Abstract
Escherichia coli is capable of synthesizing the apo-glucose dehydrogenase enzyme (GDH) but not the cofactor pyrroloquinoline quinone (PQQ), which is essential for formation of the holoenzyme. Therefore, in the absence of exogenous PQQ, E. coli does not produce gluconic acid. Evidence is presented to show that the expression of an Erwinia herbicola gene in E. coli HB101(pMCG898) resulted in the production of gluconic acid, which, in turn, implied PQQ biosynthesis. Transposon mutagenesis showed that the essential gene or locus was within a 1.8-kb region of a 4.5-kb insert of the plasmid pMCG898. This 1.8-kb region contained only one apparent open reading frame. In this paper, we present the nucleotide sequence of this open reading frame, a 1,134-bp DNA fragment coding for a protein with an M(r) of 42,160. The deduced sequence of this protein had a high degree of homology with that of gene III (M(r), 43,600) of a PQQ synthase gene complex from Acinetobacter calcoaceticus previously identified by Goosen et al. (J. Bacteriol. 171:447-455, 1989). In minicell analysis, pMCG898 encoded a protein with an M(r) of 41,000. These data indicate that E. coli HB101(pMCG898) produced the GDH-PQQ holoenzyme, which, in turn, catalyzed the oxidation of glucose to gluconic acid in the periplasmic space. As a result of the gluconic acid production, E. coli HB101(pMCG898) showed an enhanced mineral phosphate-solubilizing phenotype due to acid dissolution of the hydroxyapatite substrate.
Full text
PDF
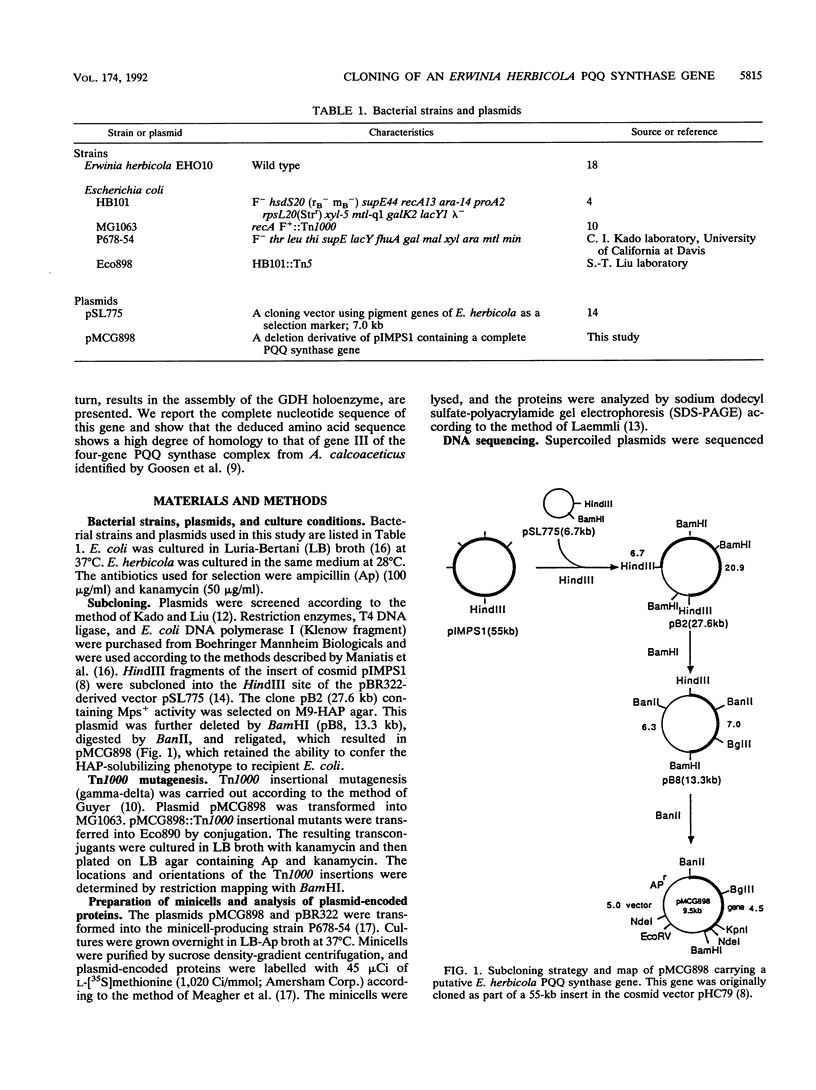

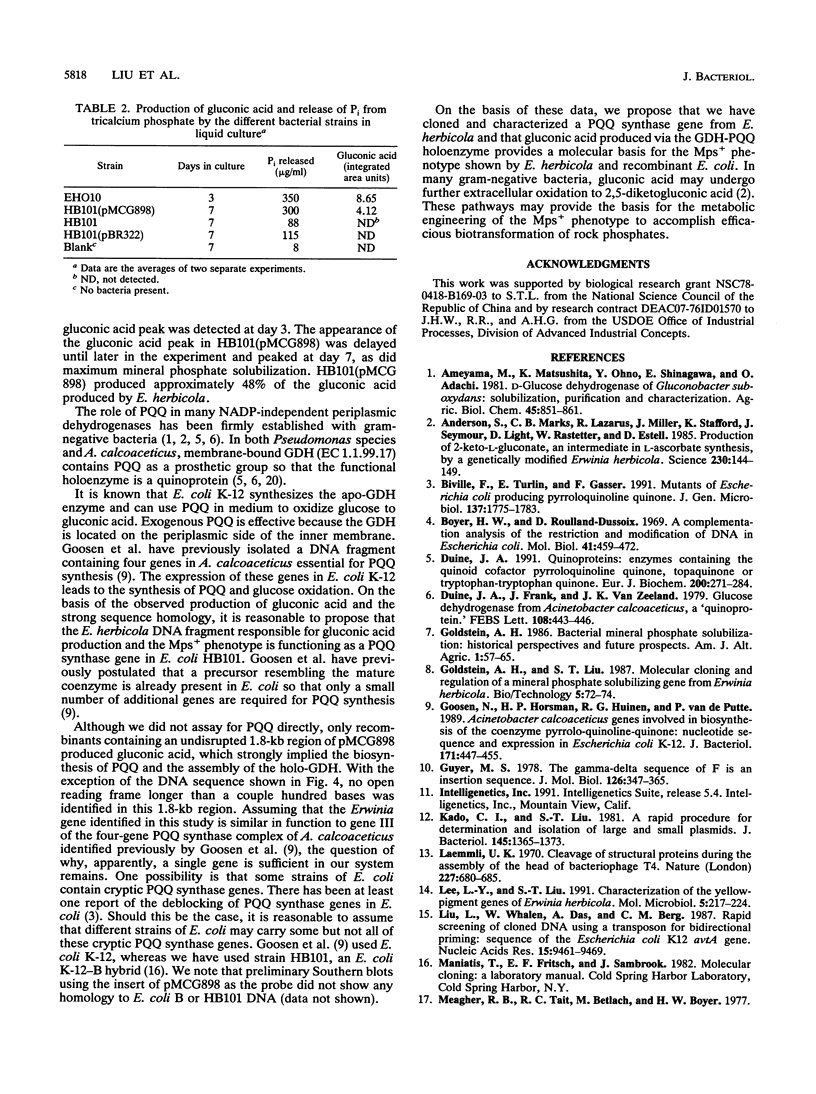

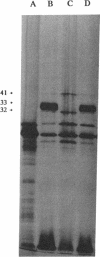
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Marks C. B., Lazarus R., Miller J., Stafford K., Seymour J., Light D., Rastetter W., Estell D. Production of 2-Keto-L-Gulonate, an Intermediate in L-Ascorbate Synthesis, by a Genetically Modified Erwinia herbicola. Science. 1985 Oct 11;230(4722):144–149. doi: 10.1126/science.230.4722.144. [DOI] [PubMed] [Google Scholar]
- Biville F., Turlin E., Gasser F. Mutants of Escherichia coli producing pyrroloquinoline quinone. J Gen Microbiol. 1991 Aug;137(8):1775–1782. doi: 10.1099/00221287-137-8-1775. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., van Zeeland J. K. Glucose dehydrogenase from Acinetobacter calcoaceticus: a 'quinoprotein'. FEBS Lett. 1979 Dec 15;108(2):443–446. doi: 10.1016/0014-5793(79)80584-0. [DOI] [PubMed] [Google Scholar]
- Duine J. A. Quinoproteins: enzymes containing the quinonoid cofactor pyrroloquinoline quinone, topaquinone or tryptophan-tryptophan quinone. Eur J Biochem. 1991 Sep 1;200(2):271–284. doi: 10.1111/j.1432-1033.1991.tb16183.x. [DOI] [PubMed] [Google Scholar]
- Goosen N., Horsman H. P., Huinen R. G., van de Putte P. Acinetobacter calcoaceticus genes involved in biosynthesis of the coenzyme pyrrolo-quinoline-quinone: nucleotide sequence and expression in Escherichia coli K-12. J Bacteriol. 1989 Jan;171(1):447–455. doi: 10.1128/jb.171.1.447-455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S. The gamma delta sequence of F is an insertion sequence. J Mol Biol. 1978 Dec 15;126(3):347–365. doi: 10.1016/0022-2836(78)90045-1. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee L. Y., Liu S. T. Characterization of the yellow-pigment genes of Erwinia herbicola. Mol Microbiol. 1991 Jan;5(1):217–224. doi: 10.1111/j.1365-2958.1991.tb01842.x. [DOI] [PubMed] [Google Scholar]
- Liu L., Whalen W., Das A., Berg C. M. Rapid sequencing of cloned DNA using a transposon for bidirectional priming: sequence of the Escherichia coli K-12 avtA gene. Nucleic Acids Res. 1987 Nov 25;15(22):9461–9469. doi: 10.1093/nar/15.22.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Perry K. L., Simonitch T. A., Harrison-Lavoie K. J., Liu S. T. Cloning and regulation of Erwinia herbicola pigment genes. J Bacteriol. 1986 Nov;168(2):607–612. doi: 10.1128/jb.168.2.607-612.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]