Abstract
The innate immune system relies on a vast array of non-clonally expressed pattern recognition receptors for the detection of pathogens. Pattern recognition receptors bind conserved molecular structures shared by large groups of pathogens, termed pathogen-associated molecular patterns. The Toll-like receptors (TLRs) are a recently discovered family of pattern recognition receptors which show homology with the Drosophila Toll protein and the human interleukin-1 receptor family. Engagement of different TLRs can induce overlapping yet distinct patterns of gene expression that contribute to an inflammatory response. The TLR family is characterized by the presence of leucine-rich repeats and a Toll/interleukin-1 receptor-like domain, which mediate ligand binding and interaction with intracellular signaling proteins, respectively. Most TLR ligands identified so far are conserved microbial products which signal the presence of an infection, but evidence for some endogenous ligands that might signal other danger conditions has also been obtained. Molecular mechanisms for pathogen-associated molecular pattern recognition still remain elusive but seem to be more complicated than initially anticipated. In most cases, direct binding of microbial ligands to TLRs still has to be demonstrated. Moreover, Drosophila TLRs bind endogenous ligands, generated through a proteolytic cascade in response to an infection. In the case of endotoxin, recognition involves a complex of TLR4 and a number of other proteins. Moreover, TLR heterodimerization further extends the spectrum of ligands and modulates the response towards specific ligands. The fact that TLR expression is regulated in both a cell type- and stimulus-dependent fashion further contributes to the complexity.
INTRODUCTION
Vertebrate immunity can be broadly categorized into adaptive and innate immunity (28). Adaptive immune responses are mediated by clonally distributed B and T lymphocytes and are characterized by specificity and memory. Recognition relies on the generation of a random and highly diverse repertoire of antigen receptors, the T- and B-cell receptors, followed by clonal selection and expansion of receptors with relevant specificities. This mechanism accounts for the generation of immunological memory, an important advantage, but has the main limitation that specific clones need to expand and differentiate into effector cells before they can participate in host defense. Therefore, adaptive immune responses are typically delayed for 4 to 7 days (28).
To control the infection during the first days, our body relies on the evolutionarily ancient and more universal innate immune system. Its main functions include opsonization, activation of complement and coagulation cascades, phagocytosis, activation of proinflammatory signaling cascades, and apoptosis (for a review, see reference 48). The innate immune system also has an important function in activation and shaping of the adaptive immune response through the induction of costimulatory molecules and cytokines (49). In contrast to the clonotypic receptors, expressed by B and T lymphocytes, the innate immune system uses nonclonal sets of recognition molecules, called pattern recognition receptors. Pattern recognition receptors bind conserved molecular structures found in large groups of pathogens, termed pathogen-associated molecular patterns (49). There are various groups of pattern recognition receptors, which can be secreted, expressed om the cell surface, or resident in intracellular compartments (48). The Toll-like receptors (TLRs) are one of the most important pattern recognition receptor families and are the main topic of this review.
TLR FAMILY CHARACTERISTICS
The first member of the TLR family identified was a Drosophila protein implicated in dorsoventral patterning during embryonal development (19). Gay and Keith (13) were the first to realize that the intracellular domain of Drosophila Toll showed striking similarities to the intracellular domain of the mammalian interleukin-1 (IL-1) receptor, and Lemaitre et al. (40) demonstrated that Drosophila Toll was also involved in the immune response of the adult fly. Different human homologues of Drosophila Toll were identified and shown to induce activation of the transcription factor nuclear factor-κB (NF-κB) upon overexpression, revealing that TLRs and IL-1 receptors trigger similar signal transduction cascades (50, 67). In 1998, Poltorak et al. (61) discovered by positional cloning that the lps gene in the lipopolysaccharide (LPS)-nonresponsive mouse strain CH3/HeJ encoded a murine member of the TLR family, providing the first clue of a function as pattern recognition receptors for mammalian TLRs.
TLRs are evolutionarily conserved proteins (the oldest TLR identified so far is expressed in Caenorhabditis elegans [66]), characterized by an extracellular leucine-rich repeat domain and an intracellular Toll/IL-1 receptor-like (TIR) domain (50). Leucine-rich repeats are found in both cytoplasmic and transmembrane proteins and are involved in ligand recognition and signal transduction (35). How leucine-rich repeats mediate ligand recognition is still puzzling, especially as it was demonstrated that 7 out of 10 leucine-rich repeat motifs of the CD14 receptor, a transmembrane protein implicated in LPS recognition, could be deleted without affecting LPS binding (30). Furthermore, each TLR can recognize the most diverse ligands, lacking any structural similarity, making it hard to conceive how one motif can interact with all these molecules (see below).
The intracellular domain of the TLRs, the TIR domain, is a conserved protein-protein interaction module which is also found in a number of transmembrane and cytoplasmic proteins in plants, worms, arthropods, and even bacteria. Interestingly, all these TIR-containing proteins seem to have a function in host defense, making the TIR domain one of the earliest signaling motifs to evolve (4). The region of homology is confined to three conserved boxes containing amino acids crucial for signaling (69). An extending loop in box 2, encompassing an RDxϕ1ϕ2G motif (where x represents any amino acid and ϕ represents a hydrophobic residue) mediates interaction with the downstream adaptor protein MyD88 (84). The LPSd nonresponder phenotype of CH3/HeJ mice results from a Pro→His mutation at the ϕ2 position in this loop in the TIR domain of TLR4, which impairs interaction with the adaptor signaling protein MyD88, resulting in abrogation of the LPS response (84). The ϕ2 proline residue is conserved in all TLRs except TLR3 (33), where it is replaced with another hydrophobic residue.
TLR SIGNALING
At present, significant efforts are focused on characterizing the complex signal transduction cascades that are activated by TLRs. The transcription factor NF-κB is a pivotal regulator of the inducible expression of key proinflammatory mediators that contribute to an immune response. NF-κB is a hetero- or homodimeric transcription factor which binds to the promoter of a wide range of different target genes (for a review, see reference 16). NF-κB dimers are kept inactive through sequestering in the cytoplasm via binding to IκB proteins, which mask their nuclear localization signal and prevent their nuclear translocation. TLR signaling cascades lead to the phosphorylation of IκB, which targets this protein for ubiquitination and proteasomal degradation, leading to the release of NF-κB dimers. Inducible phosphorylation of IκB is mediated primarily by the IκB kinase complex, a large multisubunit complex consisting of at least two catalytic subunits and a regulatory subunit. Induction of NF-κB-dependent gene expression is central to the development of a strong proinflammatory response. Many of the genes activated by NF-κB are themselves upstream activators of NF-κB, further amplifying the host defense response to microbial challenge. Proinflammatory gene expression by TLRs is also regulated by activation of mitogen-activated protein kinases, leading to the phosphorylation of multiple proteins, including several transcription factors.
TLRs rely on the recruitment and activation of intracellular adaptor molecules and kinases to transduce their signals (Fig. 1). For example, the TIR domain of the adaptor molecule MyD88 associates with the TIR domain of all TLRs and is required in most cases for signaling to NF-κB/mitogen-activated protein kinase pathways (for a review, see reference 29). MyD88 recruits IL-1 receptor-associated kinase, which then induces activation of tumor necrosis factor receptor-associated factor 6 and finally NF-κB and mitogen-activated protein kinases. Although MyD88 is a universal adaptor protein for all TLRs, recent studies revealed the existence of at least three other adaptor proteins, some of which can be used in a TLR-specific way, indicating that the signaling pathways through individual TLRs might differ from each other and thereby result in different biological responses (for a review, see reference 57).
FIG. 1.
Short overview of a TLR signaling cascade. TLR signaling relies on the function of the adaptor protein MyD88, which presumably acts in conjunction with other TLR-specific adaptor proteins, such as Tollip and Mal. These adaptor proteins are necessary for the recruitment and activation of different IL-1 receptor-associated kinase family proteins, which further transmit the signal. This leads to activation of the IκB kinase complex and mitogen-activated protein kinases (c-Jun N-terminal kinase/p38), which induce NF-κB and AP-1-dependent gene transcription, respectively. IKK, IκB kinase complex; IRAK, IL-1 receptor-associated kinase; JNK, c-Jun N-terminal kinase; MKK, mitogen-activated protein kinase kinase; P, phosphate; Ub, ubiquitin.
LIGAND RECOGNITION BY TLRS
Pathogen-Associated Molecular Patterns
TLRs, like other pattern recognition receptors, recognize so-called pathogen-associated molecular patterns, which are conserved motifs that are unique to microorganisms and are essential for their metabolism and thus survival (49). This has three major advantages. First, pathogen-associated molecular patterns are produced only by microbes and not by host cells, enabling the innate immune system to distinguish between self and nonself. Second, as pathogen-associated molecular patterns are essential for microbial survival, mutations in or loss of patterns can be lethal, and therefore these patterns are not subject to high mutation rates. And third, pathogen-associated molecular patterns are invariant between microorganisms of a given class, which implies that only a limited number of germ line-encoded pattern recognition receptors are needed to detect the presence of a microbial infection (49).
TLRs Implicated in Recognition of Pathogens
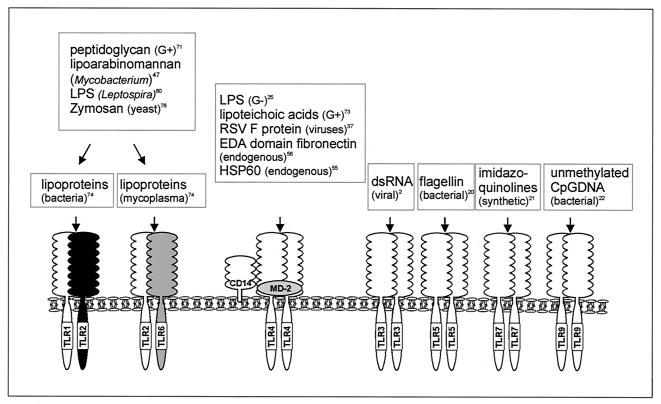
Ten different TLRs which mediate recognition of diverse classes of pathogens have been identified in humans (9, 11, 12, 50, 67, 73) (for an overview of the most important ligands, see Fig. 2). Many of the TLR ligands were identified through screening of large numbers of known pathogen-associated molecular patterns in human embryonal kidney HEK293T cells transiently transfected with one of the TLRs. HEK293T cells provide a valuable transfection model for these studies as they almost completely lack expression of any of the TLRs (24). More recently, gene disruption studies of the different TLR genes confirmed most of the results obtained in HEK293T cells. However, impurities in the commercially available pathogen-associated molecular pattern preparations have been shown to be a problem for the correct interpretation of the data. Thus, there has been confusion if TLR2 is also implicated in LPS signaling, as HEK293T cells overexpressing TLR2 induced NF-κB signaling upon LPS triggering (85), although TLR2-negative cells were still responsive to LPS (71). Repurification of the commercially available LPS preparations eliminated LPS signaling through TLR2, showing that TLR2 was probably responding to the lipoprotein contaminants and not to LPS itself (23).
FIG. 2.
Ligand specificities of TLRs. Ten different mammalian TLRs have been described, but as yet no function is known for TLR8 and TLR10 (see the text). TLR1 and TLR6 do not signal as separate entities but act in cooperation with TLR2. TLR4 acts in a complex with several other molecules, such as CD14 and MD-2. TLR3, TLR5, and TLR9 exhibit the narrowest ligand specificity. No natural ligands have been described yet for TLR7. LPS, lipopolysaccharide; RSV, respiratory syncytial virus; EDA, extra domain A; HSP60, heat shock protein 60; dsRNA, double-stranded RNA. References are indicated.
From all these data, it is now clear that one group of pathogens is not exclusively recognized by one TLR (e.g., both TLR2 and TLR4 recognize gram-positive organism-derived pathogen-associated molecular patterns) and that one TLR can respond to many structurally unrelated ligands, which are often derived from different groups of pathogens (e.g., TLR4 recognizes both viral components and gram-negative LPS). In contrast, other TLRs, like TLR3, -5, and -9, seem to be more ligand specific and at least up to now, appear to recognize only one type of ligand (Fig. 2) (48).
Most TLR ligands identified so far are conserved microbial products which signal the presence of an infection. TLR7 and TLR8 have been shown to recognize synthetic antiviral compounds with strong immunostimulatory capacity belonging to the group of imidazoquinolines (21, 31). The natural ligands of TLR7 and TLR8 remain to be identified, however. Finally, TLRs also recognize host-derived ligands such as the extra domain A of the extracellular matrix protein fibronectin and heat shock proteins (55, 56). Extracellular matrix proteins are often proteolyticly cleaved during infection to facilitate access of macrophages and other immune effector cells to the site of infection. The extra domain A (EDA) of fibronectin is encoded by an alternatively spliced exon, which is induced only upon tissue injury. Heat shock proteins are normally expressed in the cytoplasm and thus are not available for recognition by cell surface receptors but can be released by necrotic cells during tissue injury or viral infection (55). In this way, fragments of fibronectin containing the EDA region or heat shock proteins alert TLRs of an abnormal situation, e.g., tissue injury. Activation of TLRs by endogenous ligands implies that they do not distinguish between self and nonself, as defined before (49), but rather sense the presence of danger, which can be either nonself or harmful self (46).
Drosophila TLRs Are Not True Pattern Recognition Receptors
The insect immune defense involves the induction of different antimicrobial peptides in the fat body, the functional equivalent of the mammalian liver (for a review, see reference 26). Interestingly, insects are able to mount an adapted response by the specific induction of a subset of peptides which are only active against the invading pathogen (41). Two major pathways regulate the Drosophila innate immune response (Fig. 3).
FIG. 3.
(A) Drosophila Toll pathway. Drosophila Toll controls dorsoventral axis formation during development and the antifungal and anti-gram-positive organism immune response in the adult fly. In both cases, triggering relies on binding of a host-derived protein, named Spätzle, which is produced as a zymogen that is activated through a serine protease cascade. This proteolytic cascade involves different proteases, both in development and in response to fungal or bacterial challenge. In the Drosophila immune response, the protease cascade is activated by upstream pattern recognition receptors, such as soluble PGRP-SA, which circulates in the hemolymph and recognizes gram-positive pathogen-associated molecular patterns. The pattern recognition receptor responsible for fungal detection has not been characterized yet but may be another member of the PGRP family. Triggering of Toll leads to the recruitment of two adaptor proteins, a MyD88 homolog, Drosophila MyD88 (dMyD88), and tube, a protein with no known homolog in vertebrates. They further transmit the signal to other signaling intermediates and eventually induce the activation of the NF-κB homologue Dif. (B) Drosophila immune deficiency pathway. The immune deficiency (imd) pathway regulates the gram-negative organism immune response in D. melanogaster and was named after one of its intracellular signaling molecules, imd, as the upstream receptor has long been unknown. Recent studies led to the identification of PGRP-LC as the putative gram-negative pattern recognition receptor. PGRP-LC lacks intracellular signaling motifs and might act in concert with a coreceptor or might trigger a protease cascade, which then leads to generation of a ligand for the immune deficiency receptor, analogous to the Toll pathway. Triggering of the immune deficiency receptor induces a signaling cascade which leads to activation of the NF-κB-like transcription factor Relish.
The Toll pathway is activated upon fungal or gram-positive infection and results in induction of the antimicrobial peptide drosomycin, while the immune deficiency pathway is induced upon gram-negative challenge and leads to induction of the antibacterial peptide diptericin (39, 41). This implies the existence of pattern recognition receptors in Drosophila melanogaster, obvious candidates being nine different Toll transmembrane receptors, which were described recently (58, 75). However, the actual ligand for Toll, the most studied TLR in D. melanogaster, does not appear to be a microbially derived pathogen-associated molecular pattern but rather a fragment of a host-derived growth factor named Spätzle (Fig. 3A) (26). A loss-of-function mutation in a blood serine protease inhibitor (encoded by the necrotic gene) is sufficient to activate the Toll pathway, showing that Spätzle is activated by a proteolytic cascade, similar to the complement activation pathway in mammals, and that the actual pathogen recognition must occur upstream of Toll (42). Recent genetic studies identified persephone (psh) as one of the upstream proteases (44). Interestingly, psh mutants exhibited a normal drosomycin induction in response to gram-positive challenge, indicating that different protease cascades are activated in response to different pathogens (44).
Meanwhile, two different bona fide pattern recognition receptors have been identified in D. melanogaster, both belonging to the large family of peptidoglycan recognition proteins (PGRPs) (17, 51). PGRP-SA is a soluble member of this family, and flies with a loss-of-function mutation in this gene show an impaired response to gram-positive but not fungal or gram-negative infections (51) (Fig. 3A). Activation of PGRP-SA results in cleavage of Spätzle, indicating that PGRP-SA acts upstream of the serine protease cascade needed to activate Toll (51). PGRP-SA itself has no protease activity, but might activate, through conformational changes, associated proteases (51).
PGRP-LC is a transmembrane receptor, identified by three different groups as the long-sought gram-negative receptor upstream of the immune deficiency pathway (Fig. 3B) (10, 17, 63). However, PGRP-LC lacks any recognizable signaling motifs in its intracellular domain, suggesting that PGRP-LC might act in concert with another coreceptor to activate signaling. Alternatively, analogous to the Toll pathway, PGRP-LC might activate a proteolytic cascade which generates a ligand for a receptor of the Toll family (although none of the TLRs could activate diptericin upon overexpression [75]). In conclusion, Drosophila TLRs are not true pattern recognition receptors but rather bind endogenous ligands, generated through a proteolytic cascade in response to an infection. Actual pattern recognition in D. melanogaster is presumably mediated by the large family of PGRP proteins, whose versatility parallels the versatility of mammalian TLRs.
Do Mammalian TLRs Recognize Microbial Ligands Directly?
It has been speculated for a while that, analogous to the Drosophila Toll system, mammalian TLRs are activated by a host-derived protein, generated through a proteolytic cascade and that they do not recognize pathogen-associated molecular patterns directly (for a review, see reference 8). Arguments in support of this hypothesis were the fact that direct binding between TLRs and pathogen-associated molecular patterns has never been demonstrated biochemically, that several of the proteases upstream of Drosophila Toll are conserved throughout evolution and have been implicated in the LPS induced blood clotting cascade in the horseshoe crab, and that some reports have shown that LPS/TLR4 signaling can be inhibited by extracellular protease inhibitors (45), although others have doubted this (6).
Two groups elegantly showed that TLR4 directly recognizes lipid A, the active moiety of LPS (43, 62). Lipid IVa is a partial structure of lipid A which acts as an agonist of proinflammatory responses in mouse cells and as an antagonist in human cells. Transfection of mouse TLR4 into human cells gives them the ability to detect lipid IVa as an agonist, while transfection of human TLR4 into hamster CHO cells has the opposite result (43). This demonstrates that the response to lipid A is determined only by the origin of the introduced TLR, and not by that of the host cells, making this hard to reconcile with the Drosophila model of an upstream proteolytic cascade. Similarly, it has been demonstrated that human and murine TLR9 optimally respond to different CpG sequences and that this sequence specificity is determined solely by the origin of the TLR9 receptor, as transfection of murine TLR9 into a human cell line is sufficient to make the human cells respond to murine CpG motifs (7). Finally, in vitro binding and cosedimentation assays showed that the extracellular domain of TLR2 binds peptidoglycan, being the first biochemical demonstration of a direct interaction between TLRs and their ligands (27). The affinity was not as high as expected but could be increased when other factors such as soluble CD14 were included in the binding assay (27).
LPS Recognition by the TLR4 Complex: Is TLR4 the Sole LPS Receptor?
TLR4 does not need to heterodimerize with other TLRs to function but forms a complex with several other proteins on the cell surface which are needed for LPS recognition. LPS is bound in the serum by LPS binding protein, which transfers LPS to CD14 molecules (82, 83). CD14 is a glycosylphosphatidylinositol-anchored membrane protein (but also exists in a soluble form) which binds LPS binding protein complexes with high affinity, but lacks an intracellular domain to signal (82). Therefore, it has long been proposed that CD14 functions in a complex with another membrane receptor to transmit LPS signals (77). At the time Tlr4 was identified as the long-sought Lps gene, responsible for defective LPS responses in CH3/HeJ mice (61), and the confirmation that Tlr4−/− mice are hyporesponsive to LPS (25), it was presumed that CD14 complexes with TLR4 to form a functional LPS receptor complex. However, 4 years after its discovery, physical interaction between CD14 and TLR4 remains to be demonstrated, and biochemical evidence showing direct LPS binding to TLR4 has not been published yet (although genetic evidence strongly suggests that TLR4 binds LPS directly; see above). Furthermore, overexpression of TLR4 (with or without CD14) in HEK293T cells is not sufficient to confer LPS responsiveness on these cells, indicating the need for additional components (34).
One of the first additional components discovered in LPS signaling was MD-2, a homolog of MD-1, which is a B-cell-specific secretory protein that remains tethered to the membrane via interaction with RP105, a B-cell-specific leucine-rich repeat-containing molecule (52). As TLRs also express leucine-rich repeats in their extracellular domain, Miyake and colleagues reasoned that additional MD molecules might be necessary to interact with TLRs, leading to the identification of MD-2 (68). MD-2 and TLR4 interact physically on the membrane, and coexpression of MD-2 with TLR4 in HEK293T cells confers LPS responsiveness on these cells (68). MD-2 knockout mice do not respond to LPS and exhibit an impaired intracellular distribution of TLR4, showing that interaction with MD-2 is essential for proper targeting of TLR4 to the plasma membrane (54). Interestingly, B cells deficient in RP-105 or MD-1 show an impaired LPS response, indicating that in B cells both TLR4/MD-2 and RP105/MD-1 clusters are needed for an intact LPS response (54).
Biophysical approaches used to study intramolecular interactions revealed that LPS is associated with non-TLR-related molecules as well, ranging from integrins such as CD11b/CD18 to chemokine receptors, scavenger receptors, and many others (60, 76). Many of these receptors are clustered upon LPS triggering in lipid rafts, suggesting the formation of supramolecular LPS activation clusters. These could vary according to the cell type and the activation state of the cell (76). Finally, several reports suggest that LPS is not recognized as a free monomer but in the context of its packing in a membrane. E5531 is a synthetic LPS antagonist that resembles LPS but blocks its action in cells. Inversion of all 13 chiral centers of E5531 yields a mirror image, which was found to be an equally active antagonist. This observation argues against the recognition of LPS by a stoichiometric interaction with a stereospecific binding site in a receptor (reviewed in reference 81). In conclusion, the molecular mechanism for LPS recognition still remains elusive but seems to be more complicated than initially anticipated.
Heterodimerization of TLRs Extends the Spectrum of Ligands Recognized
Although dimerization of some TLRs, such as TLR4 or TLR5, is sufficient for cytokine induction in a macrophage cell line; others, such as TLR1 and TLR6, do not induce NF-κB upon overexpression, indicating that they cannot signal as homodimers (20, 59). Coimmunoprecipitation studies revealed that TLR1 and TLR6 can pair with TLR2 and that this interaction is needed for cytokine production (59). Studies in TLR1-, TLR2-, and TLR6-deficient mice demonstrated that TLR2−/− mice were impaired in their response to both mycoplasmal and bacterial lipoproteins, while TLR1−/− and TLR6−/− mice were specifically deficient in one of the two responses (72, 74). This shows that the unusual broad ligand specificity of TLR2 can be partially attributed to the fact that this receptor forms heterodimers with (at least) two other TLRs, which enable TLR2 to recognize different ligands.
TLR cooperation not only extends the spectrum of ligands but also modulates the response to specific ligands. Thus, TLR1 coexpression inhibits the TLR2-mediated response to phenol-soluble modulin, while coexpression with TLR6 enhances the TLR2 response (18). TLR1 also associates with TLR4 and inhibits its signaling in endothelial cells (70), suggesting that overall, TLR1 may have a more regulatory role through interaction with different TLRs and modulation of their function.
REGULATION OF TLR EXPRESSION
Several reports suggest that TLR expression is regulated in both a cell type- and stimulus-dependent fashion. Generally, cell surface expression of TLRs is rather low, varying in monocytes from a few hundred to a few thousand molecules per cell (for comparison, there are approximately 3 × 105 molecules of the adhesion molecule CD44 per cell) (79).
According to their cellular expression pattern, TLRs can be categorized as either ubiquitous (TLR1), restricted (TLR2, TLR4, and TLR5), or specific (TLR3) (Table 1). TLR3 shows the most restricted expression pattern, as it is predominantly detected in immature dendritic cells (53), although a recent report by Zarember et al. (86) showed a broader expression pattern. TLR1 shows the most ubiquitous expression pattern, reflecting its possible role as regulator of TLR-mediated signaling (70). There are numerous data on stimulus-dependent up- and downregulation of TLRs; the most relevant data are summarized in Table 1. Interestingly, expression of TLRs declines with age, which is a possible explanation for the increased susceptibility of elderly people to infections (65). Furthermore, TLR expression is extremely variable among individuals; e.g., TLR expression appears to be much higher in farmer's children than in non-farmer's children (38), which might again correlate with individual differences in pathogen susceptibility, although different polymorphisms at the locus of TLRs might contribute more to altered host immune responses to pathogens (5).
TABLE 1.
Cell- and stimulus-specific expression patterns of human TLRsa
| Receptor | Cell type (reference) | Regulation |
|---|---|---|
| TLR1 | Ubiquitous (63) | No significant regulation except for downregulation in T cells after exposure to PHA (53) |
| TLR2 | Restricted expression, undetectable in lymphoid cells, expressed in PMLs, DCs, and monocytes (53) | Induced by LPS (53, 85) |
| TLR3 | Expressed on DC and NK cells (24, 53) | Absent in precursor monocytes, induced by differentiation, decreased upon maturation (53) |
| TLR4 | Expressed in a variety of cell types, such as macrophages, DCs, ECs, not in lymphocytes (48, 53) | Expression enhanced by proinflammatory cytokines and bacterial products, downregulated by anti-inflammatory cytokines (53) |
| TLR5 | Expressed in monocytes, immature DCs, epithelial, NK, and T cells (14, 24, 56) | No significant modulation by cytokines or LPS (24, 53) |
| TLR6 | High expression in B cells, lower in monocytes and NK cells (24) | Not induced by proinflammatory cytokines or LPS (24) |
| TLR7 | B cells, plasmacytoid precursor DC (24, 32) | Highly induced by IL-6, moderately by other cytokines (86) |
| TLR8 | Monocytes, low in NK and T cells (24, 32) | Highly induced by gamma interferon and LPS, moderately by other cytokines (85) |
| TLR9 | Plasmacytoid precursor DCs, B-cells, macrophages, PMLs, NK cells, and microglial cells (3, 32) | Induced by gamma interferon and LPS (3, 36) |
| TLR10 | B cells, low in plasmacytoid precursor DCs (24) | No significant modulation by cytokines or LPS (86) |
Abbreviations: DC, dendritic cell; EC, endothelial cell; PML, polymorphonuclear leukocytes; NK, natural killer cell; PHA, phytohemagglutinin. References are indicated.
More and more promoter studies of Tlr genes are being published, aimed at identifying the gene-regulatory elements that control cell type specificity and inducibility of TLR gene transcription in humans and mice. Interestingly, it has been noted that there are substantial differences between the 5′ untranslated regions of TLR promoters in mice and humans, possibly indicative of a high selective pressure on these genes during evolution to adapt to a rapidly changing microbial environment (64).
TLRs also exhibit specific subcellular expression patterns, reflecting the fact that they recognize different microbial ligands, which gain access to the cell at different subcellular sites. Thus, CpG DNA and LPS were shown to activate their respective TLRs in distinct cellular compartments in macrophages (1). This was demonstrated by the use of green fluorescent protein-MyD88 constructs, which were recruited to the plasma membrane upon LPS triggering and to the lysosomes upon CpG triggering, correlating well with the subcellular expression of TLR4 and TLR9, respectively. Inhibition of endocytosis interfered with CpG but not LPS signaling, possibly indicative of the fact that bacterial cell walls must be destroyed in order to liberate DNA, a process which takes place in mature, acid endosomes, while bacterial cell wall LPS is freely accessible to cell surface-expressed TLR4.
TLR2 has been demonstrated to be recruited to yeast-loaded phagosomes (78). Phagosome expression of TLR2 and probably also other TLRs presumably enables them to sample the contents of vacuoles for different pathogen-associated molecular patterns, which are internalized by other pattern recognition receptors, like the mannose receptor (78). Finally, Gewirtz et al. showed that localized expression of TLR5, the TLR responsible for flagellin recognition (Fig. 2), in epithelial cells of the gastrointestinal tract contributes to the differential response to commensal and pathogenic bacteria (14). The intestinal epithelium is highly polarized, with two distinct compartments: the apical surface, facing the lumen, and the basolateral surface, facing the underlying lamina propria. It has been known for a long time that commensal bacteria present in the lumen of the gut do not trigger inflammatory responses, while pathogenic bacteria do. Flagellin is a conserved protein that forms bacterial flagella and is produced by both commensal and pathogenic bacteria. Flagellin only induces an immune response when it is in contact with the basolateral membrane of the gastrointestinal epithelial barrier, not when it is secreted in the lumen (15). This could be attributed to the fact that pathogenic but not commensal bacteria translocate flagellin across epithelia (15), which triggers activation of TLR5, which is expressed exclusively at the basolateral surface (14).
CONCLUDING REMARKS
The identification of mammalian TLRs has truly revolutionized the field of microbial pathogenesis and human immunology. We are just beginning to understand the complexities of this evolutionarily conserved system and the essential role it plays in innate immunity. As the basic understanding of microbially induced TLR signaling reaches a critical level, novel therapies that can effectively improve the outcome of sepsis may arise.
There are at least three strategies for interfering with TLR signaling, with the specific goal of reducing the consequences of their biological effects. The first is the generation of specific soluble TLRs to bind and neutralize their respective microbial ligands. The second is the development of small antagonistic molecules or antibodies that interfere with the extracellular domain of TLRs. This strategy awaits the elucidation of the structure of a TLR with its specific ligand. The third is the generation of small molecules that interfere with the intracellular domain of TLRs and prevent its interaction with distal intracellular signaling molecules (e.g., MyD88). Although there are reasons to be optimistic, the main problem remains the relatively late presentation of patients to the intensive care unit. Also, intervention may neutralize beneficial components of the host defense. However, if we are able to define the genetic basis of susceptibility to infection, tools will become available that might help identify patients at high risk for fatal septic shock. Such knowledge could then be used to improve established therapies.
Acknowledgments
S.J. is a research associate with the FWO-Vlaanderen. We also acknowledge the BFSK, the FWO-Vlaanderen, and the IUAP for support.
REFERENCES
- 1.Ahmad-Nejad, P., H. Hacker, M. Rutz, S. Bauer, R. M. Vabulas, and H. Wagner. 2002. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32:1958-1968. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.An, H., H. Xu, Y. Yu, M. Zhang, R. Qi, X. Yan, S. Liu, W. Wang, Z. Guo, Z. Qin, and X. Cao. 2002. Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activation of NF-κB, ERK and p38 mitogen-activated protein kinase signal pathways. Immunol. Lett. 81:165-169. [DOI] [PubMed] [Google Scholar]
- 4.Aravind, L., V. M. Dixit, and E. V. Koonin. 1999. The domains of death: evolution of the apoptosis machinery. Trends Biochem. Sci. 24:47-53. [DOI] [PubMed] [Google Scholar]
- 5.Arbour, N. C., E. Lorenz, B. C. Schutte, J. Zabner, J. N. Kline, M. Jones, K. Frees, J. L. Watt, and D. A. Schwartz. 2000. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 25:187-191. [DOI] [PubMed] [Google Scholar]
- 6.Backhed, F., S. Normark, and A. Richter-Dahlfors. 2002. TLR4-dependent lipopolysaccharide signalling in epithelial cells is independent of extracellular protease activity. Cell. Microbiol. 4:297-303. [DOI] [PubMed] [Google Scholar]
- 7.Bauer, S., C. J. Kirschning, H. Hacker, V. Redecke, S. Hausmann, S. Akira, H. Wagner, and G. B. Lipford. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 98:9237-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowie, A., and L. A. O'Neill. 2000. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for proinflammatory interleukins and microbial products. J. Leukoc. Biol. 67:508-514. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary, P. M., C. Ferguson, V. Nguyen, O. Nguyen, H. F. Massa, M. Eby, A. Jasmin, B. J. Trask, L. Hood, and P. S. Nelson. 1998. Cloning and characterization of two Toll/Interleukin-1 receptor-like genes TIL3 and TIL4: evidence for a multi-gene receptor family in humans. Blood 91:4020-4027. [PubMed] [Google Scholar]
- 10.Choe, K. M., T. Werner, S. Stoven, D. Hultmark, and K. V. Anderson. 2002. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science 296:359-362. [DOI] [PubMed] [Google Scholar]
- 11.Chuang, T., and R. J. Ulevitch. 2001. Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim. Biophys. Acta 1518:157-161. [DOI] [PubMed] [Google Scholar]
- 12.Du, X., A. Poltorak, Y. Wei, and B. Beutler. 2000. Three novel mammalian Toll-like receptors: gene structure, expression, and evolution. Eur. Cytokine Netw. 11:362-371. [PubMed] [Google Scholar]
- 13.Gay, N. J., and F. J. Keith. 1991. Drosophila Toll and IL-1 receptor. Nature 351:355-356. [DOI] [PubMed] [Google Scholar]
- 14.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 15.Gewirtz, A. T., P. O. Simon, Jr., C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 107:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109(Suppl.):S81-96. [DOI] [PubMed] [Google Scholar]
- 17.Gottar, M., V. Gobert, T. Michel, M. Belvin, G. Duyk, J. A. Hoffmann, D. Ferrandon, and J. Royet. 2002. The Drosophila immune response against gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 416:640-644. [DOI] [PubMed] [Google Scholar]
- 18.Hajjar, A. M., D. S. O'Mahony, A. Ozinsky, D. M. Underhill, A. Aderem, S. J. Klebanoff, and C. B. Wilson. 2001. Cutting edge: functional interactions between Toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 166:15-19. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto, C., K. L. Hudson, and K. V. Anderson. 1988. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52:269-279. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 21.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small antiviral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196-200. [DOI] [PubMed] [Google Scholar]
- 22.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 23.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 24.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of Toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531-4537. [DOI] [PubMed] [Google Scholar]
- 25.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 26.Imler, J. L., and J. A. Hoffmann. 2000. Signaling mechanisms in the antimicrobial host defense of Drosophila. Curr. Opin. Microbiol. 3:16-22. [DOI] [PubMed] [Google Scholar]
- 27.Iwaki, D., H. Mitsuzawa, S. Murakami, H. Sano, M. Konishi, T. Akino, and Y. Kuroki. 2002. The extracellular Toll-like receptor 2 domain directly binds peptidoglycan derived from Staphylococcus aureus. J. Biol. Chem. 277:24315-24320. [DOI] [PubMed] [Google Scholar]
- 28.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 29.Janssens, S., and R. Beyaert. 2002. A universal role for MyD88 in TLR/IL-1 receptor-mediated signaling. Trends Biochem. Sci. 27:474-482. [DOI] [PubMed] [Google Scholar]
- 30.Juan, T. S., M. J. Kelley, D. A. Johnson, L. A. Busse, E. Hailman, S. D. Wright, and H. S. Lichenstein. 1995. Soluble CD14 truncated at amino acid 152 binds lipopolysaccharide (LPS) and enables cellular response to LPS. J. Biol. Chem. 270:1382-1387. [DOI] [PubMed] [Google Scholar]
- 31.Jurk, M., F. Heil, J. Vollmer, C. Schetter, A. M. Krieg, H. Wagner, G. Lipford, and S. Bauer. 2002. Hum. TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 3:499. [DOI] [PubMed] [Google Scholar]
- 32.Kadowaki, N., S. Ho, S. Antonenko, R. W. Malefyt, R. A. Kastelein, F. Bazan, and Y. J. Liu. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaisho, T., and S. Akira. 2001. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 22:78-83. [DOI] [PubMed] [Google Scholar]
- 34.Kirschning, C. J., H. Wesche, T. Merrill Ayres, and M. Rothe. 1998. Human Toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 188:2091-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobe, B., and J. Deisenhofer. 1995. Proteins with leucine-rich repeats. Curr. Opin. Struct. Biol. 5:409-416. [DOI] [PubMed] [Google Scholar]
- 36.Krieg, A. M. 2002. From A to Z on CpG. Trends Immunol. 23:64-65. [DOI] [PubMed] [Google Scholar]
- 37.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 38.Lauener, R. P., T. Birchler, J. Adamski, C. Braun-Fahrlander, A. Bufe, U. Herz, E. von Mutius, D. Nowak, J. Riedler, M. Waser, and F. H. Sennhauser. 2002. Expression of CD14 and Toll-like receptor 2 in farmers' and non-farmers' children. Lancet 360:465-466. [DOI] [PubMed] [Google Scholar]
- 39.Lemaitre, B., E. Kromer-Metzger, L. Michaut, E. Nicolas, M. Meister, P. Georgel, J. M. Reichhart, and J. A. Hoffmann. 1995. A recessive mutation, immune deficiency (immune deficiency), defines two distinct control pathways in the Drosophila host defense. Proc. Natl. Acad. Sci. USA 92:9465-9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 41.Lemaitre, B., J. M. Reichhart, and J. A. Hoffmann. 1997. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl. Acad. Sci. USA 94:14614-41619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levashina, E. A., E. Langley, C. Green, D. Gubb, M. Ashburner, J. A. Hoffmann, and J. M. Reichhart. 1999. Constitutive activation of Toll-mediated antifungal defense in serpin-deficient Drosophila. Science 285:1917-1919. [DOI] [PubMed] [Google Scholar]
- 43.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Fenton, M. Oikawa, N. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 105:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ligoxygakis, P., N. Pelte, J. A. Hoffmann, and J. M. Reichhart. 2002. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 297:114-116. [DOI] [PubMed] [Google Scholar]
- 45.Mansell, A., A. Reinicke, D. M. Worrall, and L. A. O'Neill. 2001. The serine protease inhibitor antithrombin III inhibits LPS mediated NF-κB activation by TLR-4. FEBS Lett. 508:313-317. [DOI] [PubMed] [Google Scholar]
- 46.Matzinger, P. 2002. The danger model: a renewed sense of self. Science 296:301-305. [DOI] [PubMed] [Google Scholar]
- 47.Means, T. K., E. Lien, A. Yoshimura, S. Wang, D. T. Golenbock, and M. J. Fenton. 1999. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J. Immunol. 163:6748-6755. [PubMed] [Google Scholar]
- 48.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 49.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91:295-298. [DOI] [PubMed] [Google Scholar]
- 50.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 51.Michel, T., J. M. Reichhart, J. A. Hoffmann, and J. Royet. 2001. Drosophila Toll is activated by gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 414:756-759. [DOI] [PubMed] [Google Scholar]
- 52.Miyake, K., R. Shimazu, J. Kondo, T. Niki, S. Akashi, H. Ogata, Y. Yamashita, Y. Miura, and M. Kimoto. 1998. Mouse MD-1, a molecule that is physically associated with RP105 and positively regulates its expression. J. Immunol. 161:1348-1353. [PubMed] [Google Scholar]
- 53.Muzio, M., D. Bosisio, N. Polentarutti, G. D'Amico, A. Stoppacciaro, R. Mancinelli, C. van't Veer, G. Penton-Rol, L. P. Ruco, P. Allavena, and A. Mantovani. 2000. Differential expression and regulation of Toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 164:5998-6004. [DOI] [PubMed] [Google Scholar]
- 54.Nagai, Y., S. Akashi, M. Nagafuku, M. Ogata, Y. Iwakura, S. Akira, T. Kitamura, A. Kosugi, M. Kimoto, and K. Miyake. 2002. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3:667-672. [DOI] [PubMed] [Google Scholar]
- 55.Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J. Immunol. 164:558-561. [DOI] [PubMed] [Google Scholar]
- 56.Okamura, Y., M. Watari, E. S. Jerud, D. W. Young, S. T. Ishizaka, J. Rose, J. C. Chow, and J. F. Strauss, 3rd. 2001. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 276:10229-10233. [DOI] [PubMed] [Google Scholar]
- 57.O'Neill, L. A. 2002. Wanted: a molecular basis for specificity in Toll-like receptor signal transduction. Mol. Cell 10:969-971. [DOI] [PubMed] [Google Scholar]
- 58.Ooi, J. Y., Y. Yagi, X. Hu, and Y. T. Ip. 2002. The Drosophila Toll-9 activates a constitutive antimicrobial defense. EMBO Rep. 3:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfeiffer, A., A. Bottcher, E. Orso, M. Kapinsky, P. Nagy, A. Bodnar, I. Spreitzer, G. Liebisch, W. Drobnik, K. Gempel, M. Horn, S. Holmer, T. Hartung, G. Multhoff, G. Schutz, H. Schindler, A. J. Ulmer, H. Heine, F. Stelter, C. Schutt, G. Rothe, J. Szollosi, S. Damjanovich, and G. Schmitz. 2001. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur. J. Immunol. 31:3153-3164. [DOI] [PubMed] [Google Scholar]
- 61.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 62.Poltorak, A., P. Ricciardi-Castagnoli, S. Citterio, and B. Beutler. 2000. Physical contact between lipopolysaccharide and Toll-like receptor 4 revealed by genetic complementation. Proc. Natl. Acad. Sci. USA 97:2163-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramet, M., P. Manfruelli, A. Pearson, B. Mathey-Prevot, and R. A. Ezekowitz. 2002. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 416:644-648. [DOI] [PubMed] [Google Scholar]
- 64.Rehli, M. 2002. Of mice and men: species variations of Toll-like receptor expression. Trends Immunol. 23:375-378. [DOI] [PubMed] [Google Scholar]
- 65.Renshaw, M., J. Rockwell, C. Engleman, A. Gewirtz, J. Katz, and S. Sambhara. 2002. Cutting edge: impaired Toll-like receptor expression and function in aging. J. Immunol. 169:4697-4701. [DOI] [PubMed] [Google Scholar]
- 66.Rich, T., R. Allen, and J. Trowsdale. 2000. How low can Toll go? Trends Genet. 16:292-924. [DOI] [PubMed] [Google Scholar]
- 67.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Slack, J. L., K. Schooley, T. P. Bonnert, J. L. Mitcham, E. E. Qwarnstrom, J. E. Sims, and S. K. Dower. 2000. Identification of two major sites in the type I interleukin-1 receptor cytoplasmic region responsible for coupling to proinflammatory signaling pathways. J. Biol. Chem. 275:4670-4678. [DOI] [PubMed] [Google Scholar]
- 70.Spitzer, J. H., A. Visintin, A. Mazzoni, M. N. Kennedy, and D. M. Segal. 2002. Toll-like receptor 1 inhibits Toll-like receptor 4 signaling in endothelial cells. Eur. J. Immunol. 32:1182-1187. [DOI] [PubMed] [Google Scholar]
- 71.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 72.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 73.Takeuchi, O., T. Kawai, H. Sanjo, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, K. Takeda, and S. Akira. 1999. TLR6: a novel member of an expanding Toll-like receptor family. Gene 231:59-65. [DOI] [PubMed] [Google Scholar]
- 74.Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R. L. Modlin, and S. Akira. 2002. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10-14. [DOI] [PubMed] [Google Scholar]
- 75.Tauszig, S., E. Jouanguy, J. A. Hoffmann, and J. L. Imler. 2000. Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc. Natl. Acad. Sci. USA 97:10520-10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Triantafilou, M., and K. Triantafilou. 2002. Lipopolysaccharide recognition: CD14, TLRs and the LPS activation cluster. Trends Immunol. 23:301-304. [DOI] [PubMed] [Google Scholar]
- 77.Ulevitch, R. J. 1993. Recognition of bacterial endotoxins by receptor-dependent mechanisms. Adv. Immunol. 53:267-289. [DOI] [PubMed] [Google Scholar]
- 78.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 79.Visintin, A., A. Mazzoni, J. H. Spitzer, D. H. Wyllie, S. K. Dower, and D. M. Segal. 2001. Regulation of Toll-like receptors in human monocytes and dendritic cells. J. Immunol. 166:249-255. [DOI] [PubMed] [Google Scholar]
- 80.Werts, C., R. I. Tapping, J. C. Mathison, T. H. Chuang, V. Kravchenko, I. Saint Girons, D. A. Haake, P. J. Godowski, F. Hayashi, A. Ozinsky, D. M. Underhill, C. J. Kirschning, H. Wagner, A. Aderem, P. S. Tobias, and R. J. Ulevitch. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2:346-352. [DOI] [PubMed] [Google Scholar]
- 81.Wright, S. D. 1999. Toll, a new piece in the puzzle of innate immunity. J. Exp. Med. 189:605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 83.Wright, S. D., P. S. Tobias, R. J. Ulevitch, and R. A. Ramos. 1989. Lipopolysaccharide (LPS) binding protein opsonizes LPS bearing particles for recognition by a novel receptor on macrophages. J. Exp. Med. 170:1231-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu, Y., X. Tao, B. Shen, T. Horng, R. Medzhitov, J. L. Manley, and L. Tong. 2000. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature 408:111-115. [DOI] [PubMed] [Google Scholar]
- 85.Yang, R. B., M. R. Mark, A. Gray, A. Huang, M. H. Xie, M. Zhang, A. Goddard, W. I. Wood, A. L. Gurney, and P. J. Godowski. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395:284-288. [DOI] [PubMed] [Google Scholar]
- 86.Zarember, K. A., and P. J. Godowski. 2002. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J. Immunol. 168:554-561. [DOI] [PubMed] [Google Scholar]