Abstract
Babesia divergens is an intraerythrocytic protozoan parasite, transmitted by the tick Ixodes ricinus, and is the main agent of bovine babesiosis in Europe. It is not only a cause of significant loss to the cattle industry; it can also infect immunocompromised humans, causing medical emergencies characterized by rapid fulmination and parasitemias that may exceed 70%. The current emphasis in Europe on sustainable agriculture and extensification is likely to lead to an increase in vector tick populations with increased risk of infection. Despite the veterinary and zoonotic importance of this parasite, relatively little research has been carried out on B. divergens, and many questions regarding the parasite's epidemiology and the host's response remain unanswered. A better understanding of the species' biology and host-parasite interactions may lead to improved control mechanisms and new trends in vaccine and antibabesial drug development. This review provides the first comprehensive summary of B. divergens biology, including its morphology, life cycle, and host specificity, and the current state of knowledge of both human and bovine infections.
INTRODUCTION
Members of the genus Babesia are tick-transmitted intraerythrocytic protozoan parasites, and many species are of considerable economic importance in the livestock industry. Additionally, some species are zoonotic and affect human health. The most recent reviews of Babesia spp. primarily concern parasites of livestock in tropical and subtropical regions, or zoonotic species, focusing mainly on the rodent species B. microti (65, 93, 106, 176, 177). Relatively little attention has been paid to B. divergens, the causal agent of a widespread cattle disease in Europe and also of a dangerous zoonosis.
The significance of Babesia divergens (M'Fadyean & Stockman 1911 [132]) for today's livestock industry in Europe is almost certainly underestimated, as is the possibility of human infection. In 1981 Purnell wrote “bovine babesiosis caused by B. divergens, also known as redwater fever, is considered the most important tick-transmitted disease in cattle” (143). The following years saw a decline in disease outbreaks, probably due in part to the introduction of effective prophylactic treatment and live vaccination in some countries. However, many of the most effective drugs against B. divergens have since been withdrawn because of safety or residue problems. Concerns about the safety of live vaccines, whether they are raised in bovine or nonbovine host cells, are growing, particularly in light of recent disease outbreaks that have crossed species barriers. At the same time, the increased interest in extensive agricultural systems within the European Union is likely to cause an increase in vector tick populations. Climate changes due to global warming may add to the infection risk by prolonging the period of tick activity or by causing a change in the distribution of risk areas. The problem is compounded by the rise in cattle movement across Europe and may be further increased by the entry into the European Union of several countries where the disease is prevalent.
B. divergens is also of human medical importance and appears to be the only confirmed zoonotic Babesia sp. in Europe. Although human infections are infrequent, they are invariably fatal unless treated immediately. Recently, worldwide interest in B. divergens has increased as a result of human cases caused by identical or similar parasites outside areas where bovine babesiosis is endemic (7, 88, 135). This review provides the first comprehensive summary of the species' biology, including its life cycle, host specificity, and morphology, and the current state of knowledge about both human and bovine infections.
HOST SPECIFICITY AND PARASITE MORPHOLOGY
B. divergens was first described by M'Fadyean and Stockman, who named it Piroplasma divergens (132). The generic names Babesiella and Microbabesia were occasionally used for this species as well as for other small babesias, until Babesia was recognized to include all morphological variations of the genus (37, 129). The only two species confirmed to infect cattle in northern Europe are B. divergens and Babesia major, with B. divergens being by far the more common.
B. divergens is transmitted by Ixodes ricinus, a member of the family of hard ticks (Ixodidae). Initially it was thought of as a typical bovine babesia with a narrow host range, but this view changed when Garnham and Bray reported that splenectomized chimpanzees and rhesus monkeys inoculated with B. divergens developed fulminating infections accompanied by blackwater (hemoglobinuria) (56). It was soon discovered that while intact primates were refractory to very heavy inocula and developed no detectable antibody, splenectomized primates were highly susceptible to B. divergens (56, 58).
Within a few years, B. divergens was identified as the causative agent in the first of a growing number of cases in splenectomized humans (54). Subsequently the host range was extended experimentally by transmitting the parasite by needle injection from infected cattle to splenectomized mouflon, red, roe, and fallow deer (49) and intact reindeer (134). With the exception of the reindeer, which showed clinical signs, these animals suffered only very mild infections with low parasitemias, but their blood proved infectious to susceptible cattle. Although Enigk and Friedhoff reported that splenectomized goats and sheep were fully resistant to B. divergens (49), Chauvin et al. have since succeeded in producing very low transient parasitemias in splenectomized sheep (29).
In contrast, initial attempts to establish infections for diagnostic purposes in common laboratory animals, such as rabbits, (56), mice, hamsters, and rats (1, 25, 97) were unsuccessful in both intact and splenectomized animals, though in some cases erythrocyte invasion may have occurred. Phillips eventually succeeded in adapting the piroplasm to grow in splenectomized rats, in which peak parasitemias reached over 50% after 25 passages (138). The only laboratory animal that has been found to be fully susceptible, whether intact or splenectomized, is the Mongolian gerbil, Meriones unguiculatus (119). This discovery was made during investigations of a human case in Scotland (50), and M. unguiculatus has since been used in many laboratory studies.
The course of infection in gerbils is determined by the infective dose. High inocula (≥107 infected erythrocytes) cause fever, anemia, jaundice, anorexia, hemoglobinuria, and splenomegaly, resulting in death within a few days (121, 125). Depending on the age of the gerbil, the size of the inoculum, and the strain of the parasite, animals may recover from infections. In contrast to the bovine host, gerbils develop sterile rather than residual immunity (121, 125, 118). Rapid continuous gerbil passage may cause the selection of faster-replicating parasites resulting in higher peak parasitemias in the gerbil (125) and an increased virulence to cattle (133).
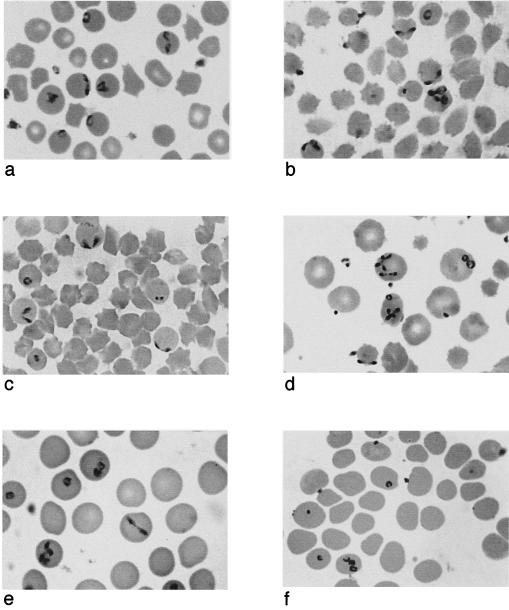
Similarly, B. divergens was found to exhibit low host specificity in vitro. Since Vayrynen and Tuomi first reported successful long-term in vitro cultivation in bovine erythrocytes (182), the parasite has been successfully maintained in human (63, 67, 141), rat (8), and sheep (29, 192) erythrocytes. Although B. divergens was also able to invade horse and donkey erythrocytes (Fig. 1f) and proliferate to some extent, neither supported long-term cultures (183, 192).
FIG. 1.
Babesia divergens in erythrocytes of different hosts. (a) Bovine erythrocytes in vivo. (b) Bovine erythrocytes in vitro. (c) Ovine erythrocytes in vitro. (d) Human erythrocytes in vitro. (e) Gerbil erythrocytes in vivo. (f) Donkey erythrocytes in vitro.
Unlike Plasmodium spp., babesia bloodstream stages do not divide simultaneously, so that all divisional stages can be observed in a single blood smear from a parasitized host. Ring or annular stages are round or oval and have nonstaining vacuoles in the center with light staining cytoplasm and a darker stained nucleus around the periphery. Pear-shaped or pyriform trophozoites occur either singly or in pairs assembled at their pointed extremities. The angle between the members of the pair is larger than in any other bovine Babesia species, resulting in the divergent appearance to which the parasite owes its name. The frequency of each of these stages is probably chiefly determined by replication rate. Filamentous or amorphous shapes are mostly observed at or just after very high levels of parasitemia and are thought to represent crisis forms (63, 79).
Merozoite size, position inside the erythrocyte, and morphological detail are dependent on the host species. Figure 1 shows B. divergens in various host cells. The dimensions and characteristics of the parasites are summarized in Table 1. Typically, the parasites in bovine erythrocyte include many that are located at the periphery (Fig. 1a) (25, 51, 79, 113), where they may cause slight protrusions of the erythrocyte membrane (63). Parasites maintained in in vitro culture in bovine erythrocytes may temporarily be larger than those found in vivo (141), but as the rate of proliferation increases with successive subcultures, merozoites tend to become smaller and more granular. Peripheral stages seem to be present in some (Fig. 1b) (43, 192) but not all cultures in bovine cells (141).
TABLE 1.
Morphological parameters of B. divergens in different host erythrocytesa
| Host cell | Position in erythrocyte | Dimensions | Polyparasitism (no. of parasite per erythrocyte) | Tetrad formsb |
|---|---|---|---|---|
| Bovine | Peripheral (25, 37, 51, 63, 79) | Pyriforms: mean length 1.5-1.91 μm (25, 37, 51, 79, 91); mean width, 0.4-1.07 μm (25, 37, 91); ring stages: mean diam, 1.48-1.8 μm (37, 51, 117) | Infrequent (63) | <0.02 (63) |
| Human | (Sub)central (50, 63, 125) | Pyriforms: mean length, 1.9 μm (125); mean width, 0.8 μm (125) | Common: up to 8 (50); up to 5 (63); up to 6 (125) | <5 (63) |
| Chimpanzee | (Sub)central (56) | Ring stages, 1-2 μm (56) | Common (≥3) (56) | |
| Rhesus monkey | Peripheral (56) | |||
| Gerbil | (Sub)central (63, 125) | Pyriforms: mean length, 1.9-2.6 μm (79, 125); mean width, 0.8 μm (125) | Common: up to 10 (63); ≥9 (125) | Present (63) |
| Rat | (Sub)central (25) | Pyriforms: mean length, 2.66 ± 0.65 μm (25); mean width, 1.18 ± 0.43 μm (25) | Occasional (5 to 6) (25) | <5 (25) |
| Hamster | (Sub)central (25) | Occasional (5 to 6) (25) | <10 (25) |
References are cited for individual items.
Percent of tetrad stages in all infected erythrocytes.
While parasites maintained in sheep erythrocytes in vitro are similar in appearance to those in cattle (Fig. 1c) (192), merozoites in all other nonbovine erythrocytes differ in size and position. In human erythrocytes, the parasite is significantly larger than in cattle (117), and ring stages are characterized by big vacuoles. Merozoites are located in a (sub)central position (50, 117) and do not protrude at the cell surface (63). In in vitro cultures, however, peripheral stages may be present (Fig. 1d) (192). Similarly, in other primates the merozoites are much larger and are rarely seen in the peripheral divergent form (56). After the parasite crisis associated with host recovery, they are largely replaced by small solid circular parasites with little cytoplasm and measuring only 1 μm or less in diameter (56).
In the rodent laboratory hosts (gerbils, rats, and hamsters), B. divergens becomes particularly large and rounded, resembling B. microti (25, 44, 79, 125, 138). Occasionally pairs are located peripherally, as in bovine erythrocytes, but a central conformation is most common (Fig. 1e) (44, 125). In infected gerbil erythrocytes, the red blood cell membrane may be invaginated locally, resulting in structures similar to Maurer's clefts in Plasmodium-infected erythrocytes (63). The authors suggest that these clefts may establish direct contact between B. divergens and the erythrocyte membrane in spite of the parasite's subcentral position.
The specific host-parasite interactions that cause these morphological and positional changes are not understood. The fact that even one passage through an abnormal host may bring about significant changes in parasite size (117) indicates that the cytological changes are an immediate response to conditions in the new host, such as immunological attack and availability of nutrients. The observation that parasite size may vary in different individuals of the same species and may also be affected by concurrent infections (91) supports this theory. It is also striking that larger host cells tend to give rise to bigger merozoites, suggesting spatial restrictions imposed by the erythrocyte surface membrane. At the same time, a selective mechanism seems to be involved, as human- and gerbil-adapted isolates that are transferred back into cattle continue to be larger than normal cattle isolates (79, 117).
Polyparasitism in the bovine host in vivo is infrequent (63), but in all other red blood cell types (Table 1) and even in bovine erythrocytes in in vitro culture (Fig. 1b and 1d), multiply infected erythrocytes are frequently observed (141, 192), with as many as 10 parasites per cell in infected gerbils (63). It is not clear whether polyparasitism is due to multiple infections or repeated multiplication in the same host cell. Apparently it is not simply a function of high parasitemia. While multiple infections are frequently observed at peak parasitemias (125), they also tend to be common at the beginning of mixed cultures (63), when certain host cells may be more suitable than others. By comparison, in well-established fast-growing cultures, where fairly uniform host cells are available to an increasingly streamlined parasite population, the number of multiply infected erythrocytes tends to decline (192). Tetrad or Maltese cross formations of four pyriforms joined at their pointed ends are not typically found in blood smears of infected cattle but may be quite common in infected human or rodent erythrocytes (Table 1).
BOVINE BABESIOSIS
Disease Symptoms and Pathology
The minimum infective dose required to produce overt disease is thought to be 103 parasites inoculated intravenously (145). Variations in the number of parasites injected result in highly significant changes to the prepatent period, peak parasitemia, and the hematological response (87, 145, 148). In addition to the number of infected ticks that feed on an animal, the immune status of the host and the virulence of the infecting strain (147) determine whether B. divergens infections take a mild, severe, or fatal course. Subclinical infections are quite common and are usually missed by the farmer and clinician. Affected animals have low parasitemia, may suffer mild fever and anorexia, and make an uneventful recovery (82).
More severe cases of redwater present with an acute onset of fever (up to 41°C), anemia, anorexia, depression, weakness, cessation of rumination, and an increase in respiratory and heart rate. The mucous membranes are pale and may be jaundiced. Intestinal and ruminal motility are increased, and spasms of the anal sphincter cause pipestem diarrhea (31, 35, 82, 159). Meanwhile, parasitemia may rise to between 30 and 45% (35, 82, 143), causing extensive erythrocyte destruction. Hemoglobinuria, frequently the clinical sign first detected by the owner, occurs at the peak of the hemolytic crisis (143, 159). As the anemia advances, the animal becomes further depressed. Dehydration is severe, and diarrhea is replaced by constipation. The very rapid heart rate with extremely loud cardiac sounds may be heard a few feet distant from the cow. The body temperature falls to near or below normal, and hemoglobinuria ceases.
Terminally, the animal is unable to rise and exhibits toxemic shock, a subnormal temperature, weak pulse, severe jaundice, constipation, and dehydration (35, 36, 82, 143, 159). Brain anoxia resulting from severe anemia may cause behavioral changes (82). Levels of carboxypeptidase B, an enzyme thought to regulate circulating vasoactive peptides, may fall during very severe infections, causing vasoactive shock (166). Death is usually attributed to cardiac failure or hepatic and renal insufficiency (35). Case fatality rates are mainly influenced by the speed of diagnosis and treatment and in Ireland in 1983 were estimated to be approximately 10% (75). Postmortem examination reveals marked jaundice, enlarged and darkened liver and kidney, and a swollen spleen of a soft pulpy consistency. Ecchymotic hemorrhages may be present under the epicardium and the endocardium, and the pericardial sac may contain a large quantity of blood-stained fluid. There may be indications of hepatocellular degeneration and necrosis (35, 82).
Even in animals which recover spontaneously, erythrocyte count, packed cell volume, and hemoglobin level continue to decline steadily after patency. Once the parasites have been eliminated, increased hematopoiesis occurs, evidenced by the presence of nucleated erythrocytes, polychromasia, and anisocytosis (155). Thus, the veterinarian can interpret the presence of reticulocytes as a sign that the hemolytic crisis has occurred 3 or 4 days previously (35, 159). Immediately after the hemolytic crisis, a brief lymphocytosis and monocytosis combine to cause a leucocytosis (82, 155). Serum changes may include increased potassium and reduced calcium and sodium levels (136). Serum iron content may vary strongly, with some animals showing low to normal levels while others have extremely high concentrations of serum iron (99).
Synchronous infections with B. divergens and Anaplasma (Ehrlichia) phagocytophila, the causative agent of tick-borne fever also transmitted by I. ricinus, appear to be very common (146), but their interaction is poorly understood (169). Apparently, concurrent infections do not generally induce more severe clinical signs than infection with either organism alone (22). In fact, babesia infections may even be suppressed by the simultaneous occurrence of A. phagocytophila, resulting in less serious disease manifestations (155). However, animals which already suffer severe neutropenia from a previous infection with tick-borne fever and subsequently contract babesiosis may suffer more serious disease symptoms (169) and be less responsive to treatment (165). This may have epidemiological implications, especially in areas with small and scattered tick populations. Since tick infection rates with B. divergens tend to be much lower than with A. phagocytophila, B. divergens infections are likely to be superimposed on tick-borne fever, giving rise to more severe cases that are more difficult to treat (71). In cattle persistently infected with bovine virus diarrhea virus, the antibody response to B. divergens may be suppressed (23). The effect of Fasciola hepatica on subsequent infection with B. divergens was negligible (94).
Distribution, Life Cycle, and Epidemiology
Of the two cattle babesias occurring in northern temperate areas, B. divergens and B. major, B. divergens is both more pathogenic and more widespread (82, 122). Epidemiological surveys have documented the presence of B. divergens throughout Europe, including Austria (89, 90), Belgium (53), Britain (2, 40, 41), France (51, 122, 123), Germany (55, 95), Ireland (35, 68, 75), Northern Ireland (165, 175), the Netherlands (13, 158), Scandinavia (33, 48), and Switzerland (5, 20, 60). A report of the parasite from Tunisia indicates that the real distribution of B. divergens may extend beyond Europe into North Africa (14). The distribution of the disease appears to be determined by the wide distribution of the tick vector, I. ricinus, which occurs from northern Scandinavia to the Mediterranean, i.e., between the −15°C January isotherm and the 30°C July isotherm (164). The ectoparasite has, however, very precise humidity requirements of at least 80% (105), restricting it to areas with an average annual rainfall of 100 cm or more (41). I. ricinus can be found in the moisture-saturated microhabitat at the base of permanent herbage in forest woodland, rough hill scrub, and damp low-lying land. Well-maintained permanent pasture seldom provides adequate continuous humidity for a favorable tick habitat, but unimproved undergrazed pastures and copses, hedges, headlands, and windbreaks bordering well-maintained land may represent foci of babesial infection (82, 103).
Each tick life cycle stage feeds only once, but all appear to be able to transmit B. divergens. Sexual reproduction occurs in the tick. While the general sequence of events during sexual reproduction has been known for some time from microscopy and electron microscopy studies of various other Babesia spp. (107, 131), Mackenstedt et al. were the first to provide a detailed account of the sexual cycle in B. divergens with cytophotometry DNA (128). They described the fusion of gametes, dissemination of kinetes throughout the tick, and invasion of the salivary glands but did not find any evidence of either pre- or postzygotic meiosis.
In summary, uninucleated gamonts taken up during the previous blood meal develop into ray bodies (Strahlenkörper; 105) inside the tick gut. Two fusing ray bodies give rise to an immobile spherical zygote, which differentiates into mobile polyploid kinetes. These kinetes enter the tick hemolymph and are disseminated throughout various tissues, including the musculature, epidermis, Malpighian tubes, and ovaries. One or two cycles of asexual reproduction (sporogony) ensue (176), resulting in vast numbers of kinetes. Developing eggs may be invaded, giving rise to infected larvae. Once the tick starts to feed on a new host, kinetes which have entered the cells of the tick salivary glands develop into numerous uninuclear, apparently haploid sporozoites. Inoculation of the infective sporozoites occurs during the latter half of the blood meal (42, 51, 103). Interestingly, the work of Mackenstedt et al. implies that B. divergens can be transmitted transstadially (128), though this is contrary to the findings of Donnelly and Peirce (42), whose exhaustive studies on cattle demonstrated that only adult females acquired infections, which were then transmitted transovarially. The transmission work of these (42) and earlier researchers (102) suggested that the infection was most readily transmitted by larvae. Gray, on the other hand, who compared the seasonality of redwater fever and tick activity, suggested that adult ticks may be most important for parasite transmission (68). Field observations also suggest that cattle are most readily parasitized by adult ticks (123).
It is generally accepted that Babesia spp. invade erythrocytes directly; there is no direct evidence for a preerythrocytic stage (128). After attachment to the host cell and orientation of the apical end towards the erythrocyte surface, secretory products released by the rhoptries cause the erythrocyte membrane to invaginate. A uniform surface coat which covers the free merozoite is removed during the process of invasion and left behind at the entering wedge (96). At first a parasitophorous vacuole encloses the sporozoite. As the vacuole membrane disintegrates, the parasite is eventually limited by one single plasma membrane which is directly in contact with the erythrocyte cytoplasm (63). At this stage the parasite is considered a trophozoite or feeding stage that divides asexually by binary fission. The study of Mackenstedt et al., consisting of quantitative measurements of DNA by fluorescence microscopy (128), suggested that, following DNA replication in the single haploid nucleus of the trophozoite, the nucleus divides, giving rise to a binucleated ring stage. Subsequently the whole cell divides into two haploid merozoites, which escape by lysing the old host cell and invading new ones. One cycle of asexual reproduction takes about 8 h in vitro (181). The vast majority of merozoites continue to multiply asexually, while a small proportion turn into nondividing spherical gamonts, which remain inside erythrocytes until they are taken up by ticks during feeding (128).
Disease occurrence typically has a bimodal seasonal distribution, with a spring peak between April and June and an autumn peak from August to October. Initially air temperature was thought to be a key factor in determining tick activity and babesiosis because a strong correlation was demonstrated between air temperature and disease incidence (41). It has since been suggested that while the temperature effect may be large at the beginning and the end of the year, determining the earliest and latest cases of the year, temperature has little effect once tick activity threshold temperatures are exceeded (68). In spring, at maximum air temperatures of 7 to 10°C, overwintering nymphs and adult ticks become active. The first cases of babesiosis are usually observed 2 weeks later, the lag phase reflecting the period of time required for host seeking, tick attachment, and incubation of the disease (39). Emergence of newly metamorphosed ticks takes place during the autumn rise (103). Those that fed the previous spring may therefore contribute to the autumn peak of babesiosis, but most autumn cases are caused by nymphs and adults that fed as larvae and nymphs the previous autumn and overwintered as engorged stages in diapause. Tick larvae are most active in the trough between the spring and autumn redwater cases. Although this instar occurs in large numbers, it tends to have a very aggregated distribution and seems to prefer nonbovine hosts. Consequently, this life cycle stage is probably of limited importance for the epidemiology of bovine babesiosis (68).
Each tick stage feeds on the host's blood for a few days and then spends several months at the base of vegetation, developing to the next stage or, in the case of the adult female, laying about 2,000 eggs (70). Because the infection persists through molts (transstadial maintenance) and is transmitted transovarially (102, 120), it appears that the vertebrate host is not essential in the short term for maintaining the parasite in the tick population. Donnelly and Peirce showed that B. divergens persisted in I. ricinus as far as the second-generation larvae even when the intervening tick stages were fed on unsusceptible nonbovine host species (42). Under field conditions, I. ricinus requires about 3 years to complete a generation. This means that the infection may be retained in the tick population for at least 4 years even in the absence of a bovine host (103). It is surprising therefore that tick infection rates with B. divergens appear to be very low.
Gern and Brossard reported an infection level of only 1.1% (59), and this study did not take into account other Babesia spp. that may have been present (45). An even more sensitive molecular analysis of ticks collected in Slovenia by PCR and subsequent sequence analysis revealed an infection rate of only 2.2% with B. divergens-like parasites (45). Because the distribution of infected ticks is bound to be highly overdispersed, these estimates of prevalence have to be regarded with caution. It is possible, however, that ticks have a resistance mechanism against B. divergens, as not all females which feed on an infected bovine will become infected, and the progeny of an infected female will not all become infected (42). It has to be borne in mind that the bite of a single tick is sufficient to transmit the disease to a susceptible host (42).
While ticks are undoubtedly the most important nonbovine reservoir (82), nonsplenectomized cervids (with the exception of reindeer [134]), caprids, bovids (29, 49), and rodents (1, 25, 56, 97) appear to be resistant to B. divergens and are unlikely to represent significant reservoirs, the parasite is also maintained in the environment by cattle which have recovered from previous infections or carry mild subclinical infections. These parasitemias, which may persist for several years following the initial infection (37, 101) and often go unnoticed, may serve as a source of parasites for the infection of new ticks (42, 102). Moreover, it has been suggested that, similar to B. bovis and B. bigemina which have both been reported as causes of abortion, B. divergens may be transmitted transplacentally (48).
Calves up to 1 year of age, although fully susceptible to infection, are resistant to disease (103). This resistance may also be observed, although to a lesser degree, in heifers and steers up to 2 to 2.5 years of age (2, 33, 122). The mechanisms responsible for this inverse age resistance have been the subject of some controversy and will be discussed further in the section on immunity. In areas with high infection pressure, most animals become infected during this protected period and acquire immunity without showing clinical symptoms (31, 33). In older cattle, immunity is reinforced by repeated tick challenge. Thus, in areas where babesiosis is endemic, clinical cases tend to be rare, although the parasite and/or specific antibodies may be detectable in most animals (80, 103).
Outbreaks of clinical babesiosis are chiefly observed when this state of enzootic stability breaks down. Where ticks occur in small and scattered pockets or their numbers fluctuate due to climatic conditions or pesticide control, cattle are exposed to infected ticks only sporadically, and a proportion of animals retain their susceptibility (68, 103). Frequently there is a mosaic pattern of infected enclosures interspersed with uninfected ones. These areas with intermediate infection pressure tend to produce most outbreaks of severe redwater fever (82, 103). Very high losses may also be sustained when groups of susceptible animals are moved into areas of endemic babesiosis with high tick infestation (3). Conversely, the introduction of tick-infested cattle from an area of endemic disease to a clean farm with suitable tick habitat may establish new foci of infection (2, 40).
In the British Isles, babesiosis is chiefly associated with small herds (75, 103, 175) and with enterprises involved in beef production, particularly 1- to 2-year-old animals that have yet to reach slaughter weight. These animals, at a highly susceptible age, are often maintained on marginal tick-infested grazings until ready to be sold for finishing (75, 175). As with most parasitic diseases, stress due to other diseases, movement, etc., may cause a breakthrough of subclinical infections (33).
The proportion of parasitized animals which develop overt infection varies greatly (2, 12, 35, 40, 41, 55, 75, 73, 122, 175), blurring the relationship between infection and clinical disease. The number of infecting ticks (3) and the virulence of the B. divergens isolate (147) seem to be the most important factors determining the outcome of the infection (143). There is some evidence that metabolic or deficiency problems such as acetonemia and hypocupremia may be contributing factors (86). No difference has been reported in the susceptibility of breeds or sex (35, 82, 103).
Parasite Detection and Diagnosis
Numerous tests have been developed for diagnostic as well as epidemiological purposes. Cases of redwater fever are usually indicated by clinical symptoms, epidemiological season, herd and farm circumstances, and individual history (82). The clinical evidence is confirmed by examining Giemsa- or acridine orange-stained blood smears (178) or by testing for specific antibabesial antibodies with the immunofluorescence antibody test (IFAT) (see below).
For epidemiological surveys, the favored tests are serological. The IFAT is the most popular serological technique used both to distinguish between Babesia spp. (83, 104, 112) and to demonstrate the presence of babesial antibodies in a population (2, 3, 11, 12, 40, 60, 110, 175). IFAT clearly differentiates between antibodies to B. divergens and other bovine babesias (72, 104, 112) but not between B. divergens and B. capreoli from red deer (Cervus elaphus) (4, 83). Joyner et al. observed no variation attributable to strain differences (104).
The only other test commonly used for B. divergens infections is the enzyme-linked immunosorbent assay (ELISA) (10, 149). The advantage that this test has over IFAT is that interpretation of results is less subjective and it is easily automated for large numbers of samples. However, far more antigen is required, and problems arise with specificity unless blocking conditions are optimized (76) or purified antigen is used (28).
Under optimum conditions, IFAT and ELISA are equally effective in the detection of positive samples (10, 76). Gray and Kaye suggested that IFAT was adequate for epidemiological studies carried out on a small scale but that ELISA was preferable for large-scale surveys (76).
Many serological studies use nonbovine animals as sources of antigen because they are easier to buy and maintain. However, this practice can be problematic, as the parasite may vary antigenically in different hosts. Phillips et al. reported that monoclonal antibodies raised to a rat-adapted strain of B. divergens showed variable reactivity with field isolates (139). Fortunately, B. divergens antigen derived from gerbils behaves no differently than that obtained from cattle (76). Alternatively, antigen may be derived from parasites raised in in vitro culture (28).
One problem associated with all serological tests for babesiosis is that the relationship between antibody titers, the presence of parasites, and the state of protective immunity is not clear (151, 193). Antibodies may persist for long periods after the disease has cleared (40, 112), giving no information as to when an infection was acquired (104) and resulting in an overestimate of disease prevalence (40). In addition, antibody titers may be observed in the absence of protective immunity (193), and negative test results can occur in the presence of parasites or after acquisition of sterile immunity (55, 193).
Xenodiagnosis and molecular detection techniques are more reliable in this respect because they directly determine the presence of viable parasites and parasite DNA, respectively. Injection of test samples into susceptible animals such as splenectomized calves or gerbils is a very sensitive detection method (78, 101, 122), although the use of calves is costly. Passage in gerbils has the added advantage that other bovine piroplasms such as B. major and Theileria mutans do not become established in these animals (118). So far, PCR has been used mainly for the diagnosis of human cases (discussed in the section on human infections). Recently, however, Caccio et al. developed a “universal” PCR assay for the detection and identification of nine of the most common pathogenic bovine, equine, and rodent piroplasms, including B. divergens (24). The assay exploits the extensive genetic variability in a fragment of the otherwise highly conserved β-tubulin gene. Following specific amplification of the parasite DNA by nested PCR, the parasite species is identified by PCR-restriction fragment length polymorphism analysis.
In the past, the chief method for detecting B. divergens in the tick vector was the laborious microscopic examination of Giemsa-stained smears of gut or other tick tissues (59, 107), which is no doubt the reason why epidemiological studies with ticks are extremely rare. Today, xenodiagnosis and particularly PCR (45) have revolutionized this approach.
Treatment
For many years, three babesicides, quinuronium sulfate (Ludobal [Bayer Ltd.] and several generics), amicarbalide isethionate (Diampron; May and Baker Ltd.), and diminazene aceturate (Berenil, Hoechst Ltd.), were available in most European countries for the treatment of bovine babesiosis in Europe. In the 1970s a fourth, imidocarb diproprionate (Imizol; Schering Plough), was introduced, and it rapidly became the product of choice in those countries that licensed it, because in addition to its therapeutic utility, it also proved to be an effective prophylactic at twice the therapeutic dose. Today this is the only product on the market in most of Europe. Quinuronium sulfate and amicarbilide were withdrawn because of manufacturing safety issues, and diminazene, which is widely used in the tropics as both a babesicide and a trypanocide, was withdrawn from Europe for marketing reasons.
Imidocarb is most toxic when given intravenously, and intramuscular or subcutaneous administration is generally recommended (189). Side effects include coughing, muscular tremors, salivation, colic, and local irritation at the site of injection following administration of high doses. While it is regarded as being slower in action than quinuronium sulfate (82) it is the only babesicide that consistently clears the host of parasites (116). In the past, the persistence of small numbers of parasites in the bloodstream was deemed necessary for the maintenance of resistance to reinfection (9, 100, 157). Today the concept of premunition is no longer accepted. While a certain period of antigenic exposure is necessary before treatment to facilitate the establishment of immunity, cattle treated with imidocarb diproprionate end up with a solid sterile immunity (116). Long-term persistence of low-level parasitemia is now considered a disadvantage. Remaining parasites may give rise to recrudescence under adverse conditions, treated cattle may act as a source of infection (154), and parasites surviving at low levels of babesicide may acquire resistance.
Blood transfusion is recommended for animals with acute anemic anoxia, indicated by jaundiced or pale mucous membranes, constipation, and a body temperature of less than 38°C (159). While incompatibility reactions are slight, at least at the first transfusion (35), transfusion therapy may fail if the transfusion is given too late, if too little blood is given, if the transfusion rate is too high, or if pyrogenic bacteria are introduced inadvertently during the procedure (136). The same donor should be employed each time if repeated transfusions are necessary (35). To aid erythropoiesis in recovered patients, nonspecific supportive therapy is recommended, involving the use of iron preparations, dextrose, vitamins (B complex), purgatives, and fluid replacements (82, 136).
Anecdotal evidence from some areas of endemic babesiosis suggests that an increasing number of acute cases are unresponsive to treatment (73, 81, 165). Although resistance to babesicides is readily induced in the laboratory, the only study on this topic so far showed that all isolates from animals that were still parasitemic on a second veterinary visit were fully susceptible to babesicides (84). Mineral deficiencies (86) or changes in parasite virulence and host susceptibility (82) have been suggested as contributing factors. For this reason, and because imidocarb is associated with residue problems (189) and is not available throughout Europe (74), research into effective chemotherapy against B. divergens continues. Today drug screening is greatly helped by the availability of gerbils as model hosts (69) and an in vitro culture system for B. divergens (141, 182). In vitro culture systems may be used for determination of parasite uptake of radioactive purines such as hypoxanthine in the presence of candidate antibabesial compounds (16, 98).
Immunity
As mentioned earlier, calves less than 9 to 12 months of age are as susceptible as adult cattle to infection with B. divergens but are less likely to respond with clinical symptoms. This phenomenon, known as inverse age resistance, has been demonstrated in numerous epidemiological studies (3, 32, 33, 60, 73, 122, 171). Even among experimentally infected gerbils, immature animals are more resistant to disease than adults (108). In the few studies that found no evidence for inverse age resistance, infections were generally mild and transient, possibly masking any differences between age groups (18, 40). One notable exception was a case of fatal babesiosis in a 6-day-old calf (48).
It is now generally accepted that inverse age resistance is due to innate resistance in calves and is independent of the maternal immune status. Although offspring of resistant dams acquire specific antibodies (mainly immunoglobulin G) via colostrum, these immunoglobulins do not seem to be necessary for protection because calves of susceptible dams without specific antibodies are equally resistant (30). In vitro studies with B. bovis have shown that erythrocytes of very young calves were unfavorable to parasitic development, possibly because of the inhibitory effect of fetal hemoglobin (114). However, this erythrocytic resistance factor was lost within a matter of weeks. A low-molecular-weight antibody-independent serum component, on the other hand, prevented parasite growth over a period of several months.
The detrimental effects of splenectomy on innate immunity suggest that nonspecific cell-mediated mechanisms are also involved. Splenectomized calves and immature gerbils become fully susceptible to disease (37, 46, 108). Likewise, several primate, deer, and rodent species with innate resistance to B. divergens may suffer severe infections if they have been splenectomized (54, 56, 58, 134, 138). The cells involved in the innate immune system, macrophages and NK cells, are thought to degrade intraerythrocytic parasites by releasing soluble mediators (79). Infected erythrocytes are removed as the blood filters through the spleen (93, 138). As Babesia spp. are curiously resistant to oxidative radicals, these probably do not contribute significantly to nonspecific effector mechanisms (109).
Animals which recover from B. divergens infections generally develop specific immunity. In the past this immunity was thought to be due to premunition maintained by a persistent subclinical infection. Once the latent infection ceased, the animal was thought to be fully susceptible to reinfection. This concept was challenged as early as 1958 by Davies et al., who found that some cattle resisted challenge even though inoculation of their blood into susceptible animals failed to cause infection (37). Today it is widely accepted that sterile immunity may develop in cattle as well as gerbils (78, 101, 184). Nevertheless, immunity does not last indefinitely, and in the absence of exposure to further infection, the animal becomes susceptible to reinfection (103).
Specific immune mechanisms include both cellular and humoral components. Monocytes and lymphocytes are thought to be the main agents of cell-mediated acquired immunity. In cattle vaccinated against B. divergens, protection was correlated with elevated mononuclear cell proliferation (180). These monocytes are thought to engage in antibody-dependent cell-mediated cytotoxicity (61).
On the whole, the protective role of antibodies in B. divergens infections is poorly understood. It has been suggested that antibodies may inhibit entry of merozoites into red blood cells, as was shown during in vitro studies with B. microti (6). In addition, as opsonins they may stimulate phagocytosis of infected erythrocytes (60). However, passive transfer of immunity to susceptible animals with sera from immune animals was only successful in some cases (82, 138), while in others recipient animals succumbed to challenge infections (184). During primary parasitemia, the humoral response seems to be of little consequence. In cattle infected with B. divergens, antibodies can be demonstrated even before infected erythrocytes appear in blood smears, indicating that they have no inhibitory effect on parasite multiplication (31). During secondary infections, protection seems to depend on the high specificity of some antibodies rather than the total level of anti-B. divergens antibodies (63), as resistant animals frequently have very low levels of specific antibodies (32, 78, 129). On the other hand, cattle may retain high levels of antibodies for long periods of time (2, 3, 40, 112, 179) even though protection has ceased (77, 123, 144).
These observations strongly suggest that, like other protozoan parasites, B. divergens has evolved immunodominant decoy antigens which induce high antibody titers that are nonprotective (190, 191). However, effective specific immunity to conserved protective antigens may be much harder to detect. Complement-mediated lysis of parasites does not seem to be an important humoral effector mechanism, as B. divergens in vitro cultures grow well in medium supplemented with bovine sera that have not been heat inactivated. The importance of the spleen in the specific immune response is illustrated by the fact that removal of the spleen following recovery may result in clinical relapse (82, 103). Moreover, release of antibodies may be delayed in splenectomized animals, although eventually the same levels are reached as in intact animals (46, 179).
A one-sided cross-immunity seems to exist between B. divergens and B. major. While splenectomized calves which had recovered from a B. major infection were fully susceptible when challenged with B. divergens (104), infections with B. divergens provided strong immunity to challenge with B. major (17).
Control
Redwater fever is often only noticed at the onset of hemoglobinuria, when the disease is far advanced. Although therapy and transfusion will generally save an afflicted animal even at an advanced stage of the disease, it may continue to be severely debilitated for several months after recovery (116). Thus, for economic and animal welfare reasons, the best option is to prevent rather than treat infections.
Tick control.
Widespread destruction of typical tick habitat is ecologically unacceptable and may also be counterproductive because pockets of ticks usually survive to parasitize fully susceptible hosts (82). Taylor et al. suggested that utilization of suitable tick habitat by sheep, which are resistant to infection with B. divergens, may reduce infection rates in ticks (175). However, these areas would have to be kept clear of cattle for considerable periods, as B. divergens infections can persist in ticks for at least two generations even in the absence of a suitable host (42). The most practicable approach is the application of synthetic acaricides to cattle with slow-release devices or as pour-ons with high residual activity during the periods of greatest exposure (82, 143). Studies in Ireland (167) showed that the synthetic pyrethroid pour-ons flumethrin and deltamethrin protected cattle from ticks for 2 and 3 weeks, respectively. In Germany, Huwer et al. obtained protection against tick infestation and babesiosis with repeated treatments with flumethrin (95). This strategic approach led to a significant decline in clinical disease but did not interfere with seroconversion.
In areas where babesiosis is endemic, integrated control may be considered. This strategy aims to maintain enzootic stability by ensuring continuous contact of livestock with ticks and babesia parasites (111). However, to ensure that cattle become infected and immune to the parasites with minimum pathological effects, chemoprophylaxis can be integrated with vaccination.
Chemoprophylaxis.
Imidocarb, the only chemoprophylactic on the market, provides protection from clinical disease for 3 weeks (82) to 6 weeks (52) but allows a sufficient level of infection for immunity to develop. This strategy is highly effective if the host is guaranteed to contract babesiosis during the period of protection, either through a tick bite in areas where babesiosis is endemic or by inoculation of live parasites (153). Acquired immunity then takes over from the passive drug protection, and the animal passes smoothly to a resistant state without an intermediate clinical stage (86). Caution is advised if infection rates are sporadic or if very high doses of imidocarb are used, as a complete inhibition of parasite development will hinder the mounting of an adequate immune response (82, 153). The main problem associated with this approach is concern about drug residues in milk and beef, which has led to the withdrawal of imidocarb in several European countries.
So far no other suitable chemoprophylactics have been identified. Ryley claimed that subcutaneous depots of sparingly soluble salts of quinuronium protected animals for up to 3 or 4 months (157), but Purnell et al. reported that administration of the drug 1 day before inoculation of the parasite had no effect on the course of the infection (154). Taylor et al. investigated the potential of long-acting oxytetracyline (Terramycin LA; Pfizer) against bovine B. divergens infections (168). Although it has no therapeutic effect if given after the parasitemia has become patent, continuous prophylactic administration allows sufficient numbers of parasites to multiply for antibodies to be produced, while clinical effects are absent. Similar observations were made in gerbils infected with B. divergens (127). Nevertheless, Taylor et al. rejected the use of continuous oxytetracyline administration as too costly and risky because of the likelihood of the development of resistance in bacterial pathogens (168).
Vaccination.
The best control option, vaccination, has been carried out with various degrees of success with live and dead whole parasites, crude parasite extracts, and isolated parasite antigens. Live vaccines have proven very effective and reasonably safe, particularly when vaccination was restricted to cattle less than 1 year of age, when they still have natural resistance to the disease. Parasites for vaccination are derived from either infected splenectomized calves (32) or intact gerbils (55, 77, 78). The use of gerbils is considered safer than that of cattle because the simultaneous transmission of other pathogens to which cattle may be susceptible is highly unlikely. Moreover, vaccination with infected gerbil erythrocytes eliminates the risk of sensitization to bovine red cell antigens and autoimmunity (55).
Although effective, live vaccines have several disadvantages, the most obvious being that the vaccine strain itself may cause babesiosis unless its virulence can be controlled. So far, all the methods used to reduce the virulence of live parasites in cattle have failed to produce consistent results. These methods have included rapid passage in splenectomized calves (171), which is the attenuation method of choice for B. bovis; rapid passage in gerbils (133); reduction of the infective dose (152); and the use of gamma irradiation (115, 150, 151, 152, 171, 174). Evidence suggests that B. divergens may be attenuated by continuous in vitro culture (186), but no trials in cattle with such material have been successful. Cattle may be protected with low doses of imidocarb injected a few days prior to vaccination (74, 77, 78, 116, 153). This approach is a controlled version of the prophylactic use of imidocarb in the field, and while effective in young cattle, it is relatively expensive and labor intensive. Furthermore, adult animals are not reliably protected (77).
As mentioned earlier, there are other problems associated with living or partially living vaccines which make a bovine source of vaccine undesirable. However, the use of alternative host species erythrocytes as vaccine carriers is also associated with difficulties. Although 150 to 200 vaccine doses for cattle may be obtained from a single gerbil (77), these animals are necessarily limiting in terms of vaccine supply. The parasite can be grown efficiently in sheep (29, 192) and human (141, 192) erythrocytes in vitro, but neither of these are considered suitable candidates, and in fact it is questionable whether any blood-based vaccine would be licensed with current concerns about pathogens breaching species barriers. The supply problem is compounded by poor shelf life and the difficulty of maintaining an intact cold chain for refrigerated material (55, 74). Cryopreservation is associated with a massive reduction in infectivity, especially if the vaccine is not used immediately after thawing (87).
In order to overcome these difficulties, many workers have used nonliving preparations to immunize cattle. These trials have used a wide variety of materials, from dead parasites to single proteins (46, 92, 172, 173, 184, 187). None of these materials proved as immunogenic as live parasites, and toxic adjuvants were often required to achieve acceptable levels of immunity. In the most promising work, an acid fraction of concentrated B. divergens-infected erythrocytes isolated by isoelectric focusing produced good immunity against a tick-transmitted heterologous strain under field conditions (169). Further separation of the acidic fraction by passage through concanavalin A-Sepharose revealed that the protective antigens were contained in the fraction which did not bind to the lectin (170). Despite this relative success, the problems of vaccine stability and supply were not resolved.
Attempts to identify protective antigens which, alone or in combination, may form the basis of a recombinant vaccine have met with various degrees of success. Early studies focused on the merozoite surface coat, which is sloughed off during the process of host cell invasion and accumulates as a soluble exoantigen in in vitro culture supernatants (184). However, the response to immunization with culture-derived exoantigens has been mixed. While Winger et al. reported that the soluble antigens provided no adequate protection against homologous challenge (184), researchers in France produced some immunity in gerbils against homologous (66) and heterologous (140) challenges. Similarly, in cattle the exoantigens induced cellular and humoral responses which were consistent with protective immunity (180, patent). These researchers identified several culture-derived immunodominant exoantigens which elicited a constant humoral response in all potential hosts of B. divergens, namely humans, cattle, and gerbils (66, 180), and were conserved among isolates from various geographical areas (140). One of them, a 37-kDa glycoprotein (Bd37), was localized to internal vesicles and the cell surface of fixed merozoites and excreted by live merozoites in culture (27). Another immunodominant antigen of the hsp70 family was mainly cytoplasmic and appeared as an early antigen during acute parasitemia and remained detectable for several months (26). The group suggested that these immunodominant antigens may be important for the induction of the protective immune response. However, it is important to remember that many parasites have developed immunodominant antigens which are nonprotective or even immunosuppressive and are used to decoy the host's protective immune response (191).
Winger et al. showed that an affinity-purified 50- to 60-kDa merozoite polypeptide conferred immunity to homologous challenge in gerbils (188) and that the corresponding monoclonal antibody inhibited merozoite invasion in in vitro culture (185). Later the same authors suggested that the protective antigen may represent the dimer or protein complex of another 24- to 29-kDa antigen which induced partial protection in gerbils against homologous challenge (187).
Rhoptry-associated proteins may become the first targets of generic recombinant vaccines. Homologues of these proteins, which are thought to be involved in host cell invasion, have been identified in several Babesia spp., including B. bovis, B. bigemina, B. canis, and B. ovis as well as B. divergens (163). In most species, rhoptry-associated protein molecules are encoded by multicopy gene families, suggesting their possible involvement in antigenic variation and host immune evasion. This has implications for the development of a vaccine, as all copies of the rhoptry-associated protein genes may be required in a combined vaccine formulation.
Attempts to control B. divergens infections by stimulating nonspecific immunity failed. Inoculation of Mycobacterium bovis bacillus Calmette-Guérin, which was found to protect mice against subsequent challenge with B. microti or B. rhodaini, had no effect on B. divergens infections in splenectomized or intact calves (19). In gerbils, M. bovis BCG was protective, but only at very high doses. Vaccination with Propionibacterium acnes or zymosan A from Saccharomyces cerevisiae failed to elicit a protective response (109).
HUMAN BABESIOSIS
Disease Symptoms and Pathology
During the incubation period of 1 to 3 weeks, patients frequently complain of general weakness and discomfort. Acute illness appears suddenly, generally with hemoglobinuria as the presenting symptom (176). The subsequent nonspecific clinical presentation can be easily confused with malaria; jaundice due to severe hemolysis is accompanied by persistent nonperiodic high fever (40 to 41°C), shaking chills, intense sweats, headaches, and myalgia as well as lumbar and abdominal pain. Vomiting and diarrhea may be present (65). Total hemoglobin levels may fall to 70 to 80 g/liter, and values as low as 40 g/liter have been reported (34). Haptoglobin is decreased dramatically, and all the usual biochemical tests of hemolysis are positive (176). The liver may be moderately enlarged and painful. In the most severe cases, patients develop shock-like symptoms, with renal failure induced by intravascular hemolysis and pulmonary edema (65).
Distribution and Epidemiology
Although at the time it was purported to be Babesia bovis, B. divergens was probably the causative agent in the first reported case of human babesiosis, which occurred in a splenectomized 33-year-old Yugoslav who grazed his babesia-infected cattle on tick-infested pastures (162). The parasite was first positively identified in a splenectomized deep-sea fisherman from Northern Ireland, who probably contracted the disease during a camping holiday (54). In contrast to North America, where human babesiosis is considered an emerging zoonosis with a mortality rate of about 5% (93), babesia infections in Europe are much less common but much more severe and require immediate aggressive treatment. To date, 31 human cases of autochthonously acquired babesia infections have come to medical attention in Europe, all of which occurred in splenectomized individuals (38, 65, 130). This surprisingly low incidence in spite of a sizeable risk group suggests that human infections are likely to be underreported (34), perhaps partly because of misdiagnosis as malaria in early cases. Indeed, over half of all cases have been reported since 1985, reflecting growing awareness of the zoonosis among the medical profession. Although B. divergens was only positively identified in 74% of all human cases—no unequivocal diagnosis was made in the remainder—it is highly probable that this species was the cause of all of them. Nine (29%) of these infections proved fatal (38, 65).
Human cases of B. divergens infection have been reported in France, Britain, Ireland, Spain, Sweden, Switzerland, the former Yugoslavia, and the former USSR (38, 65). Geographically they coincide with B. divergens-infected cattle populations and I. ricinus-infested areas (106), involving inhabitants of rural areas who are exposed to ticks by virtue of their occupation or their recreational activities (176). Most cases are reported between May and September or October, during the main season of tick activity (65, 176). Thus, although the vector has never been formally identified, I. ricinus is generally regarded as the tick species responsible for the transmission of B. divergens to humans, and experimental evidence shows that human isolates can be transmitted by I. ricinus (120). In an isolated case involving a resident of the Canary Islands, where I. ricinus is absent, a close relative, Ixodes ventalloi, was suggested as having been the vector (135).
The observations that all human cases of B. divergens infection involved splenectomized individuals (65, 38) and that susceptibility in other primates was also associated with splenectomy (56) led to the conclusion that infections in humans were normally prevented by the activity of the spleen (57). Controversially, Gorenflot et al. suggested that spleen-intact people may act as asymptomatic carriers (64). A serosurvey of 190 French blood donors, each of whom had a history of tick bite in areas where bovine infection with B. divergens is endemic, revealed two whose sera reacted with the parasite's antigen by IFAT. It is unclear, however, whether the presence of antibodies indicated a subpatent chronic infection or an asymptomatic infection which was contracted and rapidly cleared.
Attempts to demonstrate latent B. divergens infections in intact humans exposed to tick bites in an area of Ireland where the disease is enzootic by injecting their blood into splenectomized calves failed (57), and transfusion-associated human cases have never been reported (65). It is not known whether immunosuppression other than splenectomy also predisposes people to B. divergens infection, but it is likely that when immunosuppression occurs in addition to splenectomy, susceptibility is further increased, for example, in cases of Hodgkin's disease (50, 126). Transplacental or perinatal transmission in human hosts has not been documented (176).
B. divergens is understood to be restricted to Europe. However, in the United States in 1992, a 73-year-old splenectomized human in Missouri died of an infection with a parasite that was morphologically, antigenically, and genetically related to B. divergens but was not infective to gerbils (88). More recently, a case occurred in Kentucky, in which the pathogen was tentatively identified as B. divergens (7).
Diagnosis
Immediate diagnosis is essential, because the disease usually progresses extremely rapidly. The high parasitemia present during acute infections (varying from 5 to 80% of erythrocytes) is easily detected microscopically (177), making examination of thin blood smears stained with Giemsa or Wright's stain the method of choice (65). However, identification of the species may be complicated by the atypical appearance of B. divergens in human erythrocytes (63). Most significantly, the parasite may be confused with Plasmodium spp., which may be detrimental, because most antimalaria drugs are ineffective (65). A history of splenectomy, absence of recent travel to malaria-endemic areas, absence of recent blood transfusion, and particular cytological characteristics (pyriform parasites, absence of parasitic pigment, or hemozoin) are all strong indications for babesial infection (65). The diagnosis can be confirmed by xenodiagnosis with either splenectomized calves, in which the parasite assumes its typical morphology (50), or gerbils, in which B. divergens causes an acute, fatal disease within 3 to 6 days (119).
Serological diagnosis by IFAT is not practicable because specific antibodies do not become detectable until at least 1 week after the onset of illness at which point the infection may have proven fatal. IFAT can be used, however, for retrospective evaluation of human cases by distinguishing B. divergens from Plasmodium falciparum, B. microti, and WA1 (a newly recognized species first detected in a patient from Washington State) with which it has only limited cross-reactivity (93, 176). Blood autoanalyzers are unable to distinguish between uninfected erythrocytes and those infected with babesia (21). Molecular diagnostic tools, though not yet routinely used, are very promising. A PCR assay which was initially developed for the detection of B. microti (137) is based on the universal primer amplification of a fragment of the small subunit rRNA gene. Although most of the small subunit rRNA gene is highly conserved among babesias, this fragment is heterologous between Babesia spp. and other intraerythrocytic protozoal parasites as well as within the genus Babesia itself (137). Because the assay distinguishes readily between B. divergens, B. microti (160), and Plasmodium spp. (137), it provides a valuable adjunct if not an alternative to conventional methods for the diagnosis of human babesiosis.
Treatment
In early cases, treatment with well-recognized antimalarial drugs such as chloroquine, quinine, mefloquine and pyrimethamine, and pentamidine proved unsuccessful (34, 65). A mild infection was resolved after the use of pentamidine and cotrimoxazole (156), but because of its side effects and renal toxicity, pentamidine was not considered suitable for treatment of patients with acute intravascular hemolysis (176). Berenil, an antitrypanosomal compound known to be effective in veterinary cases, failed to cure a patient with a severe B. divergens infection (161). Thus, almost all cases in the past ended fatally, with general organ failure 4 to 7 days after the initial hemoglobinuria (106), coma, and death (65).
Today, fast and aggressive treatment involving prompt specific therapy to reduce parasitemia combined with supportive treatment such as blood exchange transfusion and ventilation has reduced the mortality rate due to B. divergens to approximately 40%. At present, the recommended treatment consists of massive blood exchange transfusion followed by administration of intravenous clindamycin together with oral quinine (15, 62). After clearance of parasites and resolution of hemolysis, a nonregenerative anemia may persist for at least 1 month, requiring additional blood transfusion (176). Although in vitro experiments suggest that clindamycin is effective on its own (16), the risk of rapidly increasing parasitemia, consequent hemolysis, and renal damage is generally considered great enough to make massive blood exchange prior to chemotherapy advisable (65). However, a mild infection (1% parasitemia) was resolved by administration of clindamycin and quinine without blood transfusion (38).
Further promising agents for treating B. divergens infections in humans are imidocarb diproprionate and the hydroxynaphthoquinone atovaquone. Imidocarb, although not licensed for human use, is the most effective agent for treating B. divergens infections in cattle. The drug was used successfully under special license to treat two Irish cases (E. L. Egan and C. Duggan, Int. Soc. Haematol. 23rd Congress, Am. Soc. Hematol. 32nd Annu. Meet., 1990). In contrast, atovaquone is registered for use in humans and was shown to be superior to imidocarb in clearing the parasite from human erythrocytes in vitro and from the gerbil model in vivo (142). The drug was also effective prophylactically at very low daily doses, and the authors suggested that atovaquone could be used both for the treatment of clinical disease and also for prevention in asplenic individuals exposed to infection. Certain cholesterol-lowering agents such as lovastatin and simvastatin were found to inhibit the in vitro development of B. divergens and P. falciparum in human erythrocytes (85). The authors argued that this was due to the parasites' dependence on the import of fatty acids and cholesterol from the host's plasma. For in vivo use, however, new derivatives with improved bioavailability would have to be designed because both lovastatin and simvastatin have short half-lives in plasma and are rapidly taken up by the liver.
Prevention
In the United States, public health strategies are being considered to protect human populations against zoonotic babesial infections by applying acaricides, destroying tick habitat, and removing animals which may serve as reservoirs for the zoonotic babesias and/or ticks (177). In Europe, the extremely low incidence of the infection hardly warrants such measures. However, postsplenectomy patients and other immunocompromised individuals should be cautioned about the risk of visiting areas where babesiosis is enzootic, particularly in the period from March to November, when the disease is most prevalent in cattle (34). Personal protective measures include avoidance of exposure to ticks by wearing appropriate clothing and tick repellents; thorough body inspection for the presence of ticks after being outdoors; and rapid removal of any ticks (65). Among medical personnel, awareness of the zoonosis and its malaria-like symptoms should be raised to improve prevention and treatment of this rare but life-threatening disease.
CONCLUSION
Compared to the tropical and subtropical bovine Babesia spp. and the other chief zoonotic agent in the genus, B. microti, relatively little research has been carried out on B. divergens, and many questions regarding the parasite's epidemiology and biology and the host's response remain unanswered. In order to fully understand the zoonotic potential of B. divergens, it will be necessary to determine why the incidence of human infections in Europe is apparently so low even though the vector is widespread and splenectomy is not uncommon. In this context, the taxonomic position of “B. divergens-like parasites” isolated from humans outside areas where bovine babesiosis is endemic will have to be explained.
A better understanding of some anomalies of the parasite's epidemiology in cattle, such as the low incidence of babesia infections in the tick population even in areas where babesiosis is endemic, will lead to improved control mechanisms. Little is known about the processes of host cell invasion, the parasite's metabolic requirements, and the host's immunological response, yet these parasite-host cell interactions ultimately decide whether the erythrocyte is lysed to release new infective merozoites or the parasite is destroyed within its host cell. Further research into the mechanisms may start new trends in chemotherapy and rational drug development. Recent advances in vaccine development are promising, but until we can identify conserved antigens which are vital to the functioning of the parasite, the search for protective antigens will continue in a haphazard fashion. Research on these questions is important not only for improving our general understanding of apicomplexan infections, but also because it has major implications for agriculture in Europe, with the current emphasis on extensification, sustainable agriculture, care for the environment, and avoidance of drug residues in food animals.
Acknowledgments
We are grateful to Bernard Kaye for formatting the photographs.
REFERENCES
- 1.Adam, K. M. G., and D. A. Blewett. 1974. Experimental infection of mice with Babesia divergens. J. Protozool. 21:448. [Google Scholar]
- 2.Adam, K. M. G., and D. A. Blewett. 1978. A serological survey for Babesia in cattle in Scotland. II. The occurrence of antibody in the population and the distribution of infected herds. Ann. Trop. Med. Parasitol. 72:417-428. [DOI] [PubMed] [Google Scholar]
- 3.Adam, K. M. G., D. A. Blewett, T. J. Collins, and J. T. Edgar. 1978. Outbreaks of babesiasis on two farms in Scotland. Br. Vet. J. 134:428-433. [DOI] [PubMed] [Google Scholar]
- 4.Adam, K. M. G., D. A. Blewett, D. W. Brockelsby, and G. A. M. Sharman. 1976. The isolation and characterisation of a Babesia from red deer (Cervus elaphus). Parasitology 73:1-11. [DOI] [PubMed] [Google Scholar]
- 5.Aeschlimann, A., M. Brossard, and G. Quenet. 1975. Contribution a la connaissance des piroplasmes de Suisse. Acta Trop. 32:281-289. [PubMed] [Google Scholar]
- 6.Bautista, C. R., and J. P. Kreier. 1979. Effect of immune serum on the growth of Babesia microti in hamster erythrocytes in short-term culture. Infect. Immun. 23:470-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beattie, J. F., M. L. Michelson, and P. J. Holman. 2002. Acute babesiosis caused by Babesia divergens in a resident of Kentucky. N. Engl. J. Med. 347:697-698. [DOI] [PubMed] [Google Scholar]
- 8.Ben Musa, N., and R. S. Phillips. 1991. The adaptation of three isolates of Babesia divergens to continuous culture in rat erythrocytes. Parasitology 103:165-170. [DOI] [PubMed] [Google Scholar]
- 9.Beveridge, C. G., J. Willis Thwaite, and G. Sheperd. 1960. A field trial of amicarbalide — a new babesicide. Vet. Rec. 72:383-386. [Google Scholar]
- 10.Bidwell, D. E., P. Turp, L. P. Joyner, R. C. Payne, and R. E. Purnell. 1978. Comparisons of serological tests for babesia in British cattle. Vet. Rec. 103:446-449. [DOI] [PubMed] [Google Scholar]
- 11.Blewett, D. A., and K. M. G. Adam. 1976. The distribution of Babesia divergens in cattle in Scotland. Trans. R. Soc. Trop. Med. Hyg. 70:284. [Google Scholar]
- 12.Blewett, D. A., and K. M. G. Adam. 1978. A serological survey for Babesia in cattle in Scotland. I. Assessment of the method by the results from the outlying islands. Ann. Trop. Med. Parasitol. 72:405-415. [DOI] [PubMed] [Google Scholar]
- 13.Bool, P. H., E. Goedbloed, and H. J. W. Keidel. 1961. De Babesia-soorten van het rund in Nederland: Babesia divergens en Babesia major. Tijdschr. Diergeneeskd. 1:28-37. [Google Scholar]
- 14.Bouattour, A., and M. A. Darghouth. 1996. First report of Babesia divergens in Tunisia. Vet. Parasitol. 63:161-165. [DOI] [PubMed] [Google Scholar]
- 15.Brasseur, P., S. Lecoublet, N. Kapel, L. Favennec, and J. J. Ballet. 1996. Quinine in the treatment of Babesia divergens infections in humans. Eur. J. Clin. Microbiol. Infect. Dis. 15:840-841. [DOI] [PubMed] [Google Scholar]
- 16.Brasseur, P., S. Lecoublet, N. Kapel, L. Favennec, and J. J. Ballet. 1998. In vitro evaluation of drug susceptibilities of Babesia divergens isolates. Antimicrob. Agents Chemother. 42:818-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brocklesby, D. W., D. L. Harradine, and E. R. Young. 1976. Babesia major in Britain: cross-immunity trials with Babesia divergens in splenectomised calves. Res. Vet. Sci. 21:300-302. [PubMed] [Google Scholar]
- 18.Brocklesby, D. W., E. Harness, and S. A. Sellwood. 1971. The effect of age on the natural immunity of cattle to Babesia divergens. Res. Vet. Sci. 12:15-17. [PubMed] [Google Scholar]
- 19.Brocklesby, D. W., and R. E. Purnell. 1977. Failure of BCG to protect calves against Babesia divergens infection. Nature (London). 265:343. [DOI] [PubMed] [Google Scholar]
- 20.Brossard, M., and A. Aeschlimann. 1975. Piroplasmoses bovines en Suisse italienne. Schweiz. Arch. Tierheilk. 117:287-292. [PubMed] [Google Scholar]
- 21.Bruckner, D. A., L. S. Garcia, R. Y. Shimizu, E. J. C. Goldstein, P. M. Murray, and G. S. Lazar. 1985. Babesiosis: problems in diagnosis with autoanalyzers. Am. J. Clin. Pathol. 83:520-521. [DOI] [PubMed] [Google Scholar]
- 22.Brun-Hansen, H., D. A. Christensson, F. Hardeng, and H. Gronstol. 1997. Experimental infection with Ehrlichia phagocytophila and Babesia divergens in cattle. Zentralbl. Veterinärmed. 44:235-243. [DOI] [PubMed] [Google Scholar]
- 23.Brun-Hansen, H., D. A. Christensson, D. M. Eide, and H. Gronstol. 1998. Experimental infection with Babesia divergens in cattle persistently infected with bovine virus diarrhoea virus. Zentralbl. Veterinarmed. 45:269-277. [DOI] [PubMed] [Google Scholar]
- 24.Caccio, S., C. Camma, M. Onuma, and C. Severini. 2000. The beta-tubulin gene of Babesia and Theileria parasites is an informative marker for species discrimination. Int. J. Parasitol. 30:1181-1185. [DOI] [PubMed] [Google Scholar]
- 25.Canning, E. U., R. Killick-Kendrick, and J. B. Monk. 1976. Morphology of piroplasms in abnormal hosts and the identification of piroplasms of humans. J. Trop. Med. Hyg. 79:5-8. [PubMed] [Google Scholar]
- 26.Carcy, B., E. Precigout, A. Valentin, A. Gorenflot, R. T. Reese, and J. Schrevel. 1991. Heat shock response of Babesia divergens and identification of the hsp70 as an immunodominant early antigen during ox, gerbil and human babesiosis. Biol. Cell 72:93-102. [DOI] [PubMed] [Google Scholar]
- 27.Carcy, B., E. Precigout, A. Valentin, A. Gorenflot, and J. Schrevel. 1995. A 37-kilodalton glycoprotein of Babesia divergens is a major component of a protective fraction containing low-molecular-mass culture-derived exoantigens. Infect. Immun. 63:811-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chauvin, A., M. L'Hostis, A. Valentin, E. Precigout, N. Cesbron-Zeggane, and A. Gorenflot. 1995. Babesia divergens: an ELISA with soluble parasite antigen for monitoring the epidemiology of bovine babesiosis. Parasite 2:257-262. [DOI] [PubMed] [Google Scholar]
- 29.Chauvin, A., A. Valentin, L. Malandrin, and M. L'Hostis. 2002. Sheep as a new experimental host for Babesia divergens. Vet. Res. 33:429-433. [DOI] [PubMed] [Google Scholar]
- 30.Christensson, D. A. 1987. Clinical and serological response after experimental inoculation with Babesia divergens of newborn calves with and without maternal antibodies. Acta Vet. Scand. 28:381-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensson, D. A. 1989. Inverse age resistance to experimental Babesia divergens infection in cattle. Acta Vet. Scand. 30:453-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christensson, D. A., and T. Moren. 1987. Seroresponse (IgG) after vaccination and natural infection of cattle with Babesia divergens. Acta Vet. Scand. 28:393-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensson, D. A., and M. A. Thorburn. 1987. Age distribution of naturally occurring acute babesiosis in cattle in Sweden. Acta Vet. Scand. 28:373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke, C. S., E. T. Rogers, and E. L. Egan. 1989. Babesiosis: under-reporting or case-clustering? Postgrad. Med. J. 65:591-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins, J. D., T. O Nuallain, and A. R. Ferguson. 1970. Observations of bovine babesiosis in Ireland. Ir. Vet. J. 24:42-51. [Google Scholar]
- 36.Coombs, W. G. 1977. Bovine babesiosis: a case reported in Devon, England. Can. Vet. J. 18:193-195. [PMC free article] [PubMed] [Google Scholar]
- 37.Davies, S. F. M., L. P. Joyner, and S. B. Kendall. 1958. Studies on Babesia divergens (M'Fadyean & Stockman, 1911). Ann. Trop. Med. Parasitol. 52:206-215. [DOI] [PubMed] [Google Scholar]
- 38.Denes, E., J. P. Rogez, M. L. Darde, and P. Weinbreck. 1999. Management of Babesia divergens babesiosis without a complete course of quinine treatment. Eur. J. Microbiol. Infect. Dis. 18:672-673. [DOI] [PubMed] [Google Scholar]
- 39.Donnelly, J. 1973. Epidemiology of babesia infection in cattle. Proc. R. Soc. Med. 66:774-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donnelly, J., L. P. Joyner, and P. J. Crossman. 1972. The incidence of Babesia divergens infection in a herd of cattle as measured by the indirect immunofluorescent antibody test. Res. Vet. Sci. 13:511-514. [PubMed] [Google Scholar]
- 41.Donnelly, J., and J. C. McKellar. 1970. The effect of weather and season on the incidence of redwater fever in cattle in Britain. Agric. Meteorol. 7:5-17. [Google Scholar]
- 42.Donnelly, J., and M. A. Peirce. 1975. Experiments on the transmission of Babesia divergens to cattle by the tick Ixodes ricinus. Int. J. Parasitol. 5:363-367. [DOI] [PubMed] [Google Scholar]
- 43.Donnelly, J., L. P. Phipps, and J. Konrad. 1984. Infectivity in bovine hosts of Babesia divergens and Babesia major from in vitro culture. Parasitology 89:lxxvi.
- 44.Drössigk, U., D. Mielke, and M. Adam. 1993. Morphologische Untersuchungen an Babesia divergens beim kontinuierlichen Transfer in Meriones unguiculatus. Appl. Parasitol. 34:279-282. [PubMed] [Google Scholar]
- 45.Duh, D., M. Petrovec, and T. Avsic-Zupanc. 2001. Diversity of babesia infecting European sheep ticks (Ixodes ricinus). J. Clin. Microbiol. 39:3395-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edelhofer, R., A. Kanout, M. Schuh, and E. Kutzer. 1998. Improved disease resistance after Babesia divergens vaccination. Parasitol. Res. 84:181-187. [DOI] [PubMed] [Google Scholar]
- 47.Reference deleted.
- 48.Egeli, A. K. 1996. Babesiosis in a six-day-old calf. Vet. Rec. 139:344-345. [DOI] [PubMed] [Google Scholar]
- 49.Enigk, K., and K. Friedhoff. 1962. Zur Wirtsspezifität von Babesia divergens (Piroplasmidae). Z. Parasitenkd. 21:238-256. [DOI] [PubMed] [Google Scholar]
- 50.Entrican, J. H., H. Williams, I. A. Cook, W. M. Lancaster, J. C. Clark, L. P. Joyner, and D. Lewis. 1979. Babesiosis in man: report of a case from Scotland with observations on the infecting strain. J. Infect. 1:227-234. [Google Scholar]
- 51.Euzeby, J. 1989. Sur l'epidemiologie de la babesiose humaine europeenne. Consequences prophylactiques. Bull. Acad. Natl. Med. 173:31-37. [PubMed] [Google Scholar]
- 52.Euzeby, J., and P. H. Simon. 1983. Epidémiologie de la babésiose bovine á Babesia divergens en France. Cinétique comparée des populations d'Ixodes ricinus et de fréquence de la babésiose. Conséquences pour la prophylaxie de la babésiose. Chimioprévention par un dérivé de la barbanilide, le 4 a 65 ou H. R. 2073 (Imidocarb), p. 1-17. In J. Euzeby and J. Geury (ed.), Parasitological Symposium, Lyons, France. European Commission.
- 53.Fameree, L., C. Cotteleer, and H. Antoine. 1977. La babésiose bovine en Belgique, une anthropozoonose envahissante et méconnue. Incidence des babésioses animales sur la santé humaine. Revue Med. Liege 12:383-390. [PubMed] [Google Scholar]
- 54.Fitzpatrick, J. E., C. C. Kennedy, M. G. McGeown, D. G. Oreopoulos, J. H. Robertson, and M. A. Soyannwo. 1968. Human case of piroplasmosis (babesiosis). Nature 217:861-862. [DOI] [PubMed] [Google Scholar]
- 55.Friedhoff, K. T., D. Ganse-Dumrath, C. Weber, and I. Müller. 1989. Epidemiology and control of Babesia divergens infections in northern Germany. Proc. 8th Nat. Vet. Hemoparasite Disease Conf. St. Louis, p. 441-449.
- 56.Garnham, P. C. C., and R. S. Bray. 1959. The susceptibility of the higher primates to piroplasms. J. Protozool. 6:352-355. [Google Scholar]
- 57.Garnham, P. C. C., J. Donnelly, H. Hoogstraal, C. C. Kennedy, and G. A. Walton. 1969. Human babesiosis in Ireland: Further observations and the medical significance of this infection. Br. Med. J. 4:768-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garnham, P. C. C., and A. Voller. 1965. Experimental studies on Babesis divergens in rhesus monkeys with special reference to its diagnosis by serological methods. Acta Protozool. 3:183-187. [Google Scholar]
- 59.Gern, L., and M. Brossard. 1986. Evolution annuelle de l'infestation de bovins par la tique Ixodes ricinus L. et de l'infection de ces ectoparasites par Babesia divergens dans le Clos du Doubs Jura, Suisse. Schweiz. Archiv. Tierheilkd. 128:361-363. [PubMed] [Google Scholar]
- 60.Gern, L., A. Kindler, and M. Brossard. 1988. Annual evolution of cattle immunity against Babesia divergens in Northern Switzerland. Prev. Vet. Med. 6:9-16. [Google Scholar]
- 61.Goff, W. L., G. G. Wagner, and T. M. Craig. 1984. Increased activity of bovine ADCC effector cells during acute Babesia bovis infection. Vet. Parasitol. 16:5-15. [DOI] [PubMed] [Google Scholar]
- 62.Gorenflot, A., C. Bazin, and P. Ambroise-Thomas. 1987. Babésioses humaines: traitement des formes graves. Presse Med. 16:1099. [PubMed] [Google Scholar]
- 63.Gorenflot, A., P. Brasseur, E. Precigout, M. L'Hostis, A. Marchand, and J. Schrevel. 1991. Cytological and immunological responses to Babesia divergens in different hosts: ox, gerbil, man. Parasitol. Res. 77:3-12. [DOI] [PubMed] [Google Scholar]
- 64.Gorenflot, A., M. Marjolet, M. L'Hostis, A. Couatarmanac'h, and A. Marchand. 1987. Existence probable en France de babésioses humaines asymptomatiques, p. 134. In Proceedings of the Third International Conference on Malaria and Babesiosis, Annecy, France. Fondation Marcel Merieux, Lyon, France.
- 65.Gorenflot, A., K. Moubri, E. Precigout, B. Carcy, and T. P. M. Schetters. 1998. Human babesiosis. Ann. Trop. Med. Parasitol. 92:489-501. [DOI] [PubMed] [Google Scholar]
- 66.Gorenflot, A., E. Precigout, G. Bissuel, O. Lecointre, P. Brasseur, E. Vidor, M. L'Hostis, and J. Schrevel. 1990. Identification of major Babesia divergens polypeptides that induce protection against homologous challenge in gerbils. Infect. Immun. 58:4076-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grande, N., E. Precigout, M. L. Ancelin, K. Moubri, B. Carcy, J. L. Lemesre, H. Vial, and A. Gorenflot. 1997. Continuous in vitro culture of Babesia divergens in a serum-free medium. Parasitology 115:81-89. [DOI] [PubMed] [Google Scholar]
- 68.Gray, J. S. 1980. Studies on the activity of Ixodes ricinus in relation to the epidemiology of babesiosis in Co Meath, Ireland. Br. Vet. J. 136:427-436. [DOI] [PubMed] [Google Scholar]
- 69.Gray, J. S. 1983. Chemotherapy of Babesia divergens in the gerbil, Meriones unguiculatus. Res. Vet. Sci. 35:318-324. [PubMed] [Google Scholar]
- 70.Gray, J. S. 1991. The development and seasonal activity of the tick, Ixodes ricinus: a vector of Lyme borreliosis. Rev. Med. Vet. Entomol. 79:323-333. [Google Scholar]
- 71.Gray, J. S. 1999. Tick-borne pathogen interactions. Infect. Dis. Rev. 1:117-121. [Google Scholar]
- 72.Gray, J. S., and A. J. De Vos. 1981. Studies on a bovine babesia transmitted by Hyalomma marginatum rufipes Koch 1844. Onderstepoort J. Vet. Res. 48:215-223. [PubMed] [Google Scholar]
- 73.Gray, J. S., E. Fitzgerald, and K. L. Strickland. 1983. Prevalence of clinical babesiosis in an area in north Co. Meath, Ireland. Vet. Rec. 113:537-539. [PubMed] [Google Scholar]
- 74.Gray, J. S., and P. Gannon. 1992. Preliminary development of a live drug-controlled vaccine against bovine babesiosis with the Mongolian gerbil, Meriones unguiculatus. Vet. Parasitol. 42:179-188. [DOI] [PubMed] [Google Scholar]
- 75.Gray, J. S., and L. N. Harte. 1985. An estimation of the prevalence and economic importance of clinical bovine babesiosis in the Republic of Ireland. Ir. Vet. J. 39:75-78. [Google Scholar]
- 76.Gray, J. S., and B. Kaye. 1991. Studies on the use of gerbil-derived Babesia divergens antigen for diagnosis of bovine babesiosis. Vet. Parasitol. 39:215-224. [DOI] [PubMed] [Google Scholar]
- 77.Gray, J. S., B. Kaye, P. J. Talty, and C. McSweeney. 1995. The field use of a gerbil-derived and drug-controlled live vaccine against bovine babesiosis in Ireland. Ir. Vet. J. 48:358-362. [Google Scholar]
- 78.Gray, J. S., R. J. Langley, P. O. Brophy, and P. Gannon. 1989. Vaccination against bovine babesiosis with drug-controlled live parasites. Vet. Rec. 125:369-372. [DOI] [PubMed] [Google Scholar]
- 79.Gray, J. S., R. J. Langley, and T. M. Murphy. 1985. Morphological comparisons of the bovine piroplasm, Babesia divergens, in cattle and jird (Meriones unguiculatus) erythrocytes. J. Parasitol. 71:799-802. [PubMed] [Google Scholar]
- 80.Gray, J. S., and G. Lohan. 1982. The development of a sampling method for the tick Ixodes ricinus and its use in a redwater fever area. Ann. Appl. Biol. 101:421-427. [Google Scholar]
- 81.Gray, J. S., and G. Looby. 1984. Studies on the epidemiology of acute babesiosis in Ireland, p. 140-147. In M. V. Thrusfield (ed.), Proceedings of the Society for Veterinary Epidemiology and Preventive Medicine, University of Edinburgh.
- 82.Gray, J. S., and T. M. Murphy. 1985. Bovine babesiosis in Ireland. Ir. Vet. News 9-14.
- 83.Gray, J. S., T. M. Murphy, K. A. Waldrup, G. G. Wagner, D. A. Blewett, and R. Harrington. 1991. Comparative studies of Babesia spp. from white-tailed and sika deer. J. Wildl. Dis. 27:86-91. [DOI] [PubMed] [Google Scholar]
- 84.Gray, J. S., and S. L. Parr. 1992. In vivo assays for drug resistance in Babesia divergens with the Mongolian gerbil, Meriones unguiculatus. Res. Vet. Sci. 52:126-128. [DOI] [PubMed] [Google Scholar]
- 85.Grellier, P., A. Valentin, V. Millerioux, J. Schrevel, and D. Rigomier. 1994. 3-Hydroxy-3 methylglutaryl coenzyme A reductase inhibitors lovastatin and simvastatin inhibit in vitro development of Plasmodium falciparum and Babesia divergens in human erythrocytes. Antimicrob. Agents Chemother. 38:1144-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haigh, A. J. B., and D. H. Hagan. 1974. Evaluation of imidocarb dihydrochloride against redwater disease in cattle in Eire. Vet. Rec. 94:56-59. [DOI] [PubMed] [Google Scholar]
- 87.Hentrich, B., and R. Böse. 1993. Cryopreservation of Babesia divergens from jirds as a live vaccine for cattle. Int. J. Parasitol. 23:771-776. [DOI] [PubMed] [Google Scholar]
- 88.Herwaldt, B. L., D. H. Persing, E. A. Precigout, W. L. Goff, D. A. Mathiesen, P. W. Taylor, M. L. Eberhard, and A. F. Gorenflot. 1996. A fatal case of babesiosis in Missouri: Identification of another piroplasm that infects humans. Ann. Intern. Med. 124:643-650. [DOI] [PubMed] [Google Scholar]
- 89.Hinaidy, H. K. 1981. Die Babesiose des Rindes in Österreich. I. Verbreitung und Vorkommen. Wien. Tierärztl. Monatsschr. 68:52-57.2382445 [Google Scholar]
- 90.Hinaidy, H. K. 1981. Die Babesiose des Rindes in Österreich. II Untersuchung von Blutproben natürlich erkrankter Rinder. Wien. Tierärztl. Monatsschr. 68:188-191.2382445 [Google Scholar]
- 91.Hinaidy, H. K. 1981. Die Babesiose des Rindes in Österreich. III Taxonomie und Morphologie von Babesia divergens. Zentralbl. Veterinärmed. 28:146-160. [PubMed] [Google Scholar]
- 92.Hinaidy, H. K. 1981. Die Babesiose des Rindes in Österreich. IV Versuche mit Totimpfstoffen. Berl. Münch. Tierärztl. Wochenschr. 94:121-125. [PubMed] [Google Scholar]
- 93.Homer, M. J., I. Aguilar-Delfin, S. R. Telford III, P. J. Krause, and D. H. Persing. 2000. Babesiosis. Clin. Microbiol. Rev. 13:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hughes, D. L., R. E. Purnell, and D. W. Brocklesby. 1977. The effect of initial Fasciola hepatica infection on the pathogenicity of subsequent Babesia divergens infections in intact and splenectomised calves. Vet. Rec. 100:320-321. [DOI] [PubMed] [Google Scholar]
- 95.Huwer, M., A. Schwarzmaier, H.-D. Hamel, and R. Will. 1994. Zum Vorkommen von Babesia divergens im Bezirk Freiburg i. Br. und Versuche zur Piroplasmose-prophylaxe beim Rind. Berl. Munch. Tierärztl. Wochenschr. 107:198-202. [PubMed] [Google Scholar]
- 96.Igarashi, I., M. Aikawa, and J. P. Kreier. 1988. Host cell-parasite interactions in babesiosis, p. 53-69. In M. Ristic (ed.), Babesiosis of domestic animals and man. CRC Press, Boca Raton, Fla.
- 97.Irvin, A. D., E. R. Young, and G. D. Osborn. 1978. Attempts to infect T-lymphocyte-deficient mice with Babesia species of cattle. Res. Vet. Sci. 25:245-246. [PubMed] [Google Scholar]
- 98.Irvin, A. D., E. R. Young, and R. E. Purnell. 1978. The in vitro uptake of tritiated nucleic acid precursors by Babesia spp.. of cattle and mice. Int. J. Parasitol. 8:19-24. [DOI] [PubMed] [Google Scholar]
- 99.Jerichow, H., and R. Jungmann. 1969. Untersuchungen zur Schadwirkung von Babesia divergens. 1. Mitteilung: der Gehalt an Kalium, Kalzium, Magnesium und Eisen im Blutserum bzw.-plasma bei klinisch manifester Piroplasmose unter den Bedingungen der natürlichen Infektion. Monatsh. Veterinärmed. 24:732-736. [PubMed] [Google Scholar]
- 100.Joyner, L. P., and D. W. Brocklesby. 1973. Chemotherapy of anaplasmosis, babesiasis, and theileriasis, p. 321-355. In S. Garattini, F. Hawking, A. Goldin, I. J. Kapin, and R. J. Schnitzer (ed.), Advances in pharmacology and chemotherapy. Academic Press, New York, N.Y. [DOI] [PubMed]
- 101.Joyner, L. P., and S. F. M. Davies. 1967. Acquired resistance to Babesia divergens in experimental calves. J. Protozool. 14:260-262. [DOI] [PubMed] [Google Scholar]
- 102.Joyner, L. P., S. F. M. Davies, and S. B. Kendall. 1963. The experimental transmission of Babesia divergens by Ixodes ricinus. Exp. Parasitol. 14:367-373. [DOI] [PubMed] [Google Scholar]
- 103.Joyner, L. P., and J. Donnelly. 1979. The epidemiology of babesial infections, p. 115-140. In W. H. R. Lumsden, R. Muller, and J. R. Baker (ed.), Advances in parasitology, vol. 17, Academic Press London, United Kingdom. [DOI] [PubMed]
- 104.Joyner, L. P., J. Donnelly, R. Payne, and D. W. Brocklesby. 1972. The indirect fluorescent antibody test for the differentiation of infections with Babesia divergens and Babesia major. Res. Vet. Sci. 13:515-518. [PubMed] [Google Scholar]
- 105.Kahl, O., and W. Knülle. 1988. Water vapour uptake from subsaturated atmospheres by engorged immature ixodid ticks. Exp. Appl. Acarol. 4:73-83. [DOI] [PubMed] [Google Scholar]
- 106.Kjemtrup, A. M., and P. A. Conrad. 2000. Human babesiosis: an emerging tick-borne disease. Int. J. Parasitol. 30:1323-1337. [DOI] [PubMed] [Google Scholar]
- 107.Koch, R. 1906. Beiträge zur Entwicklungsgeschichte der Piroplasmen. Z. Hyg. Infektionskrankh. 54:1-9. [Google Scholar]
- 108.Langley, R. J., and J. S. Gray. 1987. Age-related susceptibility of the gerbil, Meriones unguiculatus, to the bovine parasite, Babesia divergens. Exp. Parasitol. 64:466-473. [DOI] [PubMed] [Google Scholar]
- 109.Langley, R. J., and J. S. Gray. 1989. Nonspecific resistance to Babesia divergens in the Mongolian gerbil (Meriones unguiculatus). Int. J. Parasitol. 19:265-269. [DOI] [PubMed] [Google Scholar]
- 110.Latif, B. M. A., and E. A. Wells. 1973. Babesiasis on the Island of Arran, Scotland. Vet. Rec. 92:496-498. [DOI] [PubMed] [Google Scholar]
- 111.Lawrence, J. A. 1990. Summing-up of strategies for tick-borne disease control in Africa, especially East Africa. Parassitologia 32:113-115. [PubMed] [Google Scholar]
- 112.Leeflang, P., and N. M. Perie. 1972. Comparative immunofluorescent studies on 4 Babesia species of cattle. Res. Vet. Sci. 13:342-346. [PubMed] [Google Scholar]
- 113.Levine, N. D. 1961. Protozoan parasites of domestic animals and man, p. 295. Burgess Publishing Company, Minneapolis, Minn.
- 114.Levy, M. G., G. Clabaugh, and M. Ristic. 1982. Age resistance in bovine babesiosis: role of blood factors in resistance to Babesia bovis. Infect. Immun. 37:1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lewis, D., R. E. Purnell, and D. W. Brocklesby. 1979. Babesia divergens: the immunisation of splenectomised calves with irradiated piroplasms. Res. Vet. Sci. 26:220-222. [PubMed] [Google Scholar]
- 116.Lewis, D., R. E. Purnell, L. M. A. Francis, and E. R. Young. 1981. The effect of treatment with imidocarb dipropionate on the course of Babesia divergens infections in splenectomised calves, and their subsequent immunity to homologous challenge. J. Comp. Pathol. 91:285-292. [DOI] [PubMed] [Google Scholar]
- 117.Lewis, D., R. E. Purnell, S. R. Shaw, and J. P. Revington. 1980. The isolation and characterisation of human and bovine strains of Babesia divergens from Drumnadrochit, Scotland. Parasitology 81:145-155. [DOI] [PubMed] [Google Scholar]
- 118.Lewis, D., R. E. Purnell, E. R. Young, and G. D. Osborn. 1979. Studies on the infection of the Mongolian gerbil with piroplasms of British cattle. Proceedings. Br. Soc. Parasit. Spr. Meet., 9-11 April 1979, Keele University. Parasitology 79:3, xxviii.
- 119.Lewis, D., and H. Williams. 1979. Infection of the Mongolian gerbil with the cattle piroplasm Babesia divergens. Nature 278:170-171. [DOI] [PubMed] [Google Scholar]
- 120.Lewis, D., and E. R. Young. 1980. The transmission of a human strain of Babesia divergens by Ixodes ricinus ticks. J. Parasitol. 66:359-360. [PubMed] [Google Scholar]
- 121.Lewis, D., E. R. Young, D. G. Baggott, and G. D. Osborn. 1981. Babesia divergens infection of the Mongolian gerbil: Titration of the infective dose and preliminary observations on the disease produced. J. Comp. Pathol. 91:565-572. [DOI] [PubMed] [Google Scholar]
- 122.L'Hostis, M., A. Chauvin, A. Valentin, A. Marchand, and A. Gorenflot. 1995. Large scale survey of bovine babesiosis due to Babesia divergens in France. Vet. Rec. 14:36-38. [DOI] [PubMed] [Google Scholar]
- 123.L'Hostis, M., and A. Chauvin. 1999. Babesia divergens in France: descriptive and analytical epidemiology. Parassitologia 41:59-62 (suppl.). [PubMed]
- 124.Liddell, K. G., A. W. Joss, and H. Williams. 1982. Serological response of the Mongolian gerbil to Babesia divergens (human strain) infection. Ann. Trop. Med. Parasitol. 76:527-538. [DOI] [PubMed] [Google Scholar]
- 125.Liddell, K. G., S. B. Lucas, and H. Williams. 1980. Babesia divergens infections in the Mongolian gerbil: characteristics of a human strain. Parasitology 82:205-224. [DOI] [PubMed] [Google Scholar]
- 126.Lopez-Jimenez, L. 1997. Babesiosis in a splenectomised patient. Is it the first case in Spain? Med. Clin. (Barcelona) 108:717. [PubMed] [Google Scholar]
- 127.Losson, B., and R. Patz. 1989. Babesia divergens: activité de l'oxytetracycline retard chez la gerbille, Meriones unguiculatus. Ann. Rech. Vet. 29:501-507. [PubMed] [Google Scholar]
- 128.Mackenstedt, U., M. Gauer, H. Mehlhorn, E. Schein, and S. Hauschild. 1990. Sexual cycle of Babesia divergens confirmed by DNA measurements. Parasitol. Res. 76:199-206. [DOI] [PubMed] [Google Scholar]
- 129.Mahoney, D. F. 1977. Babesia of domestic animals, p. 1-52. In J. P. Kreier (ed.), Parasitic Protozoa, vol. IV. Academic Press, New York, N.Y.
- 130.Marsaudon, E., J. Camenen, D. Testou, P. Bourree, P. Samson, and F. Luneau. 1995. Une babésiose humaine a Babesia canis, responsable d'une anurie de 40 jours. Ann. Med. Intern. 146:451-452. [PubMed] [Google Scholar]
- 131.Mehlhorn, H., and E. Schein. 1984. The piroplasms: life cycle and sexual stages. Adv. Parasitol. 23:37-103. [DOI] [PubMed] [Google Scholar]
- 132.M'Fadyean, J., and S. Stockman. 1911. A new species of piroplasm found in the blood of British cattle. J. Comp. Pathol. 24:340-354. [Google Scholar]
- 133.Murphy, T. M., J. S. Gray, and R. J. Langley. 1986. Effects of rapid passage in the gerbil (Meriones unguiculatus) on the course of infection in the bovine piroplasm Babesia divergens in splenectomised calves. Res. Vet. Sci. 40:285-287. [PubMed] [Google Scholar]
- 134.Nilsson, O., M. Nordkvist, and L. Ryden. 1965. Experimental Babesia divergens infection in reindeer (Rangifer tarandus). Acta Vet. Scand. 6:353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Olmeda, A. S., P. M. Armstrong, B. M. Rosenthal, B. Valladares, A. Del Castillo, F. De Armas, M. Miguelez, A. Gonzalez, J. A. Rodriguez, A. Spielman, and S. R. Telford III. 1997. A subtropical case of human babesiosis. Acta Trop. 67:229-234. [DOI] [PubMed] [Google Scholar]
- 136.O'Neill, A. R. 1983. Blood transfusions in the treatment of red water. Ir. Vet. News July:4-5.
- 137.Persing, D. H., D. Mathiesen, W. F. Marshall, S. R. Telford, A. Spielman, J. W. Thomford, and P. A. Conrad. 1992. Detection of Babesia microti by polymerase chain reaction. J. Clin. Microbiol. 30:2097-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Phillips, R. S. 1984. Babesia divergens in splenectomised rats. Res. Vet. Sci. 36:251-255. [PubMed] [Google Scholar]
- 139.Phillips, R. S., G. M. Reid, A. McLean, and C. D. Pearson. 1987. Antigenic diversity in Babesia divergens: preliminary results with three monoclonal antibodies to the rat-adapted strain. Res. Vet. Sci. 42:96-100. [PubMed] [Google Scholar]
- 140.Precigout, E., A. Gorenflot, A. Valentin, G. Bissuel, B. Carcy, P. Brasseur, Y. Moreau, and J. Schrevel. 1991. Analysis of immune responses of different hosts to Babesia divergens isolates from different geographic areas and capacity of culture-derived exoantigens to induce efficient cross-protection. Infect. Immun. 59:2799-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pudney, M. 1984. Cultivation of Babesia divergens in bovine and human erythrocytes in vitro. British Society for Parasitology Proceedings. Parasitology 89:lxxvi.
- 142.Pudney, M., and J. S. Gray. 1997. Therapeutic efficacy of atovaquone against the bovine intraerythrocytic parasite, Babesia divergens. J. Parasitol. 83:307-310. [PubMed] [Google Scholar]
- 143.Purnell, R. E. 1981. Tick-borne diseases of British livestock. Vet. Med. Rev. 1:58-69. [Google Scholar]
- 144.Purnell, R. E., and D. W. Brocklesby. 1977. Babesia divergens in splenectomised calves: immunogenicity of lyophilised plasma from an infected animal. Res. Vet. Sci. 23:255-256. [PubMed] [Google Scholar]
- 145.Purnell, R. E., D. W. Brocklesby, D. J. Hendry, A. J. Stark, and E. R. Young. 1977. Babesia divergens in splenectomised calves: titration of the infective dose. Res. Vet. Sci. 23:124-125. [PubMed] [Google Scholar]
- 146.Purnell, R. E., D. W. Brocklesby, D. J. Hendry, and E. R. Young. 1976. Separation and recombination of Babesia divergens and Ehrlichia phagocytophila from a field case of redwater from Eire. Vet. Rec. 99:415-417. [DOI] [PubMed] [Google Scholar]
- 147.Purnell, R. E., D. W. Brocklesby, B. A. Kitchenham, and E. R. Young. 1976. A statistical comparison of the behaviour of five British isolates of Babesia divergens in splenectomised calves. J. Comp. Pathol. 86:609-614. [DOI] [PubMed] [Google Scholar]
- 148.Purnell, R. E., D. W. Brocklesby, A. J. Stark, and E. R. Young. 1978. Reactions of splenectomised calves to the inoculation of blood containing Babesia divergens from an infected animal during its reaction and carrier phases. J. Comp. Pathol. 88:419-423. [DOI] [PubMed] [Google Scholar]
- 149.Purnell, R. E., D. J. Hendry, D. E. Bidwell, and P. Turp. 1976. Microplate enzyme linked immunosorbent assay for antibody to Babesia divergens in cattle. Vet. Rec. 99:102. [DOI] [PubMed] [Google Scholar]
- 150.Purnell, R. E., and D. Lewis. 1981. Babesia divergens: combination of dead and live parasites in an irradiated vaccine. Res. Vet. Sci. 30:18-21. [PubMed] [Google Scholar]
- 151.Purnell, R. E., D. Lewis, A. Brabazon, L. M. A. Francis, E. R. Young, and C. Grist. 1981. Field use of an irradiated blood vaccine to protect cattle against redwater (Babesia divergens infection) on a farm in Dorset. Vet. Rec. 108:28-31. [DOI] [PubMed] [Google Scholar]
- 152.Purnell, R. E., D. Lewis, D. W. Brocklesby, and S. M. Taylor. 1979. Bovine babesiosis: steps towards an irradiated vaccine. J. South Afr. Vet. Assoc. 50:339-344. [PubMed] [Google Scholar]
- 153.Purnell, R. E., D. Lewis E. R. Young. 1980. Investigations on the prophylactic effect of treatment with imidocarb diproprionate on Babesia divergens infections in splenectomised calves. Br. Vet. J. 136:452-456. [DOI] [PubMed] [Google Scholar]
- 154.Purnell, R. E., D. Lewis, and E. R. Young. 1981. Quinuronium sulphate for the treatment of Babesia divergens infections of splenectomised calves. Vet. Rec. 108:538-539. [DOI] [PubMed] [Google Scholar]
- 155.Purnell, R. E., E. R. Young, D. W. Brocklesby, and D. J. Hendry. 1977. The haematology of experimentally induced B. divergens, and E. phagocytophila infections in splenectomised calves. Vet. Rec. 100:4-6. [DOI] [PubMed] [Google Scholar]
- 156.Raoult, D., L. Soulayrol, B. Toga, H. Duman, and P. Casanova. 1987. Babesiosis, pentamidine and cotrimoxazole. Ann. Intern. Med. 107:944. [DOI] [PubMed] [Google Scholar]
- 157.Ryley, J. F. 1964. A chemoprophylactic approach to babesiasis. Res. Vet. Sci. 5:411-418. [Google Scholar]
- 158.Schreuder, B. E., and C. Van Wijk. 1984. Een uitbraak van babesiosis in Oostelijk Flevoland. Tijdschr. Diergeneeskd. 109:549-553. [PubMed] [Google Scholar]
- 159.Sherlock, M., A. Healy, H. A. Larkin, and M. L. Doherty. 2000. Bovine babesiosis: clinical assessment and transfusion therapy. Ir. Vet. J. 53:572-578. [Google Scholar]
- 160.Skotarczak, B., and A. Cichocka. 2001. Isolation and amplification by polymerase chain reaction DNA of Babesia microti and Babesia divergens in ticks in Poland. Ann. Agric. Environ. Med. 8:187-189. [PubMed] [Google Scholar]
- 161.Skrabalo, Z. 1975. Babesiosis (piroplasmosis), p. 225-233. In R. A. Marcial-Rojas (ed.), Pathology of protozoal and helminthic diseases. Robert E. Krieger Publishing Company, Huntington, N.Y.
- 162.Skrabalo, Z., and Z. Deanovic. 1957. Piroplasmosis in man: report on a case. Doc. Med. Geogr. Trop. 9:11-16. [PubMed] [Google Scholar]
- 163.Skuce, P. J., T. R. Mallon, and S. M. Taylor. 1996. Molecular cloning of a putative associated protein homologue from Babesia divergens. Mol. Biochem. Parasitol. 77:99-102. [DOI] [PubMed] [Google Scholar]
- 164.Snow, K. R., and D. R. Arthur. 1970. Larvae of the Ixodes ricinus complex of species. Parasitology 60:27-38. [DOI] [PubMed] [Google Scholar]
- 165.Taylor, S. M. 1983. Assessment of prevalence of clinical babesiosis in cattle in Northern Ireland. Vet. Rec. 112:247-250. [DOI] [PubMed] [Google Scholar]
- 166.Taylor, S. M., and C. T. Elliott. 1983. Serum carboxypeptidase B levels during acute and mild Babesia divergens infections in adult cattle. Res. Vet. Sci. 35:370-371. [PubMed] [Google Scholar]
- 167.Taylor, S. M., and C. T. Elliott. 1987. Deltamethrin and flumethrin pour-on formulations for the control of Ixodes ricinus in cattle. Vet. Rec. 120:278. [DOI] [PubMed] [Google Scholar]
- 168.Taylor, S. M., C. T. Elliott, and J. Kenny. 1986. Inhibition of Babesia divergens in cattle by oxytetracycline. Vet. Rec. 118:98-102. [DOI] [PubMed] [Google Scholar]
- 169.Taylor, S. M., C. T. Elliott, and J. Kenny. 1986. Babesia divergens: sequential exposure to heterologous tick-borne challenge of cattle immunized with a fraction of parasitized erythrocytes. J. Comp. Pathol. 96:101-107. [DOI] [PubMed] [Google Scholar]
- 170.Taylor, S. M., C. T. Elliott, and J. Kenny. 1986. Isolation of antigenic proteins from erythrocytes parasitized with Babesia divergens and a comparison of their immunising potential. Vet. Parasitol. 21:99-105. [DOI] [PubMed] [Google Scholar]
- 171.Taylor, S. M., J. Kenny, and T. Mallon. 1983. The effect of multiple rapid passage on strains of Babesia divergens: a comparison of the clinical effects on juvenile and adult cattle of passaged and irradiated parasites. J. Comp. Pathol. 93:391-396. [DOI] [PubMed] [Google Scholar]
- 172.Taylor, S. M., J. Kenny, and T. Mallon. 1983. The effect of route of administration of a Babesia divergens inactivated vaccine on protection against homologous challenge. J. Comp. Pathol. 93:423-428. [DOI] [PubMed] [Google Scholar]
- 173.Taylor, S. M., J. Kenny, T. Mallon, and C. T. Elliott. 1984. The immunisation of cattle against Babesia divergens with fractions of parasitized erythrocytes. Vet. Parasitol. 16:235-242. [DOI] [PubMed] [Google Scholar]
- 174.Taylor, S. M., J. Kenny, R. E. Purnell, and D. Lewis. 1980. Exposure of cattle immunized against redwater to tick-induced challenge in the field: Challenge by a heterologous strain of Babesia divergens. Vet. Rec. 106:385-387. [DOI] [PubMed] [Google Scholar]
- 175.Taylor, S. M., J. Kenny, and A. Strain. 1982. The distribution of Babesia divergens infection within the cattle population of Northern Ireland. Br. Vet. J. 138:384-392. [DOI] [PubMed] [Google Scholar]
- 176.Telford, S. R., III, A. Gorenflot, P. Brasseur, and A. Spielman. 1993. Babesial infections in humans and wildlife, p. 1-45. In J. P. Kreier (ed.), Parasitic protozoa. Academic Press, San Diego, Calif.
- 177.Telford, S. R., III, and A. Spielman. 1997. Babesiosis of humans, p. 349-359. In F. E. G. Cox, J. P. Kreier, and D. Wakelin (ed.), Topley and Wilson's microbiology and microbial infections, 9th ed. Arnold, London, England.
- 178.Trees, A. J. 1974. The application of acridine orange staining to quantitate low levels of Babesia divergens parasitaemias. Trans. R. Soc. Trop. Med. Hyg. 68:277. [PubMed] [Google Scholar]
- 179.Trees, A. J. 1978. Indirect fluorescent antibody levels in experimental Babesia divergens infections in cattle. Res. Vet. Sci. 24:126-128. [PubMed] [Google Scholar]
- 180.Valentin, A., E. Precigout, M. L'Hostis, B. Carcy, A. Gorenflot, and J. Schrevel. 1993. Cellular and humoral immune response induced in cattle by vaccination with Babesia divergens culture-derived exoantigens correlate with protection. Infect. Immun. 61:734-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Valentin, A., D. Rigomier, E. Precigout, B. Carcy, A. Gorenflot, J. Schrevel. 1991. Lipid trafficking between high density lipoproteins and Babesia divergens-infected human erythrocytes. Biol. Cell 73:63-70. [DOI] [PubMed] [Google Scholar]
- 182.Vayrynen, R., and J. Tuomi. 1982. Continuous in vitro cultivation of Babesia divergens. Acta Vet. Scand. 23:471-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Westbrook, C. 2002. M.Sc. thesis. University College Dublin, Dublin, Ireland.
- 184.Winger, C. M., E. U. Canning, and J. D. Culverhouse. 1987. Induction of protective immunity to Babesia divergens in Mongolian gerbils, Meriones unguiculatus, with culture-derived immunogens. Vet. Parasitol. 26:43-53. [DOI] [PubMed] [Google Scholar]
- 185.Winger, C. M., E. U. Canning, and J. D. Culverhouse. 1987. A monoclonal antibody to Babesia divergens which inhibits merozoite invasion. Parasitology 94:17-27. [DOI] [PubMed] [Google Scholar]
- 186.Winger, C. M., E. U. Canning, and J. D. Culverhouse. 1989. A strain of Babesia divergens, attenuated after long-term culture. Res. Vet. Sci. 46:110-113. [PubMed] [Google Scholar]
- 187.Winger, C. M., E. U. Canning, and J. D. Culverhouse. 1989. A monoclonal antibody-derived antigen of Babesia divergens: characterisation and investigation of its ability to protect gerbils against virulent homologous challenge. Parasitology 99:341-348. [DOI] [PubMed] [Google Scholar]
- 188.Winger, C. M., E. U. Canning, R. H. Hartley, and A. Gunn. 1990. Evidence for surface-coat localisation of a monoclonal antibody-isolated merozoite antigen of Babesia divergens. Parasitol. Res. 76:573-577. [DOI] [PubMed] [Google Scholar]
- 189.Wood, J. C. 1971. The activity of imidocarb against Babesia infections in cattle. Ir. Vet. J. 25:254-257. [Google Scholar]
- 190.Wright, I. G. 1990. Immunodiagnosis of and immunoprophylaxis against the hemoparasites Babesia sp. and Anaplasma sp. in domestic animals. Rev. Sci. Tech. Off. Int. Epizoot. 9:345-356. [DOI] [PubMed] [Google Scholar]
- 191.Wright, I. G., R. Casu, M. A. Commins, B. P. Dalrymple, K. R. Gale, B. V. Goodger, P. W. Riddles, D. J. Waltisbuhl, I. Abetz, D. A. Berrie, Y. Bowles, C. Dommock, T. Hayes, H. Kalnins, G. Leatch, R. McCrae, P. E. Montague, I. T. Nisbet, F. Parrodi, J. M. Peters, P. C. Scheiwe, W. Smith, K. Rode-Bramanis, and M. A. White. 1992. The development of a recombinant Babesia vaccines. Vet. Parasitol. 44:3-13. [DOI] [PubMed] [Google Scholar]
- 192.Zintl, A., C. Westbrook, G. Mulcahy, H. E. Skerrett, and J. S. Gray. 2002. Invasion, and short- and long-term survival of Babesia divergens (Phylum Apicomplexa) cultures in nonbovine sera and erythrocytes. Parasitology 124:583-588. [DOI] [PubMed] [Google Scholar]
- 193.Zwart, D., and D. W. Brocklesby. 1979. Babesiosis: Nonspecific resistance, immunological factors and pathogenesis, p 49-112. In W. H. R. Lumsden, R. Muller, and J. R. Baker (ed.), Advances in parasitology, vol 17. Academic Press, London, United Kingdom. [DOI] [PubMed]