11:3482-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fraser, I. P., H. Koziel, and R. A. Ezekowitz. 1998. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin. Immunol. 10:363-372. [DOI] [PubMed] [Google Scholar]
- 96.Freerksen, E., M. Rosenfeld, R. Freerksen, and M. Kruger-Thiemer. 1977. Treatment of chronic salmonella carriers. Study with 40 cases of S. typhi, 19 cases of S. paratyphi b and 28 cases of S. enteritidis strains. Chematherapy 23:192-210. [DOI] [PubMed] [Google Scholar]
- 97.Frucht, D. M. 2002. IL-23: a cytokine that acts on memory T cells. Science's STKE: Signal Transduction Knowledge Environment 2002:PE1. [DOI] [PubMed]
- 98.Fuhlbrigge, R. C., R. Alon, K. D. Puri, J. B. Lowe, and T. A. Springer. 1996. Sialylated, fucosylated ligands for L-selectin expressed on leukocytes mediate tethering and rolling adhesions in physiologic flow conditions. J. Cell Biol. 135:837-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gallin, J. I., J. A. Klimerman, G. A. Padgett, and S. M. Wolff. 1975. Defective mononuclear leukocyte chemotaxis in the Chediak-Higashi syndrome of humans, mink, and cattle. Blood 45:863-870. [PubMed] [Google Scholar]
- 100.Gallin, J. I., M. P. Fletcher, B. E. Seligmann, S. Hoffstein, K. Cehrs, and N. Mounessa. 1982. Human neutrophil-specific granule deficiency: a model to assess the role of neutrophil-specific granules in the evolution of the inflammatory response. Blood 59:1317-1329. [PubMed] [Google Scholar]
- 101.Gallin, J. I. 1985. Neutrophil specific granule deficiency. Annu. Rev. Med. 36:263-274. [DOI] [PubMed] [Google Scholar]
- 102.Ganz, T., J. A. Metcalf, J. I. Gallin, L. A. Boxer, and R. I. Lehrer. 1988. Microbicidal/cytotoxic proteins of neutrophils are deficient in two disorders: Chediak-Higashi syndrome and “specific” granule deficiency. J. Clin. Investig. 82:552-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gazzinelli, R. T., S. Hayashi, M. Wysocka, L. Carrera, R. Kuhn, W. Muller, F. Roberge, G. Trinchieri, and A. Sher. 1994. Role of IL-12 in the initiation of cell mediated immunity by Toxoplasma gondii and its regulation by IL-10 and nitric oxide. J. Eukaryot. Microbiol. 41:9S. [PubMed]
- 104.Ghaffer, K. A., F. M. Zahran, H. M. Fahmy, and R. S. Brown. 1999. Papillon-Lefevre syndrome: neutrophil function in 15 cases fron 4 families in Egypt. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 88:320-325. [DOI] [PubMed] [Google Scholar]
- 105.Gitzelmann, R., and N. U. Bosshard. 1993. Defective neutrophil and monocyte functions in glycogen storage disease type Ib: a literature review. Eur. J. Pediatr. 152(Suppl. 1):S33-S38. [DOI] [PubMed] [Google Scholar]
- 106.Gombart, A. F., M. Shiohara, S. H. Kwok, K. Agematsu, A. Komiyama, and H. P. Koeffler. 2001. Neutrophil-specific granule deficiency: homozygous recessive inheritance of a frameshift mutation in the gene encoding transcription factor CCAAT/enhancer binding protein-epsilon. Blood 97:2561-2567. [DOI] [PubMed] [Google Scholar]
- 107.Gombart, A. F., and H. P. Koeffler. 2002. Neutrophil specific granule deficiency and mutations in the gene encoding transcription factor C/EBP(epsilon). Curr. Opin. Hematol. 9:36-42. [DOI] [PubMed] [Google Scholar]
- 108.Griffith, D. E., B. A. Brown, W. M. Girard, B. E. Griffith, L. A. Couch, and R. J. Wallace, Jr. 2001. Azithromycin-containing regimens for treatment of Mycobacterium avium complex lung disease. Clin. Infect. Dis. 32:1547-1553. [DOI] [PubMed] [Google Scholar]
- 109.Grimbacher, B., S. Holland, J. I. Gallin, F. Greenberg, S. Hill, H. L. Malech, J. A. Miller, A. C. O'Connell, B. Dent, and J. M. Puck. 1999. Hyper-IgE syndrome with recurrent infections-an autosomal dominant multisystem disorder. N. Engl. J. Med. 340:692-702. [DOI] [PubMed] [Google Scholar]
- 110.Haddad, E., F. Le Deist, S. Blanche, M. Benkerrou, P. Rohrlich, E. Vilmer, C. Griscelli, and A. Fischer. 1995. Treatment of Chediak-Higashi syndrome by allogenic bone marrow transplantation: report of 10 cases. Blood 85:3328-3333. [PubMed] [Google Scholar]
- 111.Hague, R. A., E. J. Eastham, R. E. Lee, and A. J. Cant. 1993. Resolution of hepatic abscess after interferon gamma in chronic granulomatous disease. Arch. Dis. Child. 69:443-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hampton, M. B., A. J. Kettle, and C. C. Winterbourn. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007-3017. [PubMed] [Google Scholar]
- 113.Haneke, E., O. P. Hornstein, and C. Lex. 1975. Increased susceptibility to infections in the Papillon-Lefevre syndrome. Dermatologica 150:283-286. [DOI] [PubMed] [Google Scholar]
- 114.Harris, E. S., A. O. Shigeoka, W. Li, R. H. Adams, S. M. Prescott, T. M. McIntyre, G. A. Zimmerman, and D. E. Lorant. 2001. A novel syndrome of variant leukocyte adhesion deficiency involving defects in adhesion mediated by beta1 and beta2 integrins. Blood 97:767-776. [DOI] [PubMed] [Google Scholar]
- 115.Hashimoto, C., K. L. Hudson, and K. V. Anderson. 1988. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52:269-279. [DOI] [PubMed] [Google Scholar]
- 116.Haurie, C., D. C. Dale, and M. C. Mackey. 1998. Cyclical neutropenia and other periodic hematological disorders: a review of mechanisms and mathematical models. Blood 92:2629-2640. [PubMed] [Google Scholar]
- 117.Havlir, D. V., M. P. Dube, F. R. Sattler, D. N. Forthal, C. A. Kemper, M. W. Dunne, D. M. Parenti, J. P. Lavelle, A. C. White, Jr., M. D. Witt, S. A. Bozzette, J. A. McCutchan, and the California Collaborative Treatment Group. 1996. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. N. Engl. J. Med. 335:392-398. [DOI] [PubMed] [Google Scholar]
- 118.Hayward, A. R., B. A. Harvey, J. Leonard, M. C. Greenwood, C. B. Wood, and J. F. Soothill. 1979. Delayed separation of the umbilical cord, widespread infections, and defective neutrophil mobility. Lancet i:1099-1101. [DOI] [PubMed] [Google Scholar]
- 119.Hearn, T., C. Haurie, and M. C. Mackey. 1998. Cyclical neutropenia and the peripheral control of white blood cell production. J. Theor. Biol. 192:167-181. [DOI] [PubMed] [Google Scholar]
- 120.Heltzer, M., A. F. Jawad, J. Rae, J. T. Curnutte, and K. E. Sullivan. 2002. Diminished T cell numbers in patients with chronic granulomatous disease. Clin. Immunol. 105:373-378. [DOI] [PubMed] [Google Scholar]
- 121.Hidalgo, A., S. Ma, A. J. Peired, L. A. Weiss, C. Cunningham-Rundles, and P. S. Frenette. 2003. Insights into leukocyte adhesion deficiency type 2 from a novel mutation in the GDP-fucose transporter gene. Blood 101:1705-1712. [DOI] [PubMed] [Google Scholar]
- 122.Hogg, N., M. P. Stewart, S. L. Scarth, R. Newton, J. M. Shaw, S. K. Law, and N. Klein. 1999. A novel leukocyte adhesion deficiency caused by expressed but nonfunctional beta2 integrins Mac-1 and LFA-1. J. Clin. Investig. 103:97-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Holland, S. M., S. E. Dorman, A. Kwon, I. F. Pitha-Rowe, D. M. Frucht, S. M. Gerstberger, G. J. Noel, P. Vesterhus, M. R. Brown, and T. A. Fleisher. 1998. Abnormal regulation of interferon-gamma, interleukin-12, and tumor necrosis factor-alpha in human interferon-gamma receptor 1 deficiency. J. Infect. Dis. 178:1095-1104. [DOI] [PubMed] [Google Scholar]
- 124.Holland, S. M. 2001. Immune deficiency presenting as mycobacterial infection. Clin. Rev. Allergy Immunol. 20:121-137. [DOI] [PubMed] [Google Scholar]
- 125.Holmskov, U. L. 2000. Collectins and collectin receptors in innate immunity. APMIS Suppl. 100:1-59. [PubMed] [Google Scholar]
- 126.Hopfenspirger, M. T., S. K. Parr, R. J. Hopp, R. G. Townley, and D. K. Agrawal. 2001. Mycobacterial antigens attenuate late phase response, airway hyperresponsiveness, and bronchoalveolar lavage eosinophilia in a mouse model of bronchial asthma. Int. Immunopharmacol. 1:1743-1751. [DOI] [PubMed] [Google Scholar]
- 127.Hord, J. D., J. A. Whitlock, J. C. Gay, and J. N. Lukens. 1997. Clinical features of myelokathexis and treatment with hematopoietic cytokines: a case report of two patients and review of the literature. J. Pediatr. Hematol. Oncol. 19:443-448. [DOI] [PubMed] [Google Scholar]
- 128.Hsu, H., D. L. Lacey, C. R. Dunstan, I. Solovyev, A. Colombero, E. Timms, H. L. Tan, G. Elliott, M. J. Kelley, I. Sarosi, L. Wang, X. Z. Xia, R. Elliott, L. Chiu, T. Black, S. Scully, C. Capparelli, S. Morony, G. Shimamoto, M. B. Bass, and W. J. Boyle. 1999. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA 96:3540-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguct. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 130.Hugot, J. P., M. Chamaillard, H. Zouali, S. Lesage, J. P. Cezard, J. Belaiche, S. Almer, C. Tysk, C. A. O'Morain, M. Gassull, V. Binder, Y. Finkel, A. Cortot, R. Modigliani, P. Laurent-Puig, C. Gower-Rousseau, J. Macry, J. F. Colombel, M. Sahbatou, and G. Thomas. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599-603. [DOI] [PubMed] [Google Scholar]
- 131.Ibbotson, G. C., C. Doig, J. Kaur, V. Gill, L. Ostrovsky, T. Fairhead, and P. Kubes. 2001. Functional alpha4-integrin: a newly identified pathway of neutrophil recruitment in critically ill septic patients. Nat. Med. 7:465-470. [DOI] [PubMed] [Google Scholar]
- 132.Inohara, N., T. Koseki, L. del Peso, Y. Hu, C. Yee, S. Chen, R. Carrio, J. Merino, D. Liu, J. Ni, and G. Nunez. 1999. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J. Biol. Chem. 274:14560-14567. [DOI] [PubMed] [Google Scholar]
- 132a.International Chronic Granulomatous Disease Cooperative Study Group. 1991. A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. N. Engl. J. Med. 324:509-516. [DOI] [PubMed] [Google Scholar]
- 133.Introne, W., R. E. Boissy, and W. A. Gahl. 1999. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol. Genet. Metab. 68:283-303. [DOI] [PubMed] [Google Scholar]
- 134.Ishiguro, T., M. Naito, T. Yamamoto, G. Hasegawa, F. Gejyo, M. Mitsuyama, H. Suzuki, and T. Kodama. 2001. Role of macrophage scavenger receptors in response to Listeria monocytogenes infection in mice. Am. J. Pathol. 158:179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jacobson, M. A., S. M. Hahn, J. L. Gerberding, B. Lee, and M. A. Sande. 1989. Ciprofloxacin for Salmonella bacteremia in the acquired immunodeficiency syndrome (AIDS). Ann. Intern. Med. 110:1027-1029. [DOI] [PubMed] [Google Scholar]
- 136.Jain, A., C. A. Ma, S. Liu, M. Brown, J. Cohen, and W. Strober. 2001. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat. Immunol. 2:223-228. [DOI] [PubMed] [Google Scholar]
- 137.Janssen, R., A. Van Wengen, E. Verhard, T. De Boer, T. Zomerdijk, T. H. Ottenhoff, and J. T. Van Dissel. 2002. Divergent role for TNF-alpha in IFN-gamma induced killing of Toxoplasma gondii and Salmonella typhimurium contributes to selective susceptibility of patients with partial IFN-gamma receptor 1 deficiency. J. Immunol. 169:3900-3907. [DOI] [PubMed] [Google Scholar]
- 138.Johnston, H. C., A. O. Shigeoka, D. C. Hurley, and T. J. Pysher. 1989. Nocardia pneumonia in a neonate with chronic granulomatous disease. Pediatr. Infect. Dis. J. 8:526-528. [DOI] [PubMed] [Google Scholar]
- 139.Johnston, J. J., L. A. Boxer, and N. Berliner. 1992. Correlation of messenger RNA levels with protein defects in specific granule deficiency. Blood 80:2088-2091. [PubMed] [Google Scholar]
- 140.Jouanguy, E., F. Altare, S. Lamhamedi, P. Revy, J. F. Emile, M. Newport, M. Levin, S. Blanche, E. Seboun, A. Fischer, and J. L. Casanova. 1996. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335:1956-1961. [DOI] [PubMed] [Google Scholar]
- 141.Jouanguy, E., S. Lamhamedi-Cherradi, F. Altare, M. C. Fondaneche, D. Tuerlinckx, S. Blanche, J. F. Emile, J. L. Gaillard, R. Schreiber, M. Levin, A. Fischer, C. Hivroz, and J. L. Casanova. 1997. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. J. Clin. Investig. 100:2658-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jouanguy, E., S. Lamhamedi-Cherradi, D. Lammas, S. E. Dorman, M. C. Fondaneche, S. Dupuis, R. Doffinger, F. Altare, J. Girdlestone, J. F. Emile, H. Ducoulombier, D. Edgar, J. Clarke, V. A. Oxelius, M. Brai, V. Novelli, K. Heyne, A. Fischer, S. M. Holland, D. S. Kumararatne, R. D. Schreiber, and J. L. Casanova. 1999. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat. Genet. 21:370-378. [DOI] [PubMed] [Google Scholar]
- 143.Jouanguy, E., S. Dupuis, A. Pallier, R. Doffinger, M. C. Fondaneche, C. Fieschi, S. Lamhamedi-Cherradi, F. Altare, J. F. Emile, P. Lutz, P. Bordigoni, H. Cokugras, N. Akcakaya, J. Landman-Parker, J. Donnadieu, Y. Camcioglu, and J. L. Casanova. 2000. In a novel form of IFN-gamma receptor 1 deficiency, cell surface receptors fail to bind IFN-gamma. J. Clin. Investig. 105:1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Karim, M. A., K. Suzuki, K. Fukai, J. Oh, D. L. Nagle, K. J. Moore, E. Barbosa, T. Falik-Borenstein, A. Filipovich, Y. Ishida, S. Kivrikko, C. Klein, F. Kreuz, A. Levin, H. Miyajima, J. Regueiro, C. Russo, E. Uyama, O. Vierimaa, and R. A. Spritz. 2002. Apparent genotype-phenotype correlation in childhood, adolescent, and adult Chediak-Higashi syndrome. Am. J. Med. Genet. 108:16-22. [PubMed] [Google Scholar]
- 145.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56:731-740. [DOI] [PubMed] [Google Scholar]
- 146.Kobayashi, Y., C. Miyaji, H. Watanabe, H. Umezu, G. Hasegawa, T. Abo, M. Arakawa, N. Kamata, H. Suzuki, T. Kodama, and M. Naito. 2000. Role of macrophage scavenger receptor in endotoxin shock. J. Pathol. 192:263-272. [DOI] [PubMed] [Google Scholar]
- 147.Kohbata, S., H. Yokoyama, and E. Yabuuchi. 1986. Cytopathogenic effect of Salmonella typhi GIFU 10007 on M cells of murine ileal Peyer's patches in ligated ileal loops: an ultrastructural study. Microbiol. Immunol. 30:1225-1237. [DOI] [PubMed] [Google Scholar]
- 148.Kohl, S., T. A. Springer, F. C. Schmalstieg, L. S. Loo, and D. C. Anderson. 1984. Defective natural killer cytotoxicity and polymorphonuclear leukocyte antibody-dependent cellular cytotoxicity in patients with LFA-1/OKM-1 deficiency. J. Immunol. 133:2972-2978. [PubMed] [Google Scholar]
- 149.Komiyama, A., H. Morosawa, T. Nakahata, Y. Miyagawa, and T. Akabane. 1979. Abnormal neutrophil maturation in a neutrophil defect with morphologic abnormality and impaired function. J. Pediatr. 94:19-25. [DOI] [PubMed] [Google Scholar]
- 150.Kuijpers, T. W., R. A. Van Lier, D. Hamann, M. de Boer, L. Y. Thung, R. S. Weening, A. J. Verhoeven, and D. Roos. 1997. Leukocyte adhesion deficiency type 1 (LAD-1)/variant. A novel immunodeficiency syndrome characterized by dysfunctional beta2 integrins. J. Clin. Investig. 100:1725-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kumar, A., M. T. Eby, S. Sinha, A. Jasmin, and P. M. Chaudhary. 2001. The ectodermal dysplasia receptor activates the nuclear factor-kappaB, JNK, and cell death pathways and binds to ectodysplasin A. J. Biol. Chem. 276:2668-2677. [DOI] [PubMed] [Google Scholar]
- 152.Lacy, D. E., D. A. Spencer, A. Goldstein, P. H. Weller, and P. Darbyshire. 1993. Chronic granulomatous disease presenting in childhood with Pseudomonas cepacia septicaemia. J. Infect. 27:301-304. [DOI] [PubMed] [Google Scholar]
- 153.Lammas, D. A., J. L. Casanova, and D. S. Kumararatne. 2000. Clinical consequences of defects in the IL-12-dependent interferon-gamma (IFN-gamma) pathway. Clin. Exp. Immunol. 121:417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lanza, F. 1998. Clinical manifestation of myeloperoxidase deficiency. J. Mol. Med. 76:676-681. [DOI] [PubMed] [Google Scholar]
- 155.Lefevre, C., C. Blanchet-Bardon, F. Jobard, B. Bouadjar, J. F. Stalder, S. Cure, A. Hoffmann, J. F. Prud'Homme, and J. Fischer. 2001. Novel point mutations, deletions, and polymorphisms in the cathepsin C gene in nine families from Europe and North Africa with Papillon-Lefevre syndrome. J. Investig. Dermatol. 117:1657-1661. [DOI] [PubMed] [Google Scholar]
- 156.Lehrer, R. I., and M. J. Cline. 1969. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J. Clin. Investig. 48:1478-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lekstrom-Himes, J., and K. G. Xanthopoulos. 1999. CCAAT/enhancer binding protein epsilon is critical for effective neutrophil-mediated response to inflammatory challenge. Blood 93:3096-3105. [PubMed] [Google Scholar]
- 158.Lekstrom-Himes, J. A., S. M. Holland, E. S. DeCarlo, J. Miller, S. F. Leitman, R. Chang, A. R. Baker, and J. I. Gallin. 1994. Treatment with intralesional granulocyte instillations and interferon-gamma for a patient with chronic granulomatous disease and multiple hepatic abscesses. Clin. Infect. Dis. 19:770-773. [DOI] [PubMed] [Google Scholar]
- 159.Lekstrom-Himes, J. A., S. E. Dorman, P. Kopar, S. M. Holland, and J. I. Gallin. 1999. Neutrophil-specific granule deficiency results from a novel mutation with loss of function of the transcription factor CCAAT/enhancer binding protein epsilon. J. Exp. Med. 189:1847-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Leung, D. Y. M., and R. S. Geha. 1988. Clinical and immunologic aspects of the hyperimmunoglobulin E syndrome. Hematol. Oncol. Clin. North Am. 2:81-101. [PubMed] [Google Scholar]
- 161.Leung, T., K. Chik, C. Li, M. Shing, and P. Yuen. 1999. Bone marrow transplantation for chronic granulomatous disease: long-term follow-up and review of literature. Bone Marrow Transplant. 24:567-570. [DOI] [PubMed] [Google Scholar]
- 162.Levin, M., M. J. Newport, S. D'Souza, P. Kalabalikis, I. N. Brown, H. M. Lenicker, P. V. Agius, E. G. Davies, A. Thrasher, and N. Klein. 1995. Familial disseminated atypical mycobacterial infection in childhood: a human mycobacterial susceptibility gene? Lancet 345:79-83. [DOI] [PubMed] [Google Scholar]
- 163.Levine, M. M., D. N. Taylor, and C. Ferreccio. 1989. Typhoid vaccines come of age. Pediatr. Infect. Dis. J. 8:374-381. [DOI] [PubMed] [Google Scholar]
- 164.Liese, J., S. Kloos, V. Jendrossek, T. Petropoulou, U. Wintergerst, G. Notheis, M. Gahr, and B. H. Belohradsky. 2000. Long-term follow-up and outcome of 39 patients with chronic granulomatous disease. J. Pediatr. 137:687-693. [DOI] [PubMed] [Google Scholar]
- 165.Liu, C., E. Gelius, G. Liu, H. Steiner, and R. Dziarski. 2000. Mammalian peptidoglycan recognition protein binds peptidoglycan with high affinity, is expressed in neutrophils, and inhibits bacterial growth. J. Biol. Chem. 275:24490-24499. [DOI] [PubMed] [Google Scholar]
- 166.Liu, C., Z. Xu, D. Gupta, and R. Dziarski. 2001. Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. J. Biol. Chem. 276:34686-34694. [DOI] [PubMed] [Google Scholar]
- 167.Liu, R., C. Cao, H. Meng, and Z. Tang. 2000. Leukocyte functions in 2 cases of Papillon-Lefevre syndrome. J. Clin. Periodontol. 27:69-73. [DOI] [PubMed] [Google Scholar]
- 168.Lomaga, M. A., W. C. Yeh, I. Sarosi, G. S. Duncan, C. Furlonger, A. Ho, S. Morony, C. Capparelli, G. Van, S. Kaufman, A. van der Heiden, A. Itie, A. Wakeham, W. Khoo, T. Sasaki, Z. Cao, J. M. Penninger, C. J. Paige, D. L. Lacey, C. R. Dunstan, W. J. Boyle, D. V. Goeddel, and T. W. Mak. 1999. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13:1015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Luhn, K., T. Marquardt, E. Harms, and D. Vestweber. 2001. Discontinuation of fucose therapy in LADII causes rapid loss of selection ligands and rise of leukocyte counts. Blood 97:330-332. [DOI] [PubMed] [Google Scholar]
- 170.Luhn, K., M. K. Wild, M. Eckhardt, R. Gerardy-Schahn, and D. Vestweber. 2001. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat. Genet. 28:69-72. [DOI] [PubMed] [Google Scholar]
- 171.Lukacs, N. W., S. W. Chensue, R. M. Strieter, K. Warmington, and S. L. Kunkel. 1994. Inflammatory granuloma formation is mediated by TNF-alpha-inducible intercellular adhesion molecule-1. J. Immunol. 152:5883-5889. [PubMed] [Google Scholar]
- 172.Macher, A. M., T. B. Casale, and A. S. Fauci. 1982. Chronic granulomatous disease of childhood and Chromobacterium violaceum infections in the southeastern United States. Ann. Intern. Med. 97:51-55. [DOI] [PubMed] [Google Scholar]
- 173.Macher, A. M., T. B. Casale, J. I. Gallin, H. Boltansky, and A. S. Fauci. 1983. Chromobacterium violaceum infectious and chronic granulomatous disease. Ann. Intern. Med. 98:259. [DOI] [PubMed] [Google Scholar]
- 174.Manetti, R., P. Parronchi, M. G. Giudizi, M. P. Piccinni, E. Maggi, G. Trinchieri, and S. Romagnani. 1993. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 177:1199-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mansour, S., H. Woffendin, S. Mitton, I. Jeffery, T. Jakins, S. Kenwrick, and V. A. Murday. 2001. Incontinentia pigmenti in a surviving male is accompanied by hypohidrotic ectodermal dysplasia and recurrent infection. Am. J. Med. Genet. 99:172-177. [DOI] [PubMed] [Google Scholar]
- 176.Marandian, M. H., N. Foroozanfar, H. Haghigat, S. Saket, M. Lessani, and M. Djafarian. 1979. Syndrome de Papillon-Lefevre et infections recurrentes. Arch. Fr. Pediatr. 36:819-822. [PubMed] [Google Scholar]
- 177.Marquardt, T., T. Brune, K. Luhn, K. P. Zimmer, C. Korner, L. Fabritz, N. van der Werft, J. Vormoor, H. H. Freeze, F. Louwen, B. Biermann, E. Harms, K. von Figura, D. Vestweber, and H. G. Koch. 1999. Leukocyte adhesion deficiency II syndrome, a generalized defect in fucose metabolism. J. Pediatr. 134:681-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mates, M., and C. H. Kirkpatrick. 1999. A man with multiple infections with unusual organisms. Ann. Allergy Asthma Immunol. 82:17-22. [DOI] [PubMed] [Google Scholar]
- 179.McFarland, E. J., and D. R. Kuritzkes. 1993. Clinical features and treatment of infection due to mycobacterium fortuitum/chelonae complex. Curr. Clin. Top. Infect. Dis. 13:188-202. [PubMed] [Google Scholar]
- 180.Medoff, G., A. Painter, and G. S. Kobayashi. 1987. Mycelial- to yeast-phase transitions of the dimorphic fungi Blastomyces dermatitidis and Paracoccidioides brasiliensis. J. Bacteriol. 169:4055-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Medzhitov, R., and C. A. Janeway, Jr. 2000. How does the immune system distinguish self from nonself? Semin. Immunol. 12:185-188. [DOI] [PubMed] [Google Scholar]
- 182.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 183.Michel, T., J. M. Reichhart, J. A. Hoffmann, and J. Royet. 2001. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 414:756-759. [DOI] [PubMed] [Google Scholar]
- 184.Mielke, M. E., H. Rosen, S. Brocke, C. Peters, and H. Hahn. 1992. Protective immunity and granuloma formation are mediated by two distinct tumor necrosis factor alpha- and gamma interferon-dependent T cell-phagocyte interactions in murine listeriosis: dissociation on the basis of phagocyte adhesion mechanisms. Infect. Immun. 60:1875-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Moore, R. D., and R. E. Chaisson. 1995. Survival analysis of two controlled trials of rifabutin prophylaxis against Mycobacterium avium complex in AIDS. AIDS 9:1337-1342. [DOI] [PubMed] [Google Scholar]
- 186.Moskaluk, C. A., H. W. Pogrebniak, H. I. Pass, J. I. Gallin, and W. D. Travis. 1994. Surgical pathology of the lung in chronic granulomatous disease. Am. J. Clin. Pathol. 102:684-691. [DOI] [PubMed] [Google Scholar]
- 187.Mouy, R., A. Fischer, E. Vilmer, R. Seger, and C. Griscelli. 1989. Incidence, severity, and prevention of infections in chronic granulomatous disease. J. Pediatr. 114:555-560. [DOI] [PubMed] [Google Scholar]
- 188.Mouy, R., F. Veber, S. Blanche, J. Donadieu, R. Brauner, J. C. Levron, C. Griscelli, and A. Fischer. 1994. Long-term itraconazole prophylaxis against Aspergillus infections in thirty-two patients with chronic granulomatous disease. J. Pediatr. 125:998-1003. [DOI] [PubMed] [Google Scholar]
- 189.Mulholland, M. W., J. P. Delaney, and R. L. Simmons. 1983. Gastrointestinal complications of chronic granulomatous disease: surgical implications. Surgery 94:569-575. [PubMed] [Google Scholar]
- 190.Munder, M., M. Mallo, K. Eichmann, and M. Modolell. 2001. Direct stimulation of macrophages by IL-12 and IL-18—a bridge built on solid ground. Immunol. Lett. 75:159-160. [DOI] [PubMed] [Google Scholar]
- 191.Myrup, B., N. H. Valerius, and P. B. Mortensen. 1998. Treatment of enteritis in chronic granulomatous disease with granulocyte colony stimulating factor. Gut 42:127-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nagle, D. L., M. A. Karim, E. A. Woolf, L. Holmgren, P. Bork, D. J. Misumi, S. H. McGrail, B. J. Dussault, Jr., C. M. Perou, R. E. Boissy, G. M. Duyk, R. A. Spritz, and K. J. Moore. 1996. Identification and mutation analysis of the complete gene for Chediak-Higashi syndrome. Nat. Genet. 14:307-311. [DOI] [PubMed] [Google Scholar]
- 193.Narchi, H. 2000. Ulcero-necrotic cutaneous lesions in an infant. Eur. J. Pediatr. 159:385-387. [DOI] [PubMed] [Google Scholar]
- 194.Nash, T. W., D. M. Libby, and M. A. Horwitz. 1984. Interaction between the legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone. J. Clin. Investig. 74:771-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nau, G. J., J. F. Richmond, A. Schlesinger, E. G. Jennings, E. S. Lander, and R. A. Young. 2002. Human macrophage activation programs induced by bacterial pathogens. Proc. Nat. Acad. Sci. USA 99:1503-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nauseef, W. M. 1988. Myeloperoxidase deficiency. Hematol. Oncol. Clin. North Am. 2:135-158. [PubMed] [Google Scholar]
- 197.Nauseef, W. M., B. D. Volpp, S. McCormick, K. G. Leidal, and R. A. Clark. 1991. Assembly of the neutrophil respiratory burst oxidase. Protein kinase C promotes cytoskeletal and membrane association of cytosolic oxidase components. J. Biol. Chem. 266:5911-5917. [PubMed] [Google Scholar]
- 198.Nauseef, W. M., S. Brigham, and M. Cogley. 1994. Hereditary myeloperoxidase deficiency due to a missense mutation of arginine 569 to tryptophan. J. Biol. Chem. 269:1212-1216. [PubMed] [Google Scholar]
- 199.Nestel, F. P., K. S. Price, T. A. Seemayer, and W. S. Lapp. 1992. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J. Exp. Med. 175:405-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Newman, S. L., C. Bucher, J. Rhodes, and W. E. Bullock. 1990. Phagocytosis of Histoplasma capsulatum yeasts and microconidia by human cultured macrophages and alveolar macrophages. Cellular cytoskeleton requirement for attachment and ingestion. J. Clin. Investig. 85:223-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Newport, M. J., C. M. Huxley, S. Huston, C. M. Hawrylowicz, B. A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941-1949. [DOI] [PubMed] [Google Scholar]
- 202.Nightingale, S. D., L. T. Byrd, P. M. Southern, J. D. Jockusch, S. X. Cal, and B. A. Wynne. 1992. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J. Infect. Dis. 165:1082-1085. [DOI] [PubMed] [Google Scholar]
- 203.Nogami, S., J. Watanabe, K. Nakagaki, K. Nakata, H. Suzuki, M. Fujisawa, T. Kodama, and S. Kojima. 1998. Involvement of macrophage scavenger receptors in protection against murine malaria. Am. J. Trop. Med. Hyg. 59:843-845. [DOI] [PubMed] [Google Scholar]
- 204.Nolan, C. M., and P. C. White, Jr. 1978. Treatment of typhoid carriers with amoxicillin. Correlates of successful therapy. JAMA 239:2352-2354. [DOI] [PubMed] [Google Scholar]
- 205.Ogura, Y., D. K. Bonen, N. Inohara, D. L. Nicolae, F. F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R. H. Duerr, J. P. Achkar, S. R. Brant, T. M. Bayless, B. S. Kirschner, S. B. Hanauer, G. Nunez, and J. H. Cho. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411:603-606. [DOI] [PubMed] [Google Scholar]
- 206.Ogura, Y., N. Inohara, A. Benito, F. F. Chen, S. Yamaoka, and G. Nunez. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J. Biol. Chem. 276:4812-4818. [DOI] [PubMed] [Google Scholar]
- 207.Ohga, S., K. Ikeuchi, R. Kadoya, K. Okada, C. Miyazaki, S. Suita, and K. Ueda. 1997. Intrapulmonary Mycobacterium avium infection as the first manifestation of chronic granulomatous disease. J. Infect. 34:147-150. [DOI] [PubMed] [Google Scholar]
- 208.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. de Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715-725. [DOI] [PubMed] [Google Scholar]
- 209.Orange, J. S., S. R. Brodeur, A. Jain, F. A. Bonilla, L. C. Schneider, R. Kretschmer, S. Nurko, W. L. Rasmussen, J. R. Kohler, S. E. Gellis, B. M. Ferguson, J. L. Strominger, J. Zonana, N. Ramesh, Z. K. Ballas, and R. S. Geha. 2002. Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations. J. Clin. Investig. 109:1501-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.O'Regan, S., A. J. Newman, and R. C. Graham. 1977. “Myelokathexis.” Neutropenia with marrow hyperplasia. Am. J. Dis. Child. 131:655-658. [DOI] [PubMed] [Google Scholar]
- 211.Orme, I. M., and F. M. Collins. 1983. Resistance of various strains of mycobacteria to killing by activated macrophages in vivo. J. Immunol. 131:1452-1454. [PubMed] [Google Scholar]
- 212.Ottenhoff, T. H., D. Kumararatne, and J. L. Casanova. 1998. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol. Today 19:491-494. [DOI] [PubMed] [Google Scholar]
- 213.Parham, C., M. Chirica, J. Timans, E. Vaisberg, M. Travis, J. Cheung, S. Pflanz, R. Zhang, K. P. Singh, F. Vega, W. To, J. Wagner, A. M. O'Farrell, T. McClanahan, S. Zurawski, C. Hannum, D. Gorman, D. M. Rennick, R. A. Kastelein, R. de Waal Malefyt, and K. W. Moore. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168:5699-5708. [DOI] [PubMed] [Google Scholar]
- 214.Parkos, C. A., R. A. Allen, C. G. Cochrane, and A. J. Jesaitis. 1987. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J. Clin. Investig. 80:732-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Parmley, R. T., M. Ogawa, C. P. Darby, Jr., and S. S. Spicer. 1975. Congenital neutropenia: neutrophil proliferation with abnormal maturation. Blood 46:723-734. [PubMed] [Google Scholar]
- 216.Parry, M. F., R. K. Root, J. A. Metcalf, K. K. Delaney, L. S. Kaplow, and W. J. Richar. 1981. Myeloperoxidase deficiency: prevalence and clinical significance. Ann. Intern. Med. 95:293-301. [DOI] [PubMed] [Google Scholar]
- 217.Paul-Eugene, N., D. Mossalayi, M. Sarfati, K. Yamaoka, J. P. Aubry, J. Y. Bonnefoy, B. Dugas, and J. P. Kolb. 1995. Evidence for a role of Fc epsilon RII/CD23 in the IL-4-induced nitric oxide production by normal human mononuclear phagocytes. Cell. Immunol. 163:314-318. [DOI] [PubMed] [Google Scholar]
- 218.Peerless, A. G., M. Liebhaber, S. Anderson, R. I. Lehrer, and E. R. Stiehm. 1985. Legionella pneumonia in chronic granulomatous disease. J. Pediatr. 106:783-785. [DOI] [PubMed] [Google Scholar]
- 219.Perou, C. M., K. J. Moore, D. L. Nagle, D. J. Misumi, E. A. Woolf, S. H. McGrail, L. Holmgren, T. H. Brody, B. J. Dussault, Jr., C. A. Monroe, G. M. Duyk, R. J. Pryor, L. Li, M. J. Justice, and J. Kaplan. 1996. Identification of the murine beige gene by YAC complementation and positional cloning. Nat. Genet. 13:303-308. [DOI] [PubMed] [Google Scholar]
- 220.Pettit, R. E., and K. G. Berdal. 1984. Chediak-Higashi syndrome. Neurologic appearance. Arch. Neurol. 41:1001-1002. [DOI] [PubMed] [Google Scholar]
- 221.Picard, C., C. Fieschi, F. Altare, S. Al-Jumaah, S. Al-Hajjar, J. Feinberg, S. Dupuis, C. Soudais, I. Z. Al-Mohsen, E. Genin, D. Lammas, D. S. Kumararatne, T. Leclerc, A. Rafii, H. Frayha, B. Murugasu, L. B. Wah, R. Sinniah, M. Loubser, E. Okamoto, A. Al-Ghonaium, H. Tufenkeji, L. Abel, and J. L. Casanova. 2002. Inherited interleukin-12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds. Am. J. Hum. Genet. 70:336-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pierce, M., S. Crampton, D. Henry, L. Heifets, A. LaMarca, M. Montecalvo, G. P. Wormser, H. Jablonowski, J. Jemsek, M. Cynamon, B. G. Yangco, G. Notario, and J. C. Craft. 1996. A randomized trial of clarithromycin as prophylaxis against disseminated Mycobacterium avium complex infection in patients with advanced acquired immunodeficiency syndrome. N. Engl. J. Med. 335:384-391. [DOI] [PubMed] [Google Scholar]
- 223.Pierre-Audigier, C., E. Jouanguy, S. Lamhamedi, F. Altare, J. Rauzier, V. Vincent, D. Canioni, J. F. Emile, A. Fischer, S. Blanche, J. L. Gaillard, and J. L. Casanova. 1997. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon gamma receptor deficiency. Clin. Infect. Dis. 24:982-984. [DOI] [PubMed] [Google Scholar]
- 224.Plaut, A. G. 1997. Trefoil peptides in the defense of the gastrointestinal tract. N. Engl. J. Med. 336:506-507. [DOI] [PubMed] [Google Scholar]
- 225.Playford, R. J., T. Marchbank, R. A. Goodlad, R. A. Chinery, R. Poulsom, and A. M. Hanby. 1996. Transgenic mice that overexpress the human trefoil peptide pS2 have an increased resistance to intestinal damage. Proc. Natl. Acad. Sci. USA 93:2137-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Plebani, A., A. Cantu-Rajnoldi, G. Collo, P. Allavena, A. Biolchini, A. Pirelli, M. Clerici Schoeller, and M. Masarone. 1988. Myelokathexis associated with multiple congenital malformations: immunological study on phagocytic cells and lymphocytes. Eur. J. Haematol. 40:12-17. [DOI] [PubMed] [Google Scholar]
- 227.Pocheville, I., C. Gutierrez, and J. Gonzalez. 1997. Aftas orales y esofagica en un nino con sida tratadas con exito con factores estimuladores de colonias (GM-CSF). Enferm. Infec. Microbiol. Clin. 15:439-440. [PubMed] [Google Scholar]
- 228.Pogrebniak, H. W., J. I. Gallin, H. L. Malech, A. R. Baker, C. A. Moskaluk, W. D. Travis, and H. I. Pass. 1993. Surgical management of pulmonary infections in chronic granulomatous disease of childhood. Ann. Thorac. Surg. 55:844-849. [DOI] [PubMed] [Google Scholar]
- 229.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in T1r4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 230.Qin, D., J. Wu, M. C. Carroll, G. F. Burton, A. K. Szakal, and J. G. Tew. 1998. Evidence for an important interaction between a complement-derived CD21 ligand on follicular dendritic cells and CD21 on B cells in the initiation of IgG responses. J. Immunol. 161:4549-4554. [PubMed] [Google Scholar]
- 231.Raj, P. A., and A. R. Dentino. 2002. Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol. Lett. 206:9-18. [DOI] [PubMed] [Google Scholar]
- 232.Ramirez-Duque, P., T. Arends, and F. Merino. 1982. Chediak-Higashi syndrome: description of a cluster in a Venezuelan-Andean isolated region. J. Med. 13:431-451. [PubMed] [Google Scholar]
- 233.Rausch, P. G., K. B. Pryzwansky, J. K. Spitznagel, and J. C. Herion. 1978. Immunocytochemical identification of abnormal polymorphonuclear neutrophils in patients with leukemia. Blood Cells 4:369-382. [PubMed] [Google Scholar]
- 234.Raymond, D. P., S. J. Pelletier, T. D. Crabtree, T. G. Gleason, L. L. Hamm, T. L. Pruett, and R. G. Sawyer. 2001. Impact of a rotating empiric antibiotic schedule on infectious mortality in an intensive care unit. Crit. Care Med. 29:1101-1108. [DOI] [PubMed] [Google Scholar]
- 235.Reeves, E. P., H. Lu, H. L. Jacobs, C. G. Messina, S. Bolsover, G. Gabella, E. O. Potma, A. Warley, J. Roes, and A. W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291-297. [DOI] [PubMed] [Google Scholar]
- 236.Repo, H., E. Savilahti, and M. Leirisalo-Repo. 1987. Aberrant phagocyte function in Shwachman syndrome. Clin. Exp. Immunol. 69:204-212. [PMC free article] [PubMed] [Google Scholar]
- 237.Rezende, S. A., V. R. Oliveira, A. M. Silva, J. B. Alves, A. M. Goes, and L. F. Reis. 1997. Mice lacking the gamma interferon receptor have an impaired granulomatous reaction to Schistosoma mansoni infection. Infect. Immun. 65:3457-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rivera-Matos, I. R., R. M. Rakita, M. M. Mariscalco, F. F. Elder, S. A. Dreyer, and T. G. Cleary. 1995. Leukocyte adhesion deficiency mimicking Hirschsprung disease. J. Pediatr. 127:755-757. [DOI] [PubMed] [Google Scholar]
- 239.Robertson, K. L., D. B. Drucker, J. James, A. S. Blinkhorn, S. Hamlet, and P. S. Bird. 2001. A microbiological study of Papillon-Lefevre syndrome in two patients. J. Clin. Pathol. 54:371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Roesler, J., B. Kofink, J. Wendisch, S. Heyden, D. Paul, W. Friedrich, J. L. Casanova, W. Leupold, M. Gahr, and A. Rosen-Wolff. 1999. Listeria monocytogenes and recurrent mycobacterial infections in a child with complete interferon-gamma-receptor (IFNgammaR1) deficiency: mutational analysis and evaluation of therapeutic options. Exp. Hematol. 27:1368-1374. [DOI] [PubMed] [Google Scholar]
- 241.Root, R. K., A. S. Rosenthal, and D. J. Balestra. 1972. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J. Clin. Investig. 51:649-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rosenberg, H. F., and J. I. Gallin. 1993. Neutrophil-specific granule deficiency includes eosinophils. Blood 82:268-273. [PubMed] [Google Scholar]
- 243.Rosenzweig, S., S. E. Dorman, J. Roesler, J. Palacios, M. Zelazko, and S. M. Holland. 2002. 561del4 defines a novel small deletion hotspot in the interferon-gamma receptor 1 chain. Clin. Immunol. 102:25-27. [DOI] [PubMed] [Google Scholar]
- 244.Rosh, J. R., H. B. Tang, L. Mayer, G. Groisman, S. K. Abraham, and A. Prince. 1995. Treatment of intractable gastrointestinal manifestations of chronic granulomatous disease with cyclosporine. J. Pediatr. 126:143-145. [DOI] [PubMed] [Google Scholar]
- 245.Ross, G. D., R. A. Thompson, M. J. Walport, T. A. Springer, J. V. Watson, R. H. Ward, J. Lida, S. L. Newman, R. A. Harrison, and P. J. Lachmann. 1985. Characterization of patients with an increased susceptibility to bacterial infections and a genetic deficiency of leukocyte membrane complement receptor type 3 and the related membrane antigen LFA-1. Blood 66:882-890. [PubMed] [Google Scholar]
- 246.Ruutu, P., E. Savilahti, H. Repo, and T. U. Kosunen. 1984. Constant defect in neutrophil locomotion but with age decreasing susceptibility to infection in Shwachman syndrome. Clin. Exp. Immunol. 57:249-255. [PMC free article] [PubMed] [Google Scholar]
- 247.Sakai, T., M. Matsuoka, M. Aoki, K. Nosaka, and H. Mitsuya. 2001. Missense mutation of the interleukin-12 receptor beta1 chain-encoding gene is associated with impaired immunity against Mycobacterium avium complex infection. Blood 97:2688-2694. [DOI] [PubMed] [Google Scholar]
- 248.Sammalkorpi, K., J. Lahdevirta, T. Makela, and T. Rostila. 1987. Treatment of chronic Salmonella carriers with ciprofloxacin. Lancet ii:164-165. [DOI] [PubMed] [Google Scholar]
- 249.Scharton-Kersten, T., L. C. Afonso, M. Wysocka, G. Trinchieri, and P. Scott. 1995. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J. Immunol. 154:5320-5330. [PubMed] [Google Scholar]
- 250.Schwalbe, R. A., B. Dahlback, J. E. Coe, and G. L. Nelsestuen. 1992. Pentraxin family of proteins interact specifically with phosphorylcholine and/or phosphorylethanolamine. Biochemistry 31:4907-4915. [DOI] [PubMed] [Google Scholar]
- 251.Segal, A. W. 1987. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature 326:88-91. [DOI] [PubMed] [Google Scholar]
- 252.Segal, B. H., E. S. DeCarlo, K. J. Kwon-Chung, H. L. Malech, J. I. Gallin, and S. M. Holland. 1998. Aspergillus nidulans infection in chronic granulomatous disease. Medicine 77:345-354. [DOI] [PubMed] [Google Scholar]
- 253.Segal, B. H., T. L. Leto, J. I. Gallin, H. L. Malech, and S. M. Holland. 2000. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine 79:170-200. [DOI] [PubMed] [Google Scholar]
- 254.Sengelov, H., P. Follin, L. Kjeldsen, K. Lollike, C. Dahlgren, and N. Borregaard. 1995. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J. Immunol. 154:4157-4165. [PubMed] [Google Scholar]
- 255.Shetty, A. K., A. M. Arvin, and K. M. Gutierrez. 1999. Nocardia farcinica pneumonia in chronic granulomatous disease. Pediatrics 104:961-964. [DOI] [PubMed] [Google Scholar]
- 256.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Siddiqui, F. H., J. J. Biundo, Jr., C. Moore, M. L. Ermitano, A. P. Ortigas, and F. DeFrancesch. 2000. Recombinant granulocyte macrophage colony stimulating factor (rhu-GM-CSF) in the treatment of extensive leg ulcers: a case report. Surgery 127:589-592. [DOI] [PubMed] [Google Scholar]
- 258.Skogberg, K., J. Syrjanen, M. Jahkola, O. V. Renkonen, J. Paavonen, J. Ahonen, S. Kontiainen, P. Ruutu, and V. Valtonen. 1992. Clinical presentation and outcome of listeriosis in patients with and without immunosuppressive therapy. Clin. Infect. Dis. 14:815-821. [DOI] [PubMed] [Google Scholar]
- 259.Smahi, A., G. Courtois, P. Vabres, S. Yamaoka, S. Heuertz, A. Munnich, A. Israel, N. S. Heiss, S. M. Klauck, P. Kioschis, S. Wiemann, A. Poustka, T. Esposito, T. Bardaro, F. Gianfrancesco, A. Ciccodicola, M. D'Urso, H. Woffendin, T. Jakins, D. Donnai, H. Stewart, S. J. Kenwrick, S. Aradhya, T. Yamagata, M. Levy, R. A. Lewis, D. L. Nelson, and The International Incontinentia Pigmenti (IP) Consortium. 2000. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. Nature 405:466-472. [DOI] [PubMed] [Google Scholar]
- 260.Smith, C. W., S. D. Marlin, R. Rothlein, C. Toman, and D. C. Anderson. 1989. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J. Clin. Investig. 83:2008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sorensen, R. U., M. R. Jacobs, and S. B. Shurin. 1985. Chromobacterium violaceum adenitis acquired in the northern United States as a complication of chronic granulomatous disease. Pediatr. Infect. Dis. J. 4:701-702. [DOI] [PubMed] [Google Scholar]
- 262.Speert, D. P., M. Bond, R. C. Woodman, and J. T. Curnutte. 1994. Infection with Pseudomonas cepacia in chronic granulomatous disease: role of nonoxidative killing by neutrophils in host defense. J. Infect. Dis. 170:1524-1531. [DOI] [PubMed] [Google Scholar]
- 263.Sponseller, P. D., H. L. Malech, E. F. McCarthy, Jr., S. F. Horowitz, G. Jaffe, and J. I. Gallin. 1991. Skeletal involvement in children who have chronic granulomatous disease. J. Bone Joint Surg. 73:37-51. [PubMed] [Google Scholar]
- 264.Stenger, S., and R. L. Modlin. 1999. T cell mediated immunity to Mycobacterium tuberculosis. Curr. Opin. Microbiol. 2:89-93. [DOI] [PubMed] [Google Scholar]
- 265.Strauss, R. G., K. E. Bove, J. F. Jones, A. M. Mauer, and V. A. Fulginiti. 1974. An anomaly of neutrophil morphology with impaired function. N. Engl. J. Med. 290:478-484. [DOI] [PubMed] [Google Scholar]
- 266.Tannenbaum, C. S., R. Tubbs, D. Armstrong, J. H. Finke, R. M. Bukowski, and T. A. Hamilton. 1998. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J. Immunol. 161:927-932. [PubMed] [Google Scholar]
- 267.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 268.Thomas, C., F. Le Deist, M. Cavazzana-Calvo, M. Benkerrou, E. Haddad, S. Blanche, W. Hartmann, W. Friedrich, and A. Fischer. 1995. Results of allogeneic bone marrow transplantation in patients with leukocyte adhesion deficiency. Blood 86:1629-1635. [PubMed] [Google Scholar]
- 269.Threlfall, E. J., L. R. Ward, J. A. Skinner, and B. Rowe. 1997. Increase in multiple antibiotic resistance in nontyphoidal salmonellas from humans in England and Wales: a comparison of data for 1994 and 1996. Microb. Drug Resist. 3:263-266.9270996 [Google Scholar]
- 270.Tinanoff, N., P. Tempro, and E. G. Maderazo. 1995. Dental treatment of Papillon-Lefevre syndrome: 15-year follow-up. J. Clin. Periodontol. 22:609-612. [DOI] [PubMed] [Google Scholar]
- 271.Todd, R. F., 3rd, and D. R. Freyer. 1988. The CD11/CD18 leukocyte glycoprotein deficiency. Hematol. Oncol. Clin. North Am. 2:13-31. [PubMed] [Google Scholar]
- 272.van der Sande, F. M., and H. F. Hillen. 1996. Correction of neutropenia following treatment with granulocyte colony-stimulating factor results in a decreased frequency of infections in Shwachman's syndrome. Neth. J. Med. 48:92-95. [DOI] [PubMed] [Google Scholar]
- 273.Velazco, C. H., C. Coelho, F. Salazar, A. Contreras, J. Slots, and J. J. Pacheco. 1999. Microbiological features of Papillon-Lefevre syndrome periodontitis. J. Clin. Periodontol. 26:622-627. [DOI] [PubMed] [Google Scholar]
- 274.Verhagen, C. E., T. de Boer, H. H. Smits, F. A. Verreck, E. A. Wierenga, M. Kurimoto, D. A. Lammas, D. S. Kumararatne, O. Sanal, F. P. Kroon, J. T. van Dissel, F. Sinigaglia, and T. H. Ottenhoff. 2000. Residual type 1 immunity in patients genetically deficient for interleukin 12 receptor beta1 (IL-12Rbeta1): evidence for an IL-12Rbeta1-independent pathway of IL-12 responsiveness in human T cells. J. Exp. Med. 192:517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Visser, G., J. Herwig, J. P. Rake, K. E. Niezen-Koning, A. J. Verhoeven, and G. P. Smit. 1998. Neutropenia and neutrophil dysfunction in glycogen storage disease type 1c. J. Inherited Metab. Dis. 21:227-231. [DOI] [PubMed] [Google Scholar]
- 276.Visser, G., J. P. Rake, J. Fernandes, P. Labrune, J. V. Leonard, S. Moses, K. Ullrich, and G. P. Smit. 2000. Neutropenia, neutrophil dysfunction, and inflammatory bowel disease in glycogen storage disease type Ib: results of the European Study on Glycogen Storage Disease type I. J. Pediatr. 137:187-191. [DOI] [PubMed] [Google Scholar]
- 277.von Planta, M., H. Ozsahin, H. Schroten, U. G. Stauffer, and R. A. Seger. 1997. Greater omentum flaps and granulocyte transfusions as combined therapy of liver abscess in chronic granulomatous disease. Eur. J. Pediatr. Surg. 7:234-236. [DOI] [PubMed] [Google Scholar]
- 278.Vouldoukis, I., D. Mazier, P. Debre, and M. D. Mossalayi. 1995. Nitric oxide and human infectious diseases. Res. Immunol. 146:689-692. [DOI] [PubMed] [Google Scholar]
- 279.Vouldoukis, I., V. Riveros-Moreno, B. Dugas, F. Ouaaz, P. Becherel, P. Debre, S. Moncada, and M. D. Mossalayi. 1995. The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the Fc epsilon RII/CD23 surface antigen. Proc. Natl. Acad. Sci. USA 92:7804-7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Walker, C. 1999. The supplemental use of antibiotics in periodontal therapy. Comp. Cont. Educ. Dent. 20:4-12. [PubMed] [Google Scholar]
- 281.Wang, C., L. Deng, M. Hong, G. R. Akkaraju, J. Inoue, and Z. J. Chen. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412:346-351. [DOI] [PubMed] [Google Scholar]
- 282.Wang, J., E. Guan, G. Roderiquez, and M. A. Norcross. 1999. Inhibition of CCR5 expression by IL-12 through induction of beta-chemokines in human T lymphocytes. J. Immunol. 163:5763-5769. [PubMed] [Google Scholar]
- 283.Warris, A., C. M. Weemaes, and P. E. Verweij. 2002. Multidrug resistance in Aspergillus fumigatus. N. Engl. J. Med. 347:2173-2174. [DOI] [PubMed] [Google Scholar]
- 284.Watson, R. W. 2002. Redox regulation of neutrophil apoptosis. Antiox. Redox Sig. 4:97-104. [DOI] [PubMed] [Google Scholar]
- 285.Weening, R. S., P. Kabel, P. Pijman, and D. Roos. 1983. Continuous therapy with sulfamethoxazole-trimethoprim in patients with chronic granulomatous disease. J. Pediatr. 103:127-130. [DOI] [PubMed] [Google Scholar]
- 286.Weiss, J., M. Victor, O. Stendhal, and P. Elsbach. 1982. Killing of gram-negative bacteria by polymorphonuclear leukocytes: role of an O2-independent bactericidal system. J. Clin. Investig. 69:959-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wendel, U., H. Schroten, S. Burdach, and V. Wahn. 1993. Glycogen storage disease type Ib: infectious complications and measures for prevention. Eur. J. Pediatr. 152(Suppl):S49-S51. [DOI] [PubMed] [Google Scholar]
- 288.Weston, B., R. A. Axtell, R. F. Todd, 3rd, M. Vincent, K. J. Balazovich, S. J. Suchard, and L. A. Boxer. 1991. Clinical and biologic effects of granulocyte colony stimulating factor in the treatment of myelokathexis. J. Pediatr. 118:229-234. [DOI] [PubMed] [Google Scholar]
- 289.Weston, B., R. F. Todd III, R. Axtell, K. Balazovich, J. Stewart, B. J. Locey, L. Mayo-Bond, P. Loos, R. Hutchinson, and L. A. Boxer. 1991. Severe congenital neutropenia: clinical effects and neutrophil function during treatment with granulocyte colony-stimulating factor. J. Lab. Clin. Med. 117:282-290. [PubMed] [Google Scholar]
- 290.White, J. G. 1966. The Chediak-Higashi syndrome: a possible lysosomal disease. Blood 28:143-156. [PubMed] [Google Scholar]
- 291.White, J. G., and C. C. Clawson. 1980. The Chediak-Higashi syndrome; the nature of the giant neutrophil granules and their interactions with cytoplasm and foreign particulates. I. Progressive enlargement of the massive inclusions in mature neutrophils. II. Manifestations of cytoplasmic injury and sequestration. III. Interactions between giant organelles and foreign particulates. Am. J. Pathol. 98:151-196. [PMC free article] [PubMed] [Google Scholar]
- 292.Wiekowski, M. T., M. W. Leach, E. W. Evans, L. Sullivan, S. C. Chen, G. Vassileva, J. F. Bazan, D. M. Gorman, R. A. Kastelein, S. Narula, and S. A. Lira. 2001. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J. Immunol. 166:7563-7570. [DOI] [PubMed] [Google Scholar]
- 293.Windhorst, D. B., A. S. Zelickson, and R. A. Good. 1968. A human pigmentary dilution based on a heritable subcellular structural defect--the Chediak-Higashi syndrome. J. Investig Dermatol. 50:9-18. [DOI] [PubMed] [Google Scholar]
- 294.Winkelstein, J. A., M. C. Marino, R. B. Johnston, J. Boyle, J. Curnutte, J. I. Gallin, H. L. Malech, S. M. Holland, H. Ochs, P. Quie, R. H. Buckley, C. B. Foster, S. J. Chanock, and H. Dickler. 2000. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine 79:155-169. [DOI] [PubMed] [Google Scholar]
- 295.Winkelstein, J. A., M. C. Marino, R. B. Johnston, Jr., J. Boyle, J. Curnutte, J. I. Gallin, H. L. Malech, S. M. Holland, H. Ochs, P. Quie, R. H. Buckley, C. B. Foster, S. J. Chanock, and H. Dickler. 2000. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine 79:155-169. [DOI] [PubMed] [Google Scholar]
- 296.Wright, S. D., P. S. Tobias, R. J. Ulevitch, and R. A. Ramos. 1989. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J. Exp. Med. 170:1231-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Yamamoto, A., S. Taniuchi, S. Tsuji, M. Hasui, and Y. Kobayashi. 2002. Role of reactive oxygen species in neutrophil apoptosis following ingestion of heat-killed Staphylococcus aureus. Clin. Exp. Immunol. 129:479-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Yasui, K., Y. Sekiguchi, M. Ichikawa, H. Nagumo, T. Yamazaki, A. Komiyama, and H. Suzuki. 2002. Granulocyte macrophage-colony stimulating factor delays neutrophil apoptosis and primes its function through Ia-type phosphoinositide 3-kinase. J. Leukoc. Biol. 72:1020-1026. [PubMed] [Google Scholar]
- 299.Yomtovian, R., J. Abramson, P. Quie, and J. McCullough. 1981. Granulocyte transfusion therapy in chronic granulomatous disease. Report of a patient and review of the literature. Transfusion 21:739-743. [DOI] [PubMed] [Google Scholar]
- 300.Zonana, J., M. E. Elder, L. C. Schneider, S. J. Orlow, C. Moss, M. Golabi, S. K. Shapira, P. A. Farndon, D. W. Wara, S. A. Emmal, and B. M. Ferguson. 2000. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO). Am. J. Hum. Genet. 67:1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zuany-Amorim, C., C. Manlius, A. Trifilieff, L. R. Brunet, G. Rook, G. Bowen, G. Pay, and C. Walker. 2002. Long-term protective and antigen-specific effect of heat-killed Mycobacterium vaccae in a murine model of allergic pulmonary inflammation. J. Immunol. 169:1492-1499. [DOI] [PubMed] [Google Scholar]
- 302.Zuany-Amorim, C., E. Sawicka, C. Manlius, A. Le Moine, L. R. Brunet, D. M. Kemeny, G. Bowen, G. Rook, and C. Walker. 2002. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat. Med. 8:625-629. [DOI] [PubMed] [Google Scholar]