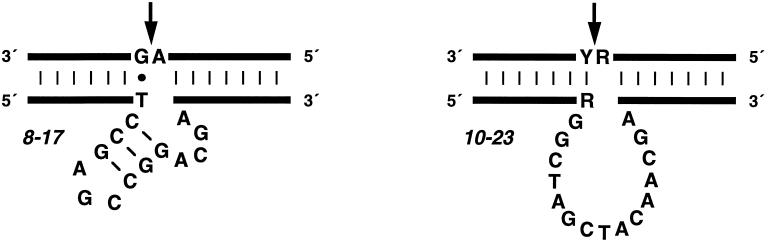Abstract
An in vitro selection procedure was used to develop a DNA enzyme that can be made to cleave almost any targeted RNA substrate under simulated physiological conditions. The enzyme is comprised of a catalytic domain of 15 deoxynucleotides, flanked by two substrate-recognition domains of seven to eight deoxynucleotides each. The RNA substrate is bound through Watson–Crick base pairing and is cleaved at a particular phosphodiester located between an unpaired purine and a paired pyrimidine residue. Despite its small size, the DNA enzyme has a catalytic efficiency (kcat/Km) of ≈109 M−1⋅min−1 under multiple turnover conditions, exceeding that of any other known nucleic acid enzyme. Its activity is dependent on the presence of Mg2+ ion. By changing the sequence of the substrate-recognition domains, the DNA enzyme can be made to target different RNA substrates. In this study, for example, it was directed to cleave synthetic RNAs corresponding to the start codon region of HIV-1 gag/pol, env, vpr, tat, and nef mRNAs.
Keywords: antisense, catalytic DNA, in vitro selection, RNA cleavage
DNA has long been regarded as a passive molecule, ideally suited for carrying genetic information but structurally monotonous and therefore functionally impoverished. After the discovery of catalytic RNA (1, 2), it became clear that nucleic acid molecules of a particular sequence and 3-dimensional structure are able to carry out specific chemical reactions, often with an efficiency comparable to that of protein enzymes (3). In recent years, the first examples of DNA enzymes have appeared, each having been obtained by in vitro selection methodology (4–7). Although it is remarkable that DNA can have catalytic activity, all of the DNA enzymes generated to date have little utility in a biological context. This is in contrast to some of the naturally occurring RNA enzymes, such as the “hammerhead” and “hairpin” ribozymes, which have been used to cleave and thereby inactivate target viral and messenger RNAs (reviewed in ref. 8).
We sought to develop a DNA enzyme that could be made to cleave almost any RNA substrate, efficiently and specifically under physiological conditions. Such a molecule could be used to inactivate a target RNA, probe a structured RNA, or assist in the manipulation of recombinant RNA. Compared with synthetic RNA enzymes, DNA enzymes are easier to prepare and less sensitive to chemical and enzymatic degradation. In a previous study, DNA was shown to catalyze the Mg2+-dependent cleavage of an RNA phosphoester embedded within an otherwise all-DNA substrate (6). Although that DNA enzyme was unable to cleave an all-RNA substrate, its properties suggested that a DNA enzyme with general purpose RNA cleavage activity might be attainable.
Using in vitro selection, we carried out an extensive search of DNA sequences, seeking molecules that best met the following criteria: (i) ability to cleave RNA with multiple turnover under simulated physiological conditions (e.g., 2 mM MgCl2/150 mM KCl, pH 7.5, 37°C); (ii) ability to recognize the RNA substrate through Watson–Crick base pairing; (iii) generalizability to other RNA substrates by changing the sequence of the substrate-recognition domain(s); (iv) catalytic efficiency meeting or exceeding that of comparable RNA enzymes; and (v) total composition of no more than 50 deoxynucleotide subunits (and preferably fewer). We obtained a variety of candidate DNA enzymes. These were examined individually, and the two most promising molecules were optimized using a combination of in vitro selection and enzyme engineering procedures. This resulted in a DNA enzyme comprised of ≈30 deoxynucleotides that met all of the above criteria.
MATERIALS AND METHODS
In Vitro Selection.
An initial library was generated by template-directed extension of 50 pmol of 5′-biotin-d(GGAAAAA)r(GUAACUAGAGAU)d(GGAAGAGATGGCGAC)-3′ on 100 pmol of 5′-GTGCCAAGCTTACCG-N50-GTCGCCATCTCTTCC-3′ (N = G, A, T, or C) in a 50-μl reaction mixture containing 10 units⋅μl−1 Superscript II reverse transcriptase (GIBCO/BRL), 3 mM MgCl2, 75 mM KCl, 50 mM Tris⋅HCl (pH 8.3), and 0.2 mM of each dNTP. A trace amount of 5′-32P-labeled primer was included in the reaction mixture to allow extension efficiency to be monitored. All components except reverse transcriptase were combined, incubated at 65°C for 5 min, and then cooled to 45°C over 10 min. Reverse transcriptase was added, and the mixture was incubated at 45°C for 45 min, then quenched by addition of Na2EDTA. NaCl was added to a final concentration of 1 M, and the extension products were immobilized by repeated passing through four streptavidin affinity columns (Genosys, The Woodlands, TX). The columns were washed with five 100-μl volumes of wash buffer (1 M NaCl/0.1 mM Na2EDTA/50 mM Tris⋅HCl, pH 7.5) followed by five 100-μl volumes of 0.1 N NaOH and five 100-μl volumes of wash buffer at 37°C and then eluted at 37°C over 1 h with three 20-μl aliquots of reaction buffer (10 mM MgCl2/1 M NaCl/50 mM Tris⋅HCl, pH 7.5). Eluted molecules were recovered and amplified by PCR (6) using the primers 5′-biotin-GGAAGAGATGGCGAC-3′ and 5′-GTGCCAAGCTTACCG-3′. The PCR products were immobilized on streptavidin columns, as above, which were washed with five 100-μl volumes of wash buffer and eluted with 40 μl of 0.1 N NaOH to obtain the nonbiotinylated strand. The isolated DNAs were ethanol precipitated and used as templates in a primer extension reaction to begin the next round of selection. Rounds 2–10 were carried out as above, except that the reaction scale was reduced 5-fold during the extension step and 2-fold during PCR.
Randomization and Reselection.
The reselections based on the 8-17 and 10-23 molecules involved six different lineages for each motif. Each lineage entailed 5–21 rounds of in vitro selection, differing with respect to the selection protocol and reaction times. All cleavage reactions were carried out in 2 mM MgCl2, 150 mM NaCl, and 50 mM Tris⋅HCl (pH 7.5) at 37°C. Reaction times varied from 60 min in early rounds to 1 min in later rounds. Each starting pool of templates was based on a sequence complementary to the prototype, with fixed binding arms of 7 nt each and a catalytic core randomized to 25% degeneracy at each nucleotide position. For the 8-17 and 10-23 motifs, the templates had the sequence 5′-gtgccaagcttaccgagtaactTCGTCCGGCTCGGRagatgggtcgtctgtccttccATCTCTAGTTACTTTTTC-3′ and 5′-gttgccaagcttaccgggaaaaaTCGTTGTAGCTAGCCtaactaggtcgtctgtccttccATCTCTAGTTACTTTTTC-3′, respectively (PCR primer sites in lower case; substrate-binding arms underlined; randomized positions italicized). The primer used in the template-directed extensions had the sequence 5′-biotin-r(GGAAAAAΔGUAACUAGAGAUGG)d(AAGAGATGGCGAC)-3′. The PCR primers for the 8-17-based selections were 5′-GTGCCAAGCTTACCGAGTAACT-3′ and 5′-d(GGAAGGACAGACGACCΔCATC)rU and for the 10-23-based selections were 5′-GTGCCAAGCTTACCGGGAAAAA-3′ and 5′-d(GGAAGGACAGACGACCTAGTT)rA. The PCR primers encompassed the binding arms, thus fixing these sequences. One of the PCR primers in each set contained a 3′-terminal ribonucleotide, allowing isolation of the template strand from the double-stranded PCR products by alkaline hydrolysis of the nontemplate strand (9) and subsequent purification by PAGE. A gel-based selection scheme was used in some of the lineages. In those cases, the PCR primers were 5′-biotin-GTGCCAAGCTTACCG-3′ and 5′-GAAAAAGTAACTAGAGATGGAAGGACAGACGACC-3′, and the extension reactions were carried out on the solid support using the primer 5′-r(GGAAAAAGUAACUAGAGAUGGAAG)-3′. A trace amount of [α-32P]-dATP was included in the mixture to label the extension products, which were eluted with alkali, purified by denaturing polyacrylamide gel purification, and recovered by electroelution. The molecules then were reacted, and those that underwent cleavage were isolated by gel electrophoresis.
Separation of Catalyst and Substrate.
After the 8th and 10th rounds of the initial selection, individual molecules were cloned and sequenced, as described (10). The 17th clone from round 8 (8-17) and 23rd clone from round 10 (10-23) had the sequence 5′-cacggttcgaΔatggcGTTATGCATCACACTATTTTTCATTGAAGCAGGCCGAGCCTTCCACCTTCcagcggtagΔagaagg-3′ and 5′-cacggttcgaatggcATGTTAAGTTCGTCCCTTTTTAGCAACATCGATCGGATTGGTTTCCCcagcggtagagaagg-3′, respectively (primer sites in lower case; substrate-binding arms underlined). For the intermolecular reaction, synthetic oligodeoxynucleotides were prepared based on the cloned sequences but lacking the regions outside of the substrate-binding arms. These were used to cleave an all-RNA substrate having the same sequence as the primer used to construct the initial library (see above). Subsequently, the substrate-binding arms of the DNA enzyme were reduced to 7 nt each and made perfectly complementary to the RNA substrate.
Analysis of Cleavage Products.
RNA substrate, prepared by in vitro transcription in the presence of [α-32P]-ATP, was cleaved by the corresponding DNA enzyme to generate two labeled products of the correct size, as judged by their electrophoretic mobility in comparison to standards that were prepared by partial alkaline hydrolysis of a 5′-32P-labeled substrate. The 5′ cleavage product could not be extended with [α-32P]-dATP and terminal deoxynucleotidyl transferase, consistent with the presence of a 2′, 3′, or 2′,3′-cyclic phosphate. The 3′ cleavage product was readily phosphorylated with [γ-32P]-ATP and T4 polynucleotide kinase, consistent with the presence of a free 5′ hydroxyl.
Kinetic Analysis.
All reported kinetic values were determined in multiple turnover reactions, typically exhibiting <20% variation for identical experiments performed on different days. Kinetic values obtained in single and multiple turnover experiments were similar; values obtained with synthetic RNA substrates were slightly less favorable than those obtained with in vitro-transcribed substrates. Reported kcat and Km values were determined from the y intercept and negative slope, respectively, of the best-fit line to a modified Eadie–Hofstee plot of kobs vs. kobs/[S]. Each plot consisted of 10 data points for a range of [S] that spanned Km, with [S] in ≥10-fold excess over [E]. kobs values were typically based on five data points obtained over the first 10% of the reaction. Substrate and enzyme molecules were preincubated separately for 10 min in reaction buffer, then combined to initiate the reaction. All reactions were carried out in the presence of 0.01% SDS to prevent adherence of material to the vessel walls. The pH was maintained by addition of 50 mM 4-(2-hydroxyethyl)-piperazine-1-propanesulfonic acid. Kinetic values did not depend on the identity of the buffer. Reaction products were separated by electrophoresis in a denaturing 20% polyacrylamide gel and were quantitated using a PhosphorImager (Molecular Dynamics).
RESULTS
In Vitro Selection.
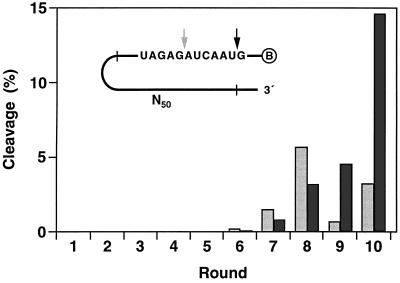
A search of random DNA sequences was undertaken, beginning with a library of 1014 different nucleic acid molecules. Each molecule contained a 5′ biotin moiety, followed (in a 5′ → 3′ direction) by a short oligodeoxynucleotide spacer, 12 target ribonucleotides, and 50 random deoxynucleotides surrounded by fixed-sequence deoxynucleotides (Fig. 1, Inset). These molecules were applied to a streptavidin-coated solid support and eluted with a solution containing 10 mM MgCl2 at pH 7.5 and 37°C. This resulted in cleavage of an RNA phosphoester within a small fraction of the bound molecules, releasing the 3′ cleavage product into the eluate. The released material was recovered and amplified by PCR, using the fixed regions surrounding the random region as sites for primer hybridization. From the PCR products, a “progeny” population was constructed, restoring the 5′ biotin, deoxynucleotide spacer, and embedded target ribonucleotides. This selective amplification procedure was repeated, progressively enriching the population with DNA sequences that best promoted the cleavage of an RNA phosphoester.
Figure 1.
In vitro selection of RNA-cleaving DNAs. A library of 1014 chimeric molecules was constructed (Inset), each containing a 5′ biotin (encircled B), 12 target ribonucleotides (sequence shown), and 50 random sequence deoxynucleotides (N50). Ten rounds of selective amplification yielded molecules that underwent phosphoester cleavage after either the first (dark arrow) or seventh (light arrow) target ribonucleotide. Self-cleavage activity was measured for each successive population. Dark and light bars correspond to cleavage at positions indicated by the dark and light arrows, respectively. Reaction conditions: 1 nM 5′-32P-labeled precursor, 10 mM MgCl2, and 1 M NaCl (pH 7.5) at 37°C for 2 h.
During the course of 10 rounds of selective amplification, two sites within the target domain emerged as the most favorable for cleavage, one dominating during rounds 6–8 and the other dominating during rounds 9 and 10 (Fig. 1). We cloned individual molecules from the population after the 8th and 10th rounds. Examination of a total of 62 cloned sequences revealed a variety of catalytic motifs, each of which promoted cleavage at one of the two favored sites within the ribonucleotide region. We surveyed the biochemical properties of many of these molecules and chose two for further analysis based on their catalytic activity and propensity to be generalized to other target sequences. Most of the selected motifs did not exhibit obvious Watson–Crick pairing between the catalytic and substrate domains. Motifs 8-17 and 10-23, however, had extensive complementarity to target nucleotides located both upstream and downstream of the cleavage site.
Refinement of Catalytic Motifs.
The 8-17 and 10-23 motifs were recast in an intermolecular cleavage format by separating the DNA catalytic domain from the RNA substrate (Fig. 2). Both catalysts directed the site-specific cleavage of the RNA substrate in a reaction that proceeded with multiple turnover under simulated physiological conditions (2 mM MgCl2/150 mM KCl, pH 7.5, 37°C; kcat ≈ 0.01 min−1). Cleavage occurred after an unpaired purine nucleotide of the substrate that was flanked by oligonucleotides complementary to the enzyme. The 5′ and 3′ cleavage products bore a 2′(3′) phosphate and 5′ hydroxyl, respectively (see Materials and Methods). The same termini are produced by several other RNA and DNA enzymes that cleave an RNA phosphoester (4, 6, 11–13), indicative of a reaction mechanism involving attack by a 2′ hydroxyl on an adjacent phosphate.
Figure 2.
Composition of the 8-17 and 10-23 catalytic motifs. The DNA enzyme (bottom strand) binds the RNA substrate (top strand) through Watson–Crick pairing. Cleavage occurs at the position indicated by the arrow. R = A or G; Y = U or C.
For both the 8-17 and 10-23 enzymes, the sequence of the substrate could be changed without loss of catalytic activity as long as the substrate-binding arms of the enzyme were changed in a complementary manner. The 8-17 enzyme had a special requirement for a rG-dT “wobble” pair located immediately downstream from the cleavage site. Substitution of a Watson–Crick pair at this position eliminated catalytic activity. The substrate-binding arms of the 10-23 enzyme interacted with the substrate entirely through standard Watson–Crick pairing. The catalytic core of the 8-17 and 10-23 enzymes, located between the two substrate-binding arms, contained 13 and 15 deoxynucleotides, respectively.
To define more precisely the sequence requirements of the catalytic core, we generated a library of 1014 variants of each motif, introducing random mutations at a frequency of 25% per nucleotide position throughout the core. Each library was subjected to six different in vitro selection protocols, involving a total of 52 rounds of selective amplification. The method and stringency of selection were varied to conduct a thorough examination of sequences related to the two prototype molecules (see Materials and Methods). Individuals from the selected populations were cloned, sequenced, and tested for catalytic activity.
In the case of the 8-17 enzyme, sequence variation among the cloned individuals suggested that the catalytic core consisted of a short internal stem-loop followed by an unpaired region of 4–5 nt (Fig. 2). The stem always contained 3 bp, at least 2 of which were G-C. The loop was invariant, having the sequence 5′-AGC-3′. Synthetic constructs in which the stem was lengthened or the sequence of the loop was altered did not exhibit catalytic activity. The unpaired region, connecting the 3′ half of the stem to the downstream substrate-binding domain, had the sequence 5′-WCGR-3′ or 5′-WCGAA-3′ (W = A or T; R = A or G). Variants having the sequence 5′-TCGAA-3′ in this region exhibited the highest level of catalytic activity, but this enhancement relative to the 8-17 enzyme was not generalizable to other substrate sequences.
The 10-23 enzyme was almost completely intolerant of variation. The eighth nucleotide position of the catalytic core was found to occur as either T, C, or A although a T at this position (as in the prototype) provided the highest level of activity. A survey of several different combinations of RNA substrate and corresponding DNA enzyme revealed that the 10-23 motif was highly generalizable with respect to substrate sequence. Cleavage occurred on the 3′ side of a single unpaired nucleotide, preferably a purine, that was followed by a pyrimidine. Target sites surrounded by A and U were cleaved most efficiently, with a catalytic rate of ≈0.1 min−1 under simulated physiological conditions.
Application to HIV-1 Targets.
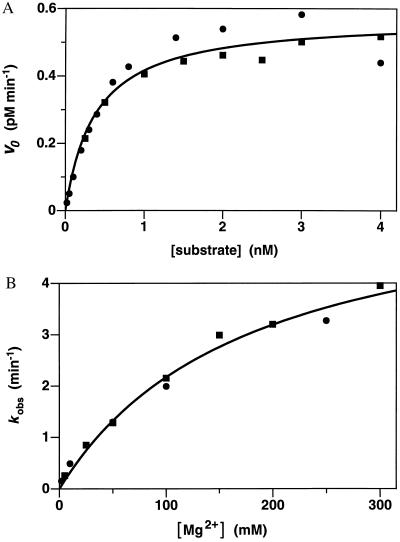
A DNA enzyme that cleaves RNA at an A⋅U site could, in principle, be used to target any mRNA start codon (A⋅UG). As a test case, we prepared both synthetic and in vitro-transcribed versions of a 17-mer RNA corresponding to the translation initiation region of HIV-1 gag/pol mRNA (5′-GGAGAGAGA⋅UGGGUGCG-3′). Both versions of the substrate were cleaved at the expected position by the corresponding 10-23 DNA enzyme in a reaction that proceeded with a kcat of 0.15 min−1 and Km of 0.47 nM under simulated physiological conditions (catalytic efficiency, kcat/Km = 3.2 × 108 M−1⋅min−1) (Fig. 3A). The catalytic rate increased with increasing MgCl2 concentration, with an apparent Km for Mg2+ of 180 mM at pH 7.5 and 37°C (Fig. 3B). The catalytic rate increased in a roughly log–linear fashion with increasing pH over the range 7.0–8.5 (data not shown), consistent with a reaction mechanism involving deprotonation of the 2′ hydroxyl that lies adjacent to the cleaved phosphoester. In the presence of 50 mM MgCl2 at pH 8.0 and 37°C, conditions that might be used in the laboratory manipulation of RNA, kcat was 3.4 min−1 and Km was 0.76 nM (Fig. 4). The catalytic efficiency of the 10-23 DNA enzyme, under both physiological and laboratory conditions, compares favorably with that of known RNA-cleaving RNA enzymes (14, 15). Compared with the protein enzyme ribonuclease A (16), the DNA enzyme has ≈104-fold lower kcat but ≈105-fold more favorable Km (Table 1).
Figure 3.
Catalytic activity of the 10-23 DNA enzyme under multiple turnover conditions. (A) Initial velocities were measured over the first 10% of the reaction, using a fixed concentration of enzyme (0.004 nM) and varying concentrations of substrate (0.02–4 nM). The 17-mer RNA substrate, corresponding to the start codon region of HIV-1 gag/pol mRNA, was prepared by in vitro transcription. Reaction conditions: 2 mM MgCl2 and 150 mM NaCl (pH 7.5) at 37°C. Data from two independent experiments are shown and were fit to the Michaelis–Menten equation: v = kcat [E]/(Km + [S]). (B) Catalytic rates were determined in the presence of saturating substrate (100 nM) and varying concentrations of MgCl2 (2–300 mM). Reaction conditions were otherwise as above. Data from two independent experiments are shown and were fit to the Michaelis–Menten equation to obtain the apparent Km for Mg2+.
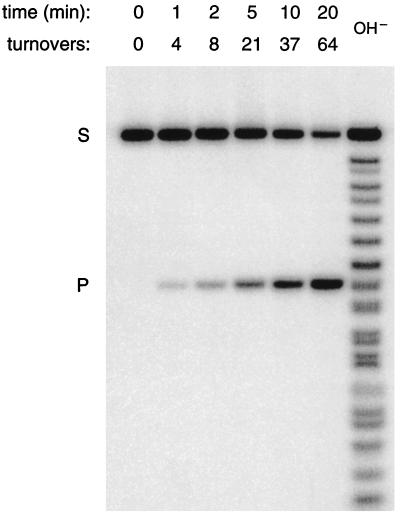
Figure 4.
DNA-catalyzed cleavage of RNA under laboratory conditions. 5′-32P-labeled RNA substrate (S), having the sequence 5′-GGAGAGAGA⋅UGGGUGCG-3′, was cleaved by the corresponding 10-23 DNA enzyme to generate a single-labeled product (P). Reaction conditions: 1 μM substrate, 10 nM enzyme, and 50 mM MgCl2 (pH 8.0) at 37°C; sampled at 0, 1, 2, 5, 10, and 20 min. Reaction products were separated by electrophoresis in a denaturing 20% polyacrylamide gel, an autoradiogram of which is shown. The ladder at the right was produced by partial alkaline hydrolysis of the substrate; at short oligonucleotide lengths, products terminated by a 2′ or 3′ phosphate have slightly faster mobility compared with those with a 2′,3′-cyclic phosphate.
Table 1.
Catalytic activity of various RNA-cleaving enzymes
| Enzyme | kcat, min−1 | Km, M | kcat/Km, M−1·min−1 |
|---|---|---|---|
| Ribonuclease A* | |||
| UpA substrate | 8.4 × 104 | 6.2 × 10−4 | 1.4 × 108 |
| Poly(C) substrate | 3.1 × 104 | 3.4 × 10−5 | 9.0 × 108 |
| Hairpin ribozyme† | 2.1 × 100 | 2.8 × 10−8 | 7.5 × 107 |
| Hammerhead ribozyme‡ | 1.4 × 100 | 4.9 × 10−8 | 2.9 × 107 |
| 10-23 DNA enzyme§ | 3.4 × 100 | 7.6 × 10−10 | 4.5 × 109 |
The 10-23 enzyme can be used to cleave a variety of biologically relevant RNAs. We prepared synthetic RNA substrates corresponding to 15–17 nt surrounding the translation initiation site of HIV-1 gag/pol, env, vpr, tat, and nef mRNA. Each was cleaved at the expected position by a synthetic DNA enzyme that contained the 10-23 catalytic core flanked by substrate-binding arms of 7 or 8 nt each (Table 2). In all cases, the catalytic rate was ≈0.1 min−1 under simulated physiological conditions. The value for Km, however, varied with the nucleotide composition of the substrate. For the guanosine-rich gag/pol substrate, Km was <1 nM when either the 7- or 8-nt substrate-binding arms were used. The env and vpr substrates were cleaved with a much less favorable Km when the 7-nt binding arms were used, but Km improved substantially when the arms were increased to 8 nt each. Previous experience with RNA-cleaving RNA enzymes suggests that the optimal arm length will vary depending on the target sequence and reaction conditions (18–20).
Table 2.
DNA-catalyzed cleavage of HIV-1 mRNA substrates under simulated physiological conditions
| Target | Substrate | Arm length | kcat, min−1 | Km, nM |
|---|---|---|---|---|
| gag/pol | (G)GAGAGAGA·UGGGUGC(G) | 7 + 7 | 0.1 | 0.9 |
| 8 + 8 | 0.1 | 0.7 | ||
| env | (C)AGUGGCAA·UGAGAGU(G) | 7 + 7 | 0.03 | 900 |
| 8 + 8 | 0.04 | 9 | ||
| vpr | (G)AGGAUAGA·UGGAACA(A) | 7 + 7 | 0.08 | 500 |
| 8 + 8 | 0.1 | 20 | ||
| tat | GCAAGAAA·UGGAGCC | 7 + 7 | 0.04 | 300 |
| nef | CUAUAAGA·UGGGUGA | 7 + 7 | 0.05 | 900 |
Kinetic values were obtained under multiple turnover conditions, with synthetic RNA substrate in >10-fold excess over synthetic DNA enzyme. Reaction conditions: 2 mM MgCl2 and 150 mM NaCl (pH 7.5), 37°C. Substrate sequences correspond to 15 or 17 nt surrounding various mRNA start codons (underlines) of the BH10 clone of HIV-1 (17).
DISCUSSION
It is remarkable that a 15-mer oligodeoxynucleotide can specify the catalytic core of a general purpose RNA-cleaving DNA enzyme. Might such a simple motif exist in nature, perhaps within the genome of a single-stranded DNA virus? A search of the available sequence database did not reveal either the 8-17 or 10-23 motif. However, the vast majority of sequences in the database are derived from double-stranded DNA, which is not expected to have enzymatic activity. Furthermore, the 8-17 and 10-23 motifs are but two of many motifs that were isolated by our in vitro selection procedure and thus need not resemble those that occur in biological systems.
The catalytic efficiency (kcat/Km) of the 10-23 enzyme is ≈109 M−1⋅min−1, measured under either single or multiple turnover conditions. In the multiple turnover experiments, initial rates were measured over the first 10% of the reaction, which involved up to 100 turnovers of the enzyme (Fig. 3A). Thus, the rate-limiting step of the reaction is not the release of the cleavage products. With subsaturating RNA substrate, the rate-limiting step is binding. The second-order rate constant of 109 M−1⋅min−1 is consistent with the rate of helix formation between two oligonucleotides, estimated to be 107-109 M−1⋅min−1 (21–23).
The Km of the 10-23 enzyme is remarkably sensitive to the length and composition of the substrate-binding arms (Table 2). This can be rationalized by considering the thermodynamic stability of the relevant RNA–DNA heteroduplexes. Assuming that the bulged adenosine of the substrate and the catalytic core of the enzyme have a constant effect on the stability of the flanking duplexes, the predicted stability (24) of the various enzyme-substrate complexes has the order: gag/pol 8 + 8 > env 8 + 8 and gag/pol 7 + 7 > vpr 8 + 8 > env 7 + 7 and vpr 7 + 7. This order mirrors that of the corresponding values for Km. The gag/pol enzyme with 7-nt binding arms is expected to bind the substrate very tightly and does not benefit when the arms are lengthened to 8 nt each. On the other hand, the env and vpr enzymes with 7-nt arms bind the substrate much less tightly, allowing substantial improvement in Km when the arms are increased to 8 nt. Further lengthening of the binding arms might eventually be counterproductive because it would slow the rate of product release and thus reduce the catalytic rate under multiple turnover conditions.
The 10-23 DNA enzyme can be made to cleave almost any target RNA that contains a purine–pyrimidine junction. Synthetic DNAs of the length necessary to specify this motif are inexpensive and readily obtainable. We expect that the 10-23 enzyme will have utility in the laboratory manipulation of RNA, acting as a sequence-specific endoribonuclease. A more intriguing possibility is that it could be used to inactivate target cellular RNAs, similar to “antisense” oligodeoxynucleotides. Antisense compounds recognize a target viral or mRNA through Watson–Crick base pairing and rely on cellular ribonuclease H or other host factors to inactivate the target (25–28). An RNA-cleaving DNA enzyme, in contrast, is responsible for both target recognition and cleavage and operates with catalytic turnover. The 10-23 enzyme has a catalytic rate of ≈0.1 min−1 and Km < 1 nM under simulated physiological conditions. Given the opportunity to express these properties, it may prove capable of playing an active role in cellular biochemistry.
Acknowledgments
We thank Ronald Breaker for helpful advice and Dan Herschlag for comments on the manuscript. This work was supported by the National Institutes of Health, the R. W. Johnson Pharmaceutical Research Institute, and the Corbin Foundation for Molecular Biology Research.
References
- 1.Kruger K, Grabowski P J, Zaug A J, Sands J, Gottschling D E, Cech T R. Cell. 1982;31:147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- 2.Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. Cell. 1983;35:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 3.Herschlag D, Cech T R. Biochemistry. 1990;29:10159–10171. doi: 10.1021/bi00496a003. [DOI] [PubMed] [Google Scholar]
- 4.Breaker R R, Joyce G F. Chem Biol. 1994;1:223–229. doi: 10.1016/1074-5521(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 5.Cuenoud B, Szostak J W. Nature (London) 1995;375:611–614. doi: 10.1038/375611a0. [DOI] [PubMed] [Google Scholar]
- 6.Breaker R R, Joyce G F. Chem Biol. 1995;2:655–660. doi: 10.1016/1074-5521(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Sen D. Nat Struct Biol. 1996;3:743–747. doi: 10.1038/nsb0996-743. [DOI] [PubMed] [Google Scholar]
- 8.Christoffersen R E, Marr J J. J Med Chem. 1995;38:2023–2037. doi: 10.1021/jm00012a001. [DOI] [PubMed] [Google Scholar]
- 9.Walder R Y, Hayes J R, Walder J A. Nucleic Acids Res. 1993;21:4339–4343. doi: 10.1093/nar/21.18.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsang J, Joyce G F. Biochemistry. 1994;33:5966–5973. doi: 10.1021/bi00185a038. [DOI] [PubMed] [Google Scholar]
- 11.Dahm S C, Derrick W B, Uhlenbeck O C. Biochemistry. 1993;32:13040–13045. doi: 10.1021/bi00211a013. [DOI] [PubMed] [Google Scholar]
- 12.Wu H-N, Lin Y-J, Lin F-P, Makino S, Chang M-F, Lai M M C. Proc Natl Acad Sci USA. 1989;86:1831–1835. doi: 10.1073/pnas.86.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowrira B M, Burke J M. Biochemistry. 1991;30:8518–8522. doi: 10.1021/bi00099a003. [DOI] [PubMed] [Google Scholar]
- 14.Hampel A, Tritz R. Biochemistry. 1989;28:4929–4933. doi: 10.1021/bi00438a002. [DOI] [PubMed] [Google Scholar]
- 15.Fedor M J, Uhlenbeck O C. Biochemistry. 1992;31:12042–12054. doi: 10.1021/bi00163a012. [DOI] [PubMed] [Google Scholar]
- 16.delCardayré S B, Raines R T. Biochemistry. 1994;33:6031–6037. doi: 10.1021/bi00186a001. [DOI] [PubMed] [Google Scholar]
- 17.Ratner L, Haseltine W, Patarca R, Livak K J, Starcich B, Josephs S F, Doran E R, Rafalski J A, Whitehorn E A, Baumeister K, Ivanoff L, Petteway Jr S R, Pearson M L, Lautenberger J A, Papas T S, Ghrayeb J, Chang N T, Gallo R C, Wong-Staal F. Nature (London) 1985;313:277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- 18.Werner M, Uhlenbeck O C. Nucleic Acids Res. 1995;23:2092–2096. doi: 10.1093/nar/23.12.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hegg L A, Fedor M J. Biochemistry. 1995;34:15813–15828. doi: 10.1021/bi00048a027. [DOI] [PubMed] [Google Scholar]
- 20.Hertel K J, Herschlag D, Uhlenbeck O C. EMBO J. 1996;15:3751–3757. [PMC free article] [PubMed] [Google Scholar]
- 21.Pörschke D, Eigen M. J Mol Biol. 1971;62:361–381. doi: 10.1016/0022-2836(71)90433-5. [DOI] [PubMed] [Google Scholar]
- 22.Craig M E, Crothers D M, Doty P. J Mol Biol. 1971;62:383–401. doi: 10.1016/0022-2836(71)90434-7. [DOI] [PubMed] [Google Scholar]
- 23.Nelson J W, Tinoco I. Biochemistry. 1982;21:5289–5295. doi: 10.1021/bi00264a026. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto N, Nakano S, Katoh M, Matsumura A, Nakamuta H, Ohmichi T, Yoneyama M, Sasaki M. Biochemistry. 1995;34:11211–11216. doi: 10.1021/bi00035a029. [DOI] [PubMed] [Google Scholar]
- 25.Zamecnik P C, Stephenson M L. Proc Natl Acad Sci USA. 1978;75:280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dash P, Lotan I, Knapp M, Kandel E R, Goelet P. Proc Natl Acad Sci USA. 1987;84:7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agrawal S, Tang J. Antisense Res Dev. 1992;2:261–266. doi: 10.1089/ard.1992.2.261. [DOI] [PubMed] [Google Scholar]
- 28.Stein C A, Cheng Y-C. Science. 1993;261:1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]