Abstract
When Bacteria, Archaea, and Eucarya separated from each other, a great deal of evolution had taken place. Only then did extensive diversity arise. The bacteria split off with the new property that they had a sacculus that protected them from their own turgor pressure. The saccular wall of murein (or peptidoglycan) was an effective solution to the osmotic pressure problem, but it then was a target for other life-forms, which created lysoymes and β-lactams. The β-lactams, with their four-member strained rings, are effective agents in nature and became the first antibiotic in human medicine. But that is by no means the end of the story. Over evolutionary time, bacteria challenged by β-lactams evolved countermeasures such as β-lactamases, and the producing organisms evolved variant β-lactams. The biology of both classes became evident as the pharmaceutical industry isolated, modified, and produced new chemotherapeutic agents and as the properties of β-lactams and β-lactamases were examined by molecular techniques. This review attempts to fit the wall biology of current microbes and their clinical context into the way organisms developed on this planet as well as the changes arising since the work done by Fleming. It also outlines the scientific advances in our understanding of this broad area of biology.
INTRODUCTION
Of the many targets for antibiosis in cells, the focus here is on the unique and quintessential element of the bacterium: its cell wall. This has been the important clinical target since the first use of penicillin in World War II. Since then, the peptidoglycan sacculus has been attacked in many different ways for medical purposes. However, the murein wall was important to other nonhuman organisms eons before World War II and will be important to mankind after more resistance mechanisms evolve and/or are laterally transferred to pathogens. Much of this review deals with the fundamental bacteriology; only a minor portion addresses specific pathogens or diseases and their treatment. The basic mechanism of bacterial wall growth and function is sophisticated. Synthesis of a structure to protect the bacterium against osmotic and other stresses and to house the cytoplasm efficiently requires conformity with engineering principles. It is only now that an understanding of the bacterial way of life is emerging based on the new experimental tools and on the insights of workers with expertise in fields that impinge on clinical microbiology.
When the domains Bacteria, Archaea, and Eucarya separated from each other, a great deal of evolution had already taken place and organisms from these domains had many skills in common. Until that time, they must have evolved as a group. Once these domains had separated, extensive diversity arose. First, the Bacteria split off, with the unique property that they had a sacculus that protected them, at least, from their own turgor pressure. Turgor becomes higher as metabolic abilities increase. The peptidoglycan saccular wall was an effective solution to the osmotic pressure problem, but it then became a target for other life-forms, which created lysozymes and β-lactams. The β-lactams, which are small organic molecules with four-member strained lactam rings, were effective agents in nature and also became the first antibiotic in human medicine; certainly, they are still important. However, that is by no means the end of the story. Over evolutionary time, bacteria challenged by β-lactams evolved countermeasures, including the synthesis of β-lactamases. As a result, the producing organisms evolved variant β-lactams. The biology of both classes became evident as the pharmaceutical industry isolated, modified, and produced new chemotherapeutic agents and as the properties of β-lactams and β-lactamases were examined by molecular techniques.
The purpose of this review is to try to look ahead to antibacterial therapy in the future. To do so, we have to look at what has happened in the past. To put the biology of the bacterial wall in context, there is a short section concerning the origin of life until bacteria originated; i.e., to the time of the evolution of the domain Bacteria. Key to this is the question of how the murein sacculus originated. It is reasonable that the development of a strong wall afforded resistance to osmotic stress (arguments for this have been presented previously [35-39]). Once initiated, the domain Bacteria greatly expanded and diversified. The other domains; i.e., Archaea and Eucarya, also arose, diversified, and interacted with bacteria. It is not surprising that all three domains saw the development of agents that acted to block bacterial wall growth (39). As mentioned above, these agents included β-lactams and lysozymes. The response of bacteria to the former was to develop β-lactamases, and there have also been some responses to the development of lysozymes.
With this background, we can appreciate the natural development of a variety of antibiotics of the β-lactam type in the biosphere, the subsequent, more rapid development of antibiotics in the pharmaceutical industry, and, as a result, the development of the β-lactamase type of resistance in bacteria in nature and in the clinics. This has been a back-and-forth battle in which the bacteria have often overcome the defenses of other organisms.
This review describes the features of the wall biology of bacteria, including some structural features that would have been important for the first bacterium. It is presented in terms of the history of a number of diverse research areas and their clinical context. The way in which microorganisms developed on this planet and the way in which changes have occurred during the antibiotic area since the time of Fleming are important aspects in our attempts to address the future of antibiotic therapy.
TERMS USED IN THIS REVIEW
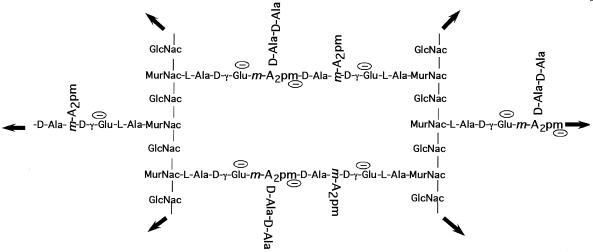
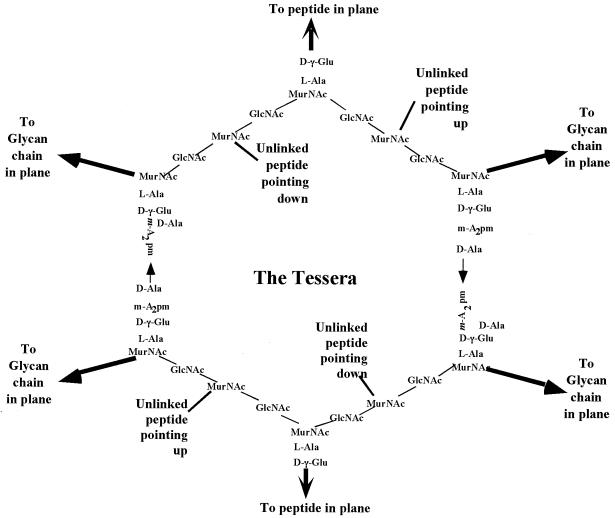
Murein or Peptidoglycan is the covalent polymer that composes the strong part of bacterial envelope, with the exception of a few bacteria such as mycoplasmas. Sacculus is the covering fabric of bacterial cells, and is composed of a large number of covalently linked disaccharide muropeptides. Disaccharide pentamuropeptide is the unit extruded through the cytoplasmic membrane and polymerized into the murein wall. Tetrasaccharide nonamuropeptide is the basic unit polymerized outside the cytoplasmic membrane that forms the individual tessera and the sacculus cell covering. Tessera is the hexagonal unit of wall area composed of two nonamuropeptides linked to each other on both sides by chains of disaccharides muropeptides: one points up from the wall plane, one points down, and one points outward from the tessera and is part of another tessera. Penicillin binding protein (PBP) is a membrane-bound protein that covalently binds penicillin. Most bacteria a have small number (less than 12) of different kinds numbered from PBP 1 (the largest) upward. Methicillin-resistant Staphylococcus aureus (MRSA) is an S. aureus mutant that has an additional PBP, i.e., PBP 2′, which binds poorly to β-lactams but still can function in the synthesis of peptidoglycan. Extended-spectrum β-lactamases (ESBL) are β-lactamases that are derived mostly from modification of TEM-1 or TEM-2 and SHV-1 β-lactamases.
HISTORY OF THE STUDY OF BACTERIAL WALLS
The first section is about the microbiologists and chemists who worked to find and exploit agents that inhibit bacterial wall synthesis in order to kill cells and/or prevent bacterial growth. These workers contributed to the elucidation of the mechanisms and growth strategies of bacteria from many different angles. Because this material is well covered elsewhere, only brief sections are provided about the history of the medical field during the antibiotic era; they were brief because the literature is extensive.
Fleming: Useful Antibiosis
Fleming was perceptive, and he saw and considered things that others had just seen and not thought about. This is the dogma, but there were many earlier workers who noted that there were antagonisms between microbes (16, 72).
Most of these instances were observed after the petri plate with solid nutrient media became available. The conclusion that a mold produced something toxic to a bacterium was a monumental insight, but its real importance depended (i) on the relative lack of toxicity of the penicillin to humans, (ii) the isolation of this chemical agent for study and modification, and (iii) its possession of sufficient stability to be useful. Its stability, however, is only barely adequate.
The Oxford School (Florey et al.): Applied Microbiology and Chemistry
Ten years later in Oxford, penicillin was isolated and purified, its chemical structure was identified, and its crystal structure was determined and modified so that it was useful as an oral antibiotic. Many people contributed from their individual perspectives, including Chain, Heatley, Jennings, Sanders, Abraham, H. W. Florey, and his wife, M. E. Florey (16). This work started a line of research that has continued until today and will continue in the future. Much of the progress dealt with chemical variations and with the related celphalosporins; both were accomplished largely by the pharmaceutical industry.
Salton, Park, and Strominger: Murein Wall
The study of the biology of the growth of bacteria also had many starting points, but the path to the understanding of the physiology of the bacterial wall was crucial. Salton (67) found a wall polymer in bacteria (now called either peptidoglycan or murein). Park (61) found uridine derivatives that were the key intermediates in biosynthesis, and this initiated the study of the biosynthetic pathway of the unit polymer formed in the cytoplasm, extruded through the cytoplasmic membrane, and inserted during the enlargement of the stress-bearing wall (16, 17, 40, 41, 70).
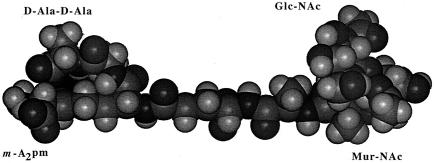
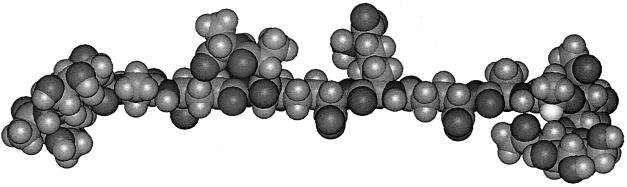
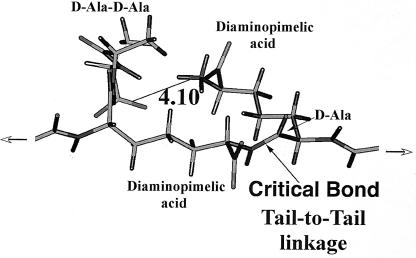
The structure of a typical unit of a disaccharide pentamuropeptide is shown in Fig. 1, while the bridge between two glycan chains through two pentamuropeptides is shown in Fig. 2. This is the necessary linkage to form a strong framework. It should be emphasized that this linkage is different from all proteins in that the linkage is not of the usual alpha amino acid type but is what I call a “tail-to-tail” bond. It contains d-amino acids and other unique chemical groupings. Tipper and Strominger (69) proposed the logical site for penicillin action. Their mechanism of action of penicillin was a proposal based on its structural similarity to acyl-d-alanyl-d-alanine. They postulated a mechanism of how penicillin-type compounds could block bacterial wall growth; this is shown in Fig. 3. They suggested that penicillin resembled the place in the wall where new tail-to-tail linkages between two disaccharide pentamuropeptides were formed. As mentioned above, penicillin is relatively unstable; this is because it is highly reactive, which in turn is because it possesses a strained four-member ring.
FIG. 1.
The unit structure for bacterial wall formation. Shown is the disaccharide pentamuropeptide as a stretched molecule. This exact sequence is present in both E. coli and B. subtilis. By attachment to the carrier, bactoprenol, it is passed through the cytoplasmic membrane. It is then inserted in the cell wall. (Reprinted from reference 40 with permission from the publisher.)
FIG. 2.
The tetrasaccharide nonamuropeptide (the peptide portion is also known as the pentatetra peptide [5 plus 4 amino acids]). This is the cross-bridge that makes possible the formation of a fabric to cover the cell. (Reprinted from reference 40 with permission from the publisher.)
FIG. 3.
The part of the tetrasaccharide nonamuropeptide where the tail-to-tail linkages have been made between two muropeptides. The ionic interaction of the d-Ala-d-Ala and the diaminopimelic acid groups that are not involved in amide (peptide) formation is shown. This aspect of structure is essential for the “stressed nonamuropeptide” model.
Jacob, Hirota, and Spratt: Two-Minute Region and PBPs
Hirota et al. (25) searched for mutants of Escherichia coli that grew only as filaments when incubated at a temperature higher than the usual temperature of 37°C, i.e., 42°C. There were a dozen types of these fts (temperature-sensitive filamentous) mutants, and the mutations involved genes that had to do with the cell division process and with wall growth. This work was done in the 1960s, and of course much molecular microbiology has followed from it.
Spratt and coworkers developed a technique 25 years ago that continues in use today (14). This technique depends on the availability of radioactively labeled penicillin. Cell membranes are prepared and treated with the penicillin, the proteins are separated by gel electrophoresis, autoradiograms are prepared, and the labeled bands are numbered from the top down. This means that the lowest number corresponds to the highest molecular weight. There are many bands in autoradiograms from all organisms. For E. coli, PBP 2 and 3 are the enzymes that catalyze side wall and pole formation, respectively. Only now are we beginning to learn the functions of the lower-molecular-weight species. The key worker in this field at present is Kevin Young (58). In particular, he is investigating the properties of PBP 5. Its function is to remove the terminal d-alanine of the d-Ala-d-Ala grouping of muropeptides. This is turning out to be very important in blocking wall growth at the poles (12, 13, 38, 40, 41).
Weidel and Pelzer: Function of the Murein Sacculus
Almost as perceptive as Fleming's original observation were the observations and conclusions of Weidel and Pelzer (71) that the murein (or peptidoglycan) polymer was the surrounding layer that gave strength and structure to the bacterial cell and was the unique enclosing fabric; they named this bag-shaped polymer the sacculus. The development of this layer is essential to the bacterial way of life, and it was this structure, I believe, that led to the development of the domain (or kingdom) Bacteria. The disaccharide pentamuropeptide unit could function in this role because it can be attached in three ways (two ways as glycosides and in a third way by connecting an amine to a d-Ala, forming an amide [or peptide] bond in one of two possible ways) (Fig. 1). Thus, thousands of these units can form a two-dimensional fabric covering the entire cell.
Schwarz et al. and Nanninga et al.: Wall Synthesis and Cellular Size Control
The important next step was to deal with the mechanism of wall enlargement. The contribution of Schwarz, Ryter, and Hirota was to make a suitable mutant and obtain a radioactive precursor so that cells could be suitably labeled and studied by autoradiography under the electron microscope (66, 68). They did this with bacteria growing at both high and low growth rates. More refined studies were done later, but it was clear that the region of the cell where division was to occur was engaged in the most rapid wall growth. Besides this septum formation, wall growth occurs in the sidewalls (see below). Nanninga's group performed the important and laborious determinations of the dimensions of E. coli cells by using electron microscopy (57). The crucial finding was the relative constancy of the size of cells as they were about to divide during exponential growth.
Koch, Higgins, and Doyle: Safe Enlargement
An engineering approach had to be introduced into what really is an engineering problem, i.e., how can a bacterial cell safely exist in an aqueous environment, and how can it grow safely? The general answer is it must (i) have a covalently linked, completely surrounding fabric (the sacculus); (ii) it must be able to enlarge the sacculus by addition of units, like those shown in Fig. 1; and (iii) it must break bonds only after new covalent attachments have been made. Making strong bonds first allows the cell to be structurally secure at all times. One can think of several mechanisms by which this might be accomplished. The first one to be suggested, the “inside-to-outside growth mechanism,” is the strategy that is indeed used in the formation of the sidewall of the gram-positive rod-shaped bacteria (30, 45, 46).
In this case, new layers of murein are added just outside the cytoplasmic membrane and just under the previously added layer of wall. Then a new layer is laid below this and the older layer becomes stretched as the cell becomes elongated and as each layer is expanded outward. Eventually, a given layer becomes stretched to its elastic limit. At this point, autolysins created and extruded by the cell hydrolyze the most stressed layer, and the fragments are discarded. In some organisms they are reutilized. The net effect is that the cell can elongate indefinitely by inside-to-outside growth, septation, and division.
This inside-to-outside mechanism is now established in many different ways. Merad et al. (54) carried out the most critical work.
Cole, Doyle, Archibald, Koch, Woldringh, and De Pedro: Special Role of Poles
How can bacteria grow and divide and still retain their size? They need some sort of measuring apparatus, otherwise they will grow ever bigger and rounder like a balloon into which air is pumped (41). Clearly, there are templates that do maintain the diameter of a bacterial cell; they are, by necessity or default, the old poles. For gram-positive cocci, the fact that the old poles are retained intact and the new poles are formed each generation by new synthesis was first established for a Streptococcus strain in the 1960s (10).
For the gram-positive rod Bacillus subtilis, this mechanism was shown in the 1970s and 1980s by several clever manipulations of pulse-chase types of experiments, along with several ways to differentially stain the bacteria (54, 56).
For a rod such as E. coli, a template for diameter is needed. Consequently, it also had to be the case that the old pole determined the size of a new pole. Retaining a constant diameter for the cylinder portion in a constant environment implied that as growth took place, the cylinder diameter was equal to the diameter of the poles on both ends. Attempts to show this concept experimentally failed until sufficiently accurate autoradiographs became available in the late 1980s (50); however, the critical experiments were performed in the 1990s; these involved replacing the terminal d-Alanine of the disaccharide pentamuropeptide with stainable d-cysteine before a chase (12, 13). These experiments showed that the old poles contained no new murein and that the forming poles contained only new material. An example is shown in Fig. 4.
FIG. 4.
Inertness of the poles in a growing E. coli cell. The old poles are shown in dark shading, and the new murein synthesized in the last generation is shown in white. Data from reference 12, but shown as examined by the NIH-Image software (13).
Höltje and Schwarz: Critical Analysis of Murein Composition
In their laboratory in Tübingen, Germany, Höltje and Schwarz and their associates applied modern methods to the structure of murein, as it exists in E. coli. Much enzymology was needed to separate the sacculus, which is the world's largest macromolecule, into fragments that could be analyzed to understand the pattern of the fabric. A key technique was to use high-pressure liquid chromatography to examine the mixture produced after enzyme treatment (19). This allowed the investigators to analyze the composition much more precisely than had their predecessors. They found that the wall was composed of units generated from the basic building block shown in Fig. 1 but arranged in the wall in a quite irregular ways. Additionally, they decomposed the fabric into just the glycan chains with no peptides and measured the distribution of lengths (59). They noted a novel aspect: the existence of an anhydride structure at the end of the chains. With respect to the peptide portion, they analyzed the variation and distribution of muropeptide composition. These findings led to a very important model of how the bacterial wall grows (22-24).
Abramson and Chain: Bacterial Resistance to Antibiotics
The first infection resistant to penicillin was reported in 1940. Abramson and Chain were the first to observe the development of resistant bacterial strains (1). Other reports soon followed (28, 65). Unknown at the time were the role and function of the sacculus, the enzymology of its formation, and the existence of substances, primarily lactamases, to block wall growth. The detection of resistance initiated a pattern of responses from the pharmaceutical community; new derivatives were made, which later lost their efficacy, and still newer antibiotics were developed. Through the several generations of penicillin derivatives developed by the pharmaceutical industry interleaving with the development (evolution) of clinically relevant resistant bacteria, much of the practical aspects of chemotherapy have developed.
The Industrial Contribution: Course of β-Lactam Antibiotic Development
Bush has devoted her scientific life to charting the role of the development of β-lactams and β-lactamases as the pharmaceutical industry and bacterial community battled each other. Many workers were involved in developing new and different antibiotics; today, both Bush's and Mobashery's studies are opening up the phylogenetic relationships of the wall variants and the β-lactam inhibitors (4, 5, 51, 52).
FROM THE EVOLUTION OF LIFE TO THE DEVELOPMENT OF DIVERSITY
A brief consideration of the origin and evolution of life on this planet until the time that bacteria separated from the main line of life is necessary because it is likely that these events set the stage for the evolution of the bacterial cell wall and then the evolution and role of agents preventing peptidoglycan synthesis. Although treatments with antibiotics began with Fleming's concepts, that was at least 3 billion years after the development of the first cell and probably 2 billion years since the time of the last universal common ancestor. This was the time that the first cell with a strong cell wall would have arisen to be the first bacterium. This development would have made it sensitive to penicillin, but there would have been no penicillin in existence at that time. The relevant points are that the murein wall must have developed after the development of much of the cell physiology that is common to all cells and that the creation of the bacterial wall was an important contributor to the development of diversity on this planet.
There is by no means unanimity about what transpired at the time of the last universal common ancestor because none of us was there to observe. The explanation presented above is that of Koch (29, 32, 34, 37, 48), but that is not the only opinion. Probably that of Woese (73, 74) carries the most weight, while Cavalier-Smith gives the most extensive discussion (7, 8). His ideas have some logical flaws (49) and are not consistent with the recent work of Gupta (see reference 18 and Gupta's earlier papers). Gupta's deductions from the sequences of various bacteria show that gram-positive cells arose first and that gram-negative cells arose last, the reverse of the order assumed by Cavalier-Smith. Cavalier-Smith’s results are not consistent with the findings of Brown et al. (3).
The creation of the peptidoglycan (murein) sacculus was likely to have been an important part of the development of diversified life on this planet. This needed to occur in combination with the development of the other two domains, Archaea and Eucarya. The split of these two happened a little later and led to different solutions to the same osmotic problem that the bacteria had faced and had solved with development of the murein sacculus. This biological diversification led to competition between individual kinds of organisms. This natural antibiosis included the naturally occurring β-lactams, which were the precursors of the antibiotics manufactured for medical purposes. The final sections of this review include some discussion of the countermeasures from bacteria and our potential future strategy.
Preamble to Life
Considerable organic chemistry had to occur on the early Earth after it cooled sufficiently for organic reactions to create a variety of stable compounds (for reviews, see references 11, 32, and 49). The formation of vesicles, polymers, and catalysts had to occur abiotically. Before the first cell was formed, the same chemical developments would occur over and over many times. This would be fruitless because there would be no mechanism to retain these developments and pass them on by replication, and they must have been simply “reinvented and then lost” many times over. Only when a single vesicle arose by chance that could carry out at least three essential functions could the replication of life start. Subsequently, the biology of that cell's descendents becomes more and more elaborate and the world's biomass expanded (32, 37).
The First Cell
The community of students studying the origin of life is coming more and more to the conclusion that life capable of Darwinian evolution started on Earth in an anaerobic world inside a single, very rare vesicle. That vesicle by chance was equipped with a few functional essential facilities needed for ongoing life. The minimum list of special facilities needed for growth and evolution was basically that list first presented by Darwin; it consisted of a way to trap and use exogenously available energy (chemiosmosis), a way to replicate information (complementary replication of nucleic acid strands), and a way for an informational molecule (enzyme or a riboenzyme) to catalyze some useful chemical reactions. It was important to Darwin that variability was present and could be propagated. Obviously the first cell capable of growing was without competition and would grow to the limit of the available resources. In the resulting stationary phase, faster-growing variants would take over the population. This would happen according to the ecological principle of Gause, called the competitive exclusion principle, and it would have tended to maintain a monophyletic biosphere and blocked the evolution of diversity but not of progress to develop cellular physiology.
Development of Cell Physiology and, Finally, Diversity
Once a cell capable of growth, reproduction, and evolution existed, further open-ended development was possible. Considering that even the most primitive cell that exists today has a great many features and abilities common to all cells, these extensive developments must have been the results of laborious and time-consuming processes (35-37). It has been argued that these developments occurred without the help of lateral gene transfer mechanisms. While this point can be argued, it is clear that diversity on Earth was minimal until after the time of the last universal common ancestor (35). From phylogenetic studies (3, 73, 74), it is thought that Bacteria was the first of the three domains to have split off the main trunk.
There is a logical reason for no stable splits having occurred earlier (29), but why the domains developed at all is a logical conundrum. One suggestion is that as life developed and cells grew faster and became more complex, they accumulated higher concentration of internal osmolytes. This created a completely new and different problem from those with which life-forms had previously had to cope. Cells had now acquired too high a cellular turgor pressure and therefore were at risk of rupture. The first solution that arose to combat this was the development of a sacculus that completely covered the cell, was strong, and was able to aid the cell in resisting turgor pressure. This development was probably the one that resulted in the generation of the domain Bacteria. That in itself was not a cause of the creation of diversity, but when independent and different solutions to the same problem developed in the Archaea and Eucarya, stable diversity was established on Earth (29). From this stage onward, elaboration of the diversity of all three domains flowered.
STRUCTURE OF THE FABRIC THAT ENCLOSES THE BACTERIUM
Once a sacculus had been developed, what are the chemical and biological problems faced by a bacterium in enlarging and in dividing its sacculus between two daughters? Certainly the construction of the sacculus through the cell cycle and renovation through division is far from trivial. It is a project that requires “multiple contractors.” From knowledge of the process (17, 41, 70) in modern bacteria, it must have been the case that before the development of bacteria, evolution had had produced at least five totally different mechanisms for other, quite various, biological purposes unrelated to formation of the cell wall. Then evolution developed separate variants of these. These five components functioned for different processes but could work in concert in such a way that they could make a functional wall. Consequently, these five mechanisms meshed to allow the safe sacculi formation. These partial processes, no doubt, included those listed below. (i) Polyfunctional wall units had to be synthesized. This is accomplished with the aid of variants of the enzymatic and intermediate metabolism of the cell needed for other processes to produce disaccharide penta-muropeptides. The disaccharide portion allows it to be integrated into a glycan chain, and the peptide portion allows it to form a third linkage with another muropeptide unit. In this way, saccular fabric formation occurs and covers the surface area of the cell. The muropeptide portion is unique; the one shown in the figures is present in E. coli and B. subtilis. There are many variants in the amino acids in the walls of different bacteria. (ii) The units had to be transported from the cytoplasm to the outside of the cell's cytoplasmic membrane. This is accomplished with bactoprenol, a carrier that is integral to the membrane. This sophisticated transfer mechanism is not fully understood. (iii) The units then had to be polymerized by insertion into the existing wall. This is done with a complex of enzymes. It must occur in a way that does not render the cell at risk of being burst by the extant hydrostatic (turgor) pressure or of risking the cell's integrity during division. (iv) Enzymes had to be secreted through the membrane to accomplish the various necessary functions. This transport mechanism is the usual one for protein secretion but is totally different from the transport mechanism of the disaccharide pentamuropeptide. (v) The wall needed to be selectively degraded under strict cellular control, to permit continued growth and division. This requires autolysins, transglycosylases and transpeptidases, all with well-controlled and coordinated actions.
Covalent Bonds and the Tensile Strength of Biomaterials
The osmosis problems faced by most bacterial cells would place severe limitations on the structural materials that conceivably could be used to form the sacculus of cells. Hydrogen bonds, apolar bonds, and hydrophobic bonds would not be strong enough. Instead, a sufficiently dense layer of a polymer connected by covalent bonds had to be formed in a two-dimensional enclosing sheet wrapping around the entire cell. This is only a portion of the biological problem. Another problem occurs because the polymerization is outside of the cell proper while the energy for creating covalent bonds has to be supplied from the inside. This is done, in part, by having an expendable amino acid (a d-Ala residue) as part of the pentamuropeptide (see below).
Structure of a Disaccharide Pentamuropeptide
For more details about wall structure and function, see references 12, 26, 27, 33, and 69. These references will lead you back to the extensive background material. A short outline is presented here. The conformation of a sample disaccharide pentamuropeptide as formed inside the cell is shown in Fig. 1. There are many variations of the muropeptide; this is only a representative sample and the pentamuropeptides are slightly different in various species. The conformation of two fused copies of the same structure, but as a tetrasaccharide nonamuropeptide functioning as part of the wall, is shown in Fig. 2. The tetrasaccharide nonamuropeptide exists as part of the wall, and its four saccharides would be the integral part of two different glycan chains. In Fig. 1 and 2, the muropeptide structures simulated by computer modeling (40) are shown. The ways in which the tetrasaccharide nonamuropeptides are integrated in the tessera, the unit of wall area, and these tesserae are integrated in the cell sacculus are discussed below.
A brief outline of the details given in references 17, 41, and 70 is as follows. The fabric of the covering is made of carbohydrate chains in which N-acetylglucosamine alternates with N-acetylmuramic acid residues. These are N-acetylglucosamines to which has been added an additional functional group of a d-lactyl residue. The carboxyl group of this is linked to a special pentamuropeptide. This peptide has unique amino acids, some with d configurations; one is a glutamic acid residue connected by its γ carboxyl group instead of the usual α carboxyl group, and one is diaminopimelic acid, which does not occur in proteins (and other unusual amino acids are used in other species). An important aspect, which has been alluded to above, is that the terminal amino acid of the chain is polyfunctional, possessing an extra amino function. A simulation of this portion of nonamuropeptide is shown in Fig. 3 (40).
Insertion of a Muropeptide into the Wall Fabric
After the disaccharide pentamuropeptide is extruded though the cytoplasmic membrane, this unit is added to elongate a glycan chain. The peptide portion is linked by cross-bridging it to another peptide belonging to another chain in a tail-to-tail linkage. This is probably the pattern and structure in all bacteria with walls. Such tail-to-tail peptide bonds, or amide bonds, are very uncommon in living systems. The peptidoglycan synthesis, no doubt, is a most unusual process; this process is unique to the biochemistry of bacteria and is not found in other organisms. However, it is crucial to the physiology and structure of bacteria.
Figure 1 (40) shows a computer-modeled structure of the unit building block that is fabricated inside the cell and is transported through the cytoplasmic membrane in a special way as a UDP derivative (possibly in an especially compact structural form). Once outside, it is extended and becomes linked into the saccular fabric. The detailed structure shown in Fig. 1 and 2 is the sequence present in both E. coli and B. subtilis. Figure 2 shows the computer simulation of the results of tail-to-tail linkage of two such disaccharide penta-muropeptides. Note again that to achieve this linkage, energy is supplied by the transpeptidation and loss of the terminal d-Ala of one of the muropeptides. The structure in Fig. 2 is referred to in this review as a nonamuropeptide, but it was also called the pentatetrapeptide in earlier literature. The structure is shown in the extended configuration that it would achieve when it had become part of the sacculus and had then become moderately stretched by cellular growth. The d-Ala residues of the other muropeptide are often subsequently hydrolyzed (decarboxylated) to create an octa- or even a heptamuropeptide.
Structure of the Tessera: Unit Structure of the Stress-Bearing Part of the Wall
The disaccharide portions of the exported units are linked in two different ways. The sugar-to-sugar bonds are formed of glycoside linkages, and the nonamuropeptide portions, as mentioned above, are formed of two pentamuropeptides joined by tail-to-tail linkages. In this way, a fabric is formed and enlarged. However, in almost all microbiology textbooks, the structure of the cell wall is depicted as a grid of parallel chains of polymerized disaccharides that are cross-bridged at right angles by peptides (8 or 9 amino acids long) parallel to each other to make a rectangular grid, such as that depicted in Fig. 5. This is certainly inaccurate because the stresses when the fabric is part of the living wall would cause it to become stretched and distorted into hexagons because the tessera is pulled in six directions by the rest of the wall. A better representation of the unit of surface area is shown in Fig. 6.
FIG. 5.
Structure of a unit of surface area of the sacculus of E. coli and B. subtillis. While the structure of the tessera is correct, the rectilinear arrangement, although typical of the presentation in textbooks, is certainly wrong. It would be correct if a murein fabric had been synthesized under stress-free conditions in the absence of the organisms. However, since the forces acting on the sacculus when it is an integral part of a functioning wall surface protecting the cell would stress it in six directions, it would become distorted to become hexagonal (see Fig. 6).
FIG. 6.
Tessera structure of a unit of cell surface in a growing bacterium. The chemical groups are the same as Fig. 5, but because of the turgor stresses in the wall, the fabric would be distorted into something that more closely resembles a hexagon.
Sacculus: Raison d'Être, Its Formation and Function
Cells have a higher osmotic pressure than their environment. Water therefore tends to enter and causes the cell to expand; under some circumstances, it could rupture the cell. A strong elastic covering would protect the cell, and bacterial evolution has created the sacculus for this purpose.
This appears to be a perfect solution for the bacterial style of growth, and it could be surmised that the bacteria would grow rapidly to the limit of the available resources. Consequently, in a diverse biosphere, it is also not surprising that bacteria, fungi, plants, animals, and now modern humans have devised countermeasures against this growth facility. One obvious countermeasure is to produce a compound that blocks the tail-to-tail bond formation. This can be done with highly reactive molecules containing a strained four-member lactam ring; these are special β-lactams antibiotics. A second strategy was also developed in the distant past; this was the construction of lysozymes to cleave the glycan chains of the bacterial wall. Note that in plants, animals, and fungi there are many varieties of lysozymes; there are different ones in different parts of a metazoan animal (27). Lysozymes are very effective at counteracting bacteria in many sites and situations. Although both approaches, i.e., the inhibitors of tail-to-tail transpeptidation and the hydrolyase of the murein wall, have been developed by many kinds of organisms, only the former approach is so far useful clinically. The lysozymes are not clinically useful since they are small proteins, but in the future they may well be medically significant for special applications in special conditions.
Poles of Bacterial Cells
For cells to grow and divide and span the same range of dimensions, some parts of their anatomy must serve as a ruler for the new growth (30, 42). Logically, these are the poles in both rod-shaped and in coccal cells. An example of the inert mature polar wall is presented for E. coli in Fig. 4. A cell is shown from a microfluograph of a cell in which all the old wall is shown dark and the new centrally located murein of the new poles is shown in white (13). The inertness of the poles was referred to above; it is a logical deduction from what we know about bacteria, but here the evidence is shown as the results from staining the chased cell with d-cysteine and using computer enhancement of the resultant image file with the NIH-Image program to examine the details of the wall development. The conclusion can be drawn that the wall of the extant poles is old (and rigid) and that of the septal region is entirely new and somewhat plastic (41) and that the sidewalls turn over (44).
DEVELOPMENT OF WALL ANTIBIOTICS AND COUNTERMEASURES
β-Lactam Production
After the initial evolution of Bacteria, Archaea, and Eucarya, there was a great explosion of biodiversity. For their own benefit, some organisms developed chemical agents to harm other organisms. These classes of agents included phytoalexins, antibiotics, lysozymes, proteases, and immunoglobulins. From this list, the antibiotics were the most amenable for medical usage. The important fact for this review is that the main evolution of the antibiotics preceded the success of the pharmaceutical industry in drug manufacture by a billion years.
The DNAs of many PBPs and related molecules have been sequenced, and from the phylogenetic tree that developed from these studies, it has been concluded that a Streptomyces organism developed the first penicillin derivative or a very similar antibiotic (for a review, see reference 39). This compound probably was isopenicillin N. The genes were subsequently transferred into Penicillium, Aspergillus, and Cephalosporium species and then modified. In the vicinity of a producing organism, the outpouring β-lactams could inhibit bacterial growth and/or kill bacteria so that their contents could be utilized.
Usually it has been taught, and accepted, that bacteria could not produce penicillin while, in contrast, Penicillium species could do so because they are not bacteria and do not have a bacterial type of wall and thus are immune to the action of penicillin. However, there are bacteria that produce β-lactams, but the mechanisms by which they avoid the toxic effects in any particular case is not clear. A plausible explanation is that β-lactams and β-lactamases could have originated in the first antibiotic-producing bacterium and the hydrolytic enzyme protected the producing cell, which could then live but excrete enough of the functional antibiotic. It is generally the case that a toxin-producing cell has (in fact, must have) ways to protect itself against its own toxin. Streptomyces species, of course, do have PBPs and do produce β-lactamases. We can imagine that the some Streptomyces organisms developed a β-lactamase from variation of a gene for a PBP, which, as we will see in the next section, is not too difficult an evolutionary task. This could serve the producing organism's needs because the antibiotic would diffuse much faster than the protein, which would remain near the producing cell, or the β-lactamase protein might not be liberated from the producing cell at all.
While very many variant small molecules with the strained four-member ring initially could presumably have been effective as antibiotics, it is more likely that subsequent events led to the diversification in different cases (assorted penicillins and cephalosorins) so that different molecular species with the same β-lactam grouping could be more effective under some particular circumstances.
β-Lactamases
The bacteria targeted by the secreted β-lactams were consequently under strong selective pressure to counteract these agents, and thus the development of resistance mechanisms became essential. There is something special about β-lactam antibiotics that do not apply to most biologically generated toxic substances. For most of the rest of the naturally produced toxic substances, the evolution of a resistance mechanism must have involved very difficult step-by-step processes and long times because a series of mutations and very complex evolutionary pathways are generally required to create a totally new protein structure.
It is surprising that no antibiotic resistance mechanisms have appeared to be newly developed and to become important in our antibiotic era (29). Rather, for most antibiotics used by humans, the resistance mechanisms are not new but already existed somewhere in the world and the effect of antibiotic production by humans was only to lead to lateral transfer of the genes to pathogenic and agriculturally relevant microorganisms. For the important cases of the β-lactamases and MRSA (see the next section), the mutations are old.
The β-lactamase proteins are special chemically because there is very little biochemical difficulty in slightly modifying a gene that codes for an endotranspeptidase so that the chemistry of the enzyme is changed and it becomes a hydrolase (39). An endotranspeptidase functions to form the tail-to-tail bond from a d-Ala-d-Ala to an amine, such as diaminopimelic acid, both of which are parts of separate pentamuropeptides. It is important to reemphasize that this occurs because the “transpeptidation” event is powered by the elimination of one of its d-Ala residues.
Only a few point mutations suffice to let water into the active site, so that instead of reacting only with an amine, the site would react with the much more ubiquitous H2O. This would convert the enzyme from a transpeptidase into a hydrolase (39). The main way in which transpeptidases (transamidases) act is by binding a substrate, cleaving the critical bond, and reforming the linkage with a new partner. In the case of bacterial cell wall enlargement, the d-Ala-d-Ala bond is cleaved and the tetramuropeptide remains bound to the enzyme until another muropeptide supplies a replacement amino group, which for the case typified in Fig. 1 to 3 is the free amine end of a diaminopimelic acid residue.
Since hydrolases use H2O instead of an amino group, it is possible that rare errors would be made by the endotranspeptidases so that they use water by mistake and thus cause d-Ala liberation and only hydrolysis. This would prevent local cross-bridge formation but would destroy only a small part of the wall. On the other hand, hydrolysis of the β-lactam antibiotic by the β-lactamase of the four-member strained ring could occur repeatedly at a high rate, and a few molecules of β-lactamase would destroy many drug molecules and could protect the cell. After this evolutionary change, there would be little action on the tail-to-tail bond but the enzyme would cleave any β-lactams that bind to the corresponding site on the β-lactamase hydrolase.
This is an important idea for the future of clinical microbiology, and it will be stated a little differently: because β-lactamase action is similar (except for the acceptor) to the transpeptidase action that is needed for saccular expansion, with just a few changes a functional PBP for growth could have been duplicated and one copy could have been turned from a endotranspeptidase into a β-lactamase by changes allowing the H2O entry to the active site. Such enzymes are also known as d,d-hydrolases or d,d-carboxypeptidases. Considering how easy this is theoretically, it is not surprised that this has happened many times, in many ways, and in many lines of descent. The available amino acid sequences (2, 3, 6, 39, 51-53) provide evidence that make it clear that all the β-lactamases came from PBPs and did not arise independently. The amino acid sequences of the genes for this large family of penicillin-recognizing enzymes contain several highly conserved sequences of amino acids. This group includes the PBPs per se, penicillin-sensing proteins, and lactamases. This has led to the development of evolutionary trees (2, 51-53, 55). Evidently, all these proteins together form a superfamily. The point relevant to the evolution of the development of resistance, however, is that the β-lactamases branch later than most PBP types. This clearly suggests that these β-lactamases did not exist until some time after the murein walls became ubiquitous and only, presumably, after penicillin-type antibiotics became common. It can be concluded that they are old enough to be in place in the bacterial world millennia before the time of Fleming. Quite recently, this has also been found to be the case for a different group of β-lactamases, the OXA group (2).
β-Lactamase-Resistant β-Lactams Extant before Medicinal Chemists
In the preantibiotic era, there was further evolution in various lactam-producing organisms to create novel and variant compounds; this probably occurred to overcome the resistance due to the β-lactamases arising in the target organisms. These variants still had the four-member lactam ring characteristic of the penicillin and still are classified as penicillin or cephalosporin compounds. There appears to be a large variety of compounds based on a theme with variation of the side groups of the basic four-member ring, presumably with different secondary effects. It is also clear that natural antibiotic production entered only a few lines of bacteria and fungi and subsequently developed many variations within these lines. This is consistent with the idea that these variations were driven in response to β-lactamases in the target organisms.
Overview of Early β-Lactam Evolution
The argument reviewed here is that the development of lactams was inevitable (i) after the development of the peptidoglycan sacculus surrounding bacteria occurred and (ii) after biodiversity became extensive. Competition for space and resources and the potential that bacterial organisms could serve as resources for other organisms forced the issue. Since they first arose, bacteria have had a point of weakness stemming from the fact that their life strategy required the application of mechanical and chemical engineering principles for the formation of a strong wall outside the cell proper. This mandated that free-energy resources be built into the precursor units as constructed inside the cell, and in the previous section it was pointed out that it almost had to be the case that the prestrained, reactive, covalently binding inhibitors of the PBPs would be antibiotics and would irreversibly inactive the PBPs and prevent bacterial growth. Said differently, when a cell is subject to attack on its essential PBPs, the attack could be made less effective if the cell also had a similar PBP molecule with a poorer ability to conserve chemical energy, i.e., that it would be a hydrolyase and not a transpeptidase. The important point is that the β-lactamase could cycle over and over whereas the endotranspeptidase PBP would react irreversibly with the penicillin derivative. Retrospectively, the β-lactams and β-lactamases interacted with each other in evolutionary history and caused each other's modification and diversification over a long time span.
Turgor Pressure Responses to Wall Antibiotics
It is obvious that turgor pressure inside cells is very important for many aspects of bacterial physiology and for the action of wall antibiotics on cells. However, methods to measure turgor pressure are lacking. Because of the importance of turgor pressure, a light-scattering apparatus and method were developed and used to study turgor pressure and the effect of wall antibiotics on it (41, 47, 62, 64). Only a short summary can be given here; reference 43 gives a more detailed review.
The technique is not applicable to any known pathogen or to any bacteria usually studied in the laboratory. Consequently a freshwater organism that had a useful special property, was studied. This property was that the cell contained a number of gas-filled vesicles. On exposure to adequate pressure, these vesicles would collapse, the gas would rapidly dissolve in the cell contents, and the light scattered by the cell would be greatly reduced. The apparatus exposed the cells to a pressure ramp and recorded the light scattered at right angles to an incident laser beam. The experiment involved exposing the cells to various agents and measuring the pressure that resulted in the collapse of half of the vesicles.
Before the experiments were done, the expected results were almost trivial. Taking ampicillin as the prototype, the normal assumption is that although it would prevent wall enlargement, other cellular processes, such as uptake of solutes from the medium and synthesis of macromolecule would continue. This unbalancing of cell processes should lead to progressively increasing turgor pressure. This would decrease the pressure needed for the apparatus to crush the vesicles.
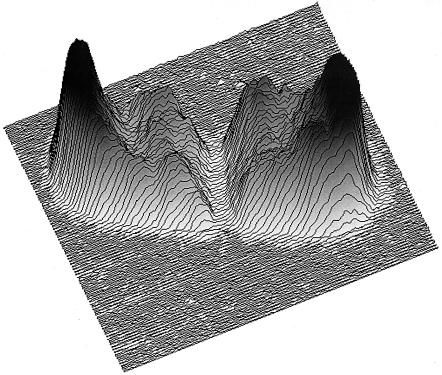
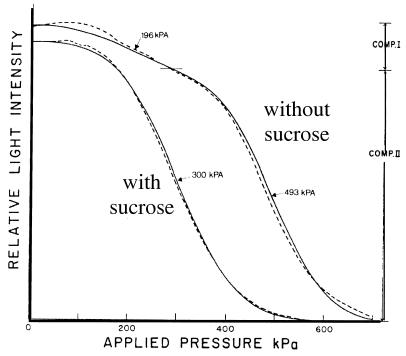
Under antibiotic action, the cell would rupture and the turgor pressure would abruptly become zero. Then a higher hydrostatic pressure would have to be supplied by the apparatus to crush the vesicles. As can be seen in Fig. 7, that is not at all what happened, at least not to all of the cells. Rather, the collapse curves 20 min after treatment changed from a monophasic to a biphasic shape. From the pressure at the midpoint of the two components, it could be concluded that some bacteria had done what was expected and their vesicles now had a collapse pressure similar to that found in the presence of high concentrations of sucrose, where the cell turgor pressure would be zero; it followed, therefore, that the cell envelope must have ruptured. Surprisingly, the collapse curve of the remaining cells had a stable turgor pressure but one higher than that of the untreated cells. This had to mean that in the face of a challenge that prevented enlargement of the stress-bearing wall, these latter cells had implemented a strategy of stopping further increase in turgor pressure. To prevent a blowout, the cells must have blocked many active transport systems or opened channels to let ions and solutes return to the medium. Probably they would also have had to shut down synthesis of macromolecular species of all kinds.
FIG. 7.
Two classes of cell pressure responses in cells treated with ampicillin. Ancylobacter aquaticus cells were examined in a light-scattering apparatus exposed to a range of pressures that at some point crushed their gas-filled vesicles. Without ampicillin treatment, the collapse curve was monophasic like that marked “with sucrose” but required a higher pressure for collapse. After 20 min of treatment, the curve changed to the one marked “without sucrose.” This shows two branches, one higher and one lower than the original curve. The conclusion to be drawn is that different cells in the population exhibited different behaviors. Most of the cells ruptured, and the gas vesicles were exposed to the growth medium and were no longer compressed by the cell's turgor pressure. A smaller number of cells apparently stopped growing and acquired a higher turgor pressure. These cells were immune to the action of the antibiotic because the wall was not growing. (Modified from reference 47.)
If this type of mechanism is general for bacteria, including pathogens, and for many antibiotic agents that prevent wall growth, then this is quite a different problem from the one that has been recognized, and it will have to be addressed by the medical community in the future.
METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS
The other case of a seemingly recent evolution of an antibiotic is the emergence of MRSA. Although MRSA has been very important clinically since 1961, there is reason to believe that the basic mecA gene evolved from an existing PBP in the distant past as a supplement to the normal group of PBPs (20, 21; see the review in reference 39). This gene codes for PBP 2′. It is reasonable to imagine that PBP 2′ would protect against natural β-lactam antibiosis when the inhibition of wall synthesis by natural β-lactams is modest. When present in most (nonclinical) organisms, PBP 2′ is a supplement to usual PBPs and not a replacement. Selection for function in these cases occurs when the organisms are subjected to a mild blockade by β-lactams. PBP 2′ probably is not fully efficient in wall synthesis, and therefore its presence is an extra operating cost to the cell, but not a severe one.
To understand the role of PBP 2′, it is necessary to appreciate that ordinarily in the preantibiotic era, it was not maximally expressed because there was repressor control over its synthesis. This repression resulted from the presence of mecI and mecR1 in the relevant operon. These genes moderated and controlled the production of PBP 2′ but presumably allowed enough to be made under environmental conditions when low levels of β-lactams were being produced by nearby organisms. However, once methicillin and oxacillin were being commercially produced and their medical use expanded in recent times, the partially repressive conditions that had previously evolved did not lead to a high enough level of PBP 2′ for resistance of the target organisms to these antibiotics. As a result, the regulatory genes were lost and the genetic material was fused to a lactamase gene cluster that had the now appropriate regulatory capability. Such changes could occur easily and rapidly. The reason for thinking that the basic evolution of MRSA occurred prior to 1960 is that mecA is found in a gene cluster in other organisms together with its regulatory genes, suggesting that a long evolutionary time was needed to create the gene and the controls for its action in preantibiotic times. The critical point is that mecA did not evolve in the antibiotic era; rather, the regulatory genes were lost by inactivation or deletion, which subsequently permitted resistance to stronger challenges mounted near the beginning of the antibiotic era with the use of methicillin.
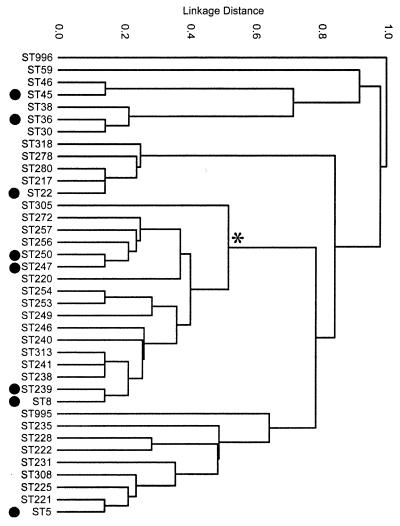
This is not to say that there have not been many changes in MRSA in our era. Spratt and coworkers (14) analyzed 359 MRSA strains and separated them into 38 families. These changes are all changes that happened recently, but presumably the parent of all, the original mecA, goes very far back in evolution. This pedigree of existing organisms is shown in Fig. 8.
FIG. 8.
Phylogenetic tree of MRSA strains. It shows the divergence of modern clinical strains from the original MRSA strain, which is shown on the top of the figure. It can be seen that from this original strain at least eight major strains (black circles) evolved and diverged but retained this MRSA character. The implication is that mecA arose only once, but variations on it arose much more often, probably in the current antibiotic era. (Reprinted from reference 14 with permission from the publisher.)
LACTAM ANTIBIOTICS AND VARIANTS TODAY
β-Lactamases Destroying β-Lactams in the Current Antibiotic Era
No one could foresee the consequences, both the great advances and the great failures, that we now can see with hindsight. The literature on the development of the penicillin story is fascinating (15, 16). The conclusion to be drawn is that this is a case where history repeated itself many times and may repeat itself much more forcibly in the time to come. The defeats came from genes already existing in the world biosphere, lateral transfer that mobilized these genes, and mutations that modified antibiotic action in pathogens. The action of such extant genes, no matter how remote their host organisms are from being human pathogens, has defeated many potential clinical agents and is defeating specific antibiotic actions. This kind of event is likely to happen again and again. How to avoid this in the future is not obvious, but there are certain design features that may be important (26, 38).
Initial Manufacture of Penicillins and the Microbiological Sequelae
When penicillin G was first used to treat infected people, it was so valuable and its amounts were so limited that it was recovered from the urine of the recipients and reused in other patients. Its use was restricted to military persons in certain locations. Then penicillin V, which was more acid resistant and therefore could be taken orally, was developed. However, resistance soon became apparent. The first indication was the finding by Abraham and Chain (1) that some strains of E. coli were already resistant; shortly after that, Kirby (28) found resistant strains of S. aureus. These resistance mechanisms, with hindsight, were no doubt generated by the prior existence of β-lactamases in various places in microbial world. Of course, then and especially now, the vast majority of the staphylococci have β-lactamases borne on plasmids. In is important to note from the above discussion that the creation of MRSA was a man-made event. This accusation can be made because streptococci that did not have β-lactamases were treated with penicillin, while, on the other hand, staphylococci, which only rarely had β-lactamases but coexisted in the same environment, did have rare transmissible plasmids. The consequence was that quickly and commonly, staphylococci acquired the MRSA gene (6).
While the β-lactamases are all generally related, indicating that they all go back to the primordial PBP, there are many variants, and their sequences suggest that a large number of β-lactamases genes in the world's pool existed prior to human involvement in chemotherapy (51, 52). Bush and Mobashery (6) counted 255 primary sequences, and it is clear that many of these arose before our era and took time to be transferred to medically significant bacteria. Of course, variations arose or were subject to minor modifications after the human exploitation of β-lactams. This is also the known history of the OXA β-lactamases (2) as deduced from available sequences.
Novel β-Lactams
In the late 1950s, new β-lactams were discovered, such as cephalosporin C and 6-aminopenicillianic acid. These permitted chemists to produce β-lactams that resisted some of the β-lactamases. These included the semisynthetic isoxazolyly penicillins, such as methicillin, oxacillin, and the “first-generation” cephalosporins. The use of cepalothin, cephaloridine, and cefazolin significantly decreased the role of β-lactamases, which was then prominent in gram-positive organisms.
Extended-Spectrum β-Lactamases and Cephalosporinases
Not too surprisingly with hindsight, antibiotics including methicillin and oxacillin were associated with the appearance of ESBL and cephalosporinases. In the mid-1960s new plasmids encoding β-lactamases appeared in gram-negative organisms that had been treated with cephalosporins. These enzymes have been designated SHV-1, TEM-1, and TEM-2. Of these, TEM-1 has caused particularly grave medical problems. This danger led to a concerted attack on TEM-1 by the pharmaceutical industry and resulted in a two-pronged approach. One approach was the development of β-lactams containing clavulanic acid and penicillin-derived sulfones. These were offered to the patient together with aβ-lactam that blocked certain PBPs. This combination worked because the clavulanic acid irreversibly tied up the β-lactamase, thus protecting the β-lactam antibiotic, which was then not able to inhibit PBPs and block wall growth. The alternative prong was to find and use new lactams that were resistant to the β-lactamases like the carbapenems. Later, some of the other “third-generation” cephalosphorins were developed, and still later, the monobactam aztreonam was developed.
CONCLUSIONS AND THE FUTURE
The development of a new genetic capability can be rapid or extremely slow, and it can be a common and an often-repeated change or a unique occurrence. Considering first rare events, the origin of life, the origin of bacteria, and the origin of the first MRSA mutation were events that apparently happened only once in the history of the world. Once these “macromutations” took place, then propagation, expansion, and variation did occur. A variety of more common mutations could occur more easily or more often to tune variants to create distinct organisms. This, in general, led to the generation of improved measures but also to countermeasures to these improvements. All these smaller changes usually are relevant only in the context of the preceding macromutation. Although the subsequent changes are usually minor, they still would be of selective advantage, although usually would not constitute “a great leap forward.” Figure 8 shows an example of this: one mutation in S. aureus made it resistant to β-lactamase with the creation of the original mecA mutation, but then further evolution in various target strains as well as in the antibiotic-producing organisms made further changes necessary and readily possible.
Once a β-lactamase had originated from existing PBPs, it was evolutionarily easy to develop many slightly different β-lactamases (39). Once the first MRSA organism had arisen, it was relatively easy to develop MRSA variants. From these two revolutionary ancient starts, these macromutations also made the bacterial responses in the present era to manmade agents evolutionarily easy. This led to a human-created field of variation of possible lactams, using the methods of genetics, natural products chemistry, semisynthetic chemistry, and total synthetic chemistry. Now we are now finding that it is hard to develop counter-countermeasures to these bacterial responses that have functioned for a long time.
It seems clear (29) from the history of antibiotic successes and failures that the easy things like small changes in chemistry or many small changes will not yield an antibiotic that will solve our medical problem in the long run because countermeasures by bacteria are also easy. For an antibiotic to have a long, useful life, it must be able to pose a problem that has never been faced before by the bacterium. The PBPs must have arisen from lengthy evolution to empower sacculus formation. However, only relatively minor chemical developments created functional β-lactamases in the first place. (They had to arise in a bacterium that had PBPs and not, for example, in a fungus that did not.) Evolutionarily, the development of β-lactamases from PBPs was essentially trivial compared to the de novo evolution of a new protein structure.
If we can choose a target that has not been exploited in nature, then the bacterial response will be slow because lateral gene transfer cannot function and a countermeasure will arise only as a result of rare or multiple accumulation of mutations. An adequate response to our weapon by de novo evolution could occur only slowly, and therefore we will have considerable time—many decades or more—during which infections can be effectively held at bay before an entirely new resistance mechanism evolves in the pathogen population. A selective pressure would not act on the much larger number of microorganisms of the world that are not human pathogens and are not subject to my imagined challenge by the new “wonder drug.” Resistance would evolve much more slowly or not at all.
For this review, the crux of the matter is that the wall and its function permitted the domain Bacteria to be quite different from Archaea and Eucarya. However, the response from the rest of the living community was that the murein sacculus was a simple target for attack, and many organisms did so. It was a long time before there were medical applications of antibiotics, but penicillin-type agents were important in the biosphere. They still are the example and the paradigm for the pattern of generation and use of antibiotics in medicine.
This review has omitted many topics that are relevant from the viewpoint of wall biology. Some of these are the advantages and disadvantages of cocci versus rods and other shapes; wall growth strategies of gram-positive and gram-negative cells; advantages and disadvantages of the strategies of curved and spiral cells; and advantages and disadvantages of the strategies of bacteria with weak walls or no walls at all. It also has not covered the topic of tolerance. I hope to cover these things in a book in preparation (A. L. Koch, unpublished data). However, I have tried to describe the antibiosis and countermeasures of the preantibiotic and antibiotic eras.
REFERENCES
- 1.Abramson, E. P., and E. Chain. 1940. An enzyme from bacteria able to destroy penicillin. Nature 146:837. [PubMed] [Google Scholar]
- 2.Barlow, M., and B. G. Hall. 2002. Phylogenetic analysis shows that OXA β-lactamase genes have been on plasmids for millions of years. J. Mol. Evol. 55:314-321. [DOI] [PubMed] [Google Scholar]
- 3.Brown, J. T. R., C. J. Douady, M. J. Italia, W. E. Marshall, and M. J. Stanhope. 2001. Universal trees based on large combined protein sequence data sets. Nat. Genet. 28:281-285. [DOI] [PubMed] [Google Scholar]
- 4.Bush, K. 1999. β-Lactamases of increasing clinical importance. Curr. Pharm. Design 5:839-845. [PubMed] [Google Scholar]
- 5.Bush, K. 2001. New beta-lactamases in gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin. Infect. Dis. 32:1085-1089. [DOI] [PubMed] [Google Scholar]
- 6.Bush, K., and S. Mobashery. 1998. How β-lactamases have driven pharmaceutical drug discovery, p. 71-98. In B. Rosen and S. Mobashery (ed.), Resolving the antibiotic paradox. Kluwer Academics, Dordrecht, The Netherlands.
- 7.Cavalier-Smith, T. 2001. Obcells as proto-organisms: membrane heredity, lithophosphorylation, and the origins of the genetic code, the first cells, and photosynthesis. J. Mol. Evol. 53:555-595. [DOI] [PubMed] [Google Scholar]
- 8.Cavalier-Smith, T. 2002. The Neomuran origin of Archaebacteria, the negibacterial root of the universal tree and the bacterial megaclassification. Int. J. Syst. E vol. Microbiol. 52:7-76. [DOI] [PubMed] [Google Scholar]
- 9.Chadwick, D., and J. Goode (ed). 1997. Antibiotic resistance: origins, evolution, selection, and spread. John Wiley & Sons, Chichester, United Kingdom.
- 10.Cole, R. M., and J. J. Hahn. 1962. Cell wall replication in Streptoccocus pyogenes Science 135:722. [DOI] [PubMed] [Google Scholar]
- 11.Deamer, D. W. 1997. The first living systems: a bioenergetic perspective. Microbiol. Mol. Biol. Rev. 61:239-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Pedro, M. A., J. C. Quintela, J.-V. Höltje, and H. Schwarz. 1997. Murein segregation in Escherichia coli. J. Bacteriol. 179:2823-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Pedro, M. A., H. Schwarz, and A. L. Koch. 2003. Patchiness of insertion of murein in the sidewall of E. coli. Microbiology, in press. [DOI] [PubMed]
- 14.Enright, M. C., D. A. Robinson, R. Gaynor, E. J. Feil, H. Grundmann, and B. W. Spratt. 2002. The evolutionary history of methicillin-resistantStaphylococcus aureus (MRSA) Proc. Natl. Acad. Sci. USA 99:7687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming, A. 1946. Chemotherapy, yesterday, today, and tomorrow, Symp. Soc. Gen. Microbiol. 53:1-18. [Google Scholar]
- 16.Florey, H. W., E. Chain, N. G. Heatley, M. A. Jennings, A. G. Sanders, E. P. Abraham, and M. E. Florey. 1949. Antibiotics, vol. 1. Oxford University Press, Oxford, United Kingdom.
- 17.Ghuysen, J.-M., and R. Hakenbeck (ed.). 1994. Bacterial cell wall. Elsevier, Amsterdam, The Netherlands.
- 18.Gupta, E. S. 2002. Phylogeny of bacteria; are we now close to understanding it? ASM News 68:284-291. [Google Scholar]
- 19.Glauner, B., and J.-V. Höltje. 1990. Growth pattern of the murein sacculi of Escherichia coli. J. Biol. Chem. 263:18988-18996. [PubMed] [Google Scholar]
- 20.Hiramatsu, K., L. Cui, M. Kuroda, and T. Ito. 2001. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol 9:486-493. [DOI] [PubMed] [Google Scholar]
- 21.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 22.Höltje, J.-V. 1993. “Three for one” — a simple growth mechanism that guarantees a precise copy of the thin, rod-shaped sacculus of E. coli. p. 419-426. In M. A. de Pedro, J. V. Höltje, and W. Löffëlhardt (ed.), Bacterial growth and lysis. Metabolism and structure of the bacterial sacculus. Plenum Press, New York, N.Y.
- 23.Höltje, J.-V. 1996. A hypothetical holoenzyme involved in the replication of the murein sacculus of Escherichia coli. Microbiology 142:1991-1919. [DOI] [PubMed] [Google Scholar]
- 24.Höltje, J.-V. 1998. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 62:181-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirota, Y., J. Mordoh, I. Scheffler, and F. Jacob. 1972. Genetic approach to DNA replication and its control in Escherichia coli. Fed. Proc. 31:1422-1427. [PubMed] [Google Scholar]
- 26.Hughes, D., and D. I. Andersson. 2001. Antibiotic development and resistance. Taylor and Francis, London, United Kingdom.
- 27.Jollès, P. (ed.). 1996. Lysozymes: model enzymes in biochemistry. Birkhäuser Verlag, Basel, Switzerland.
- 28.Kirby, W. M. M. 1944. Extraction of a potent penicillin inactivator from penicillin-resistant staphylococci. Science 99:452-453. [DOI] [PubMed] [Google Scholar]
- 29.Koch, A. L. 1981. Evolution of antibiotic resistance gene function. Microbiol. Rev. 45:355-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch, A. L. 1982. On the growth and form of Escherichia coli. J. Gen. Microbiol. 128:2527-2540. [DOI] [PubMed] [Google Scholar]
- 31.Koch, A. L. 1983. The surface stress theory of microbiol morphogenesis. Adv. Microb. Physiol. 24:301-366. [DOI] [PubMed] [Google Scholar]
- 32.Koch, A. L. 1985. Primeval cells: possible energy-generating and cell-division mechanisms. J. Mol. Evol. 21:270-277. [DOI] [PubMed] [Google Scholar]
- 33.Koch, A. L. 1988. Biophysics of bacterial wall viewed as a stress-bearing fabric. Microbiol. Rev. 52:337-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch, A. L. 1993. Microbiol genetic responses to extreme challenges. J. Theor. Biol. 160:1-21. [DOI] [PubMed] [Google Scholar]
- 35.Koch, A. L. 1994. Development and diversification of the Last Universal Ancestor. J. Theor. Biol. 168:269-280. [DOI] [PubMed] [Google Scholar]
- 36.Koch, A. L. 1995. Origin of intracellular and intercellular pathogens. Q. Rev. Biol. 70:423-437. [Google Scholar]
- 37.Koch, A. L. 1998. How did Bacteria come to be? Adv. Microb. Physiol. 40:354-399. [DOI] [PubMed] [Google Scholar]
- 38.Koch, A. L. 2000. The exoskeleton of bacterial cells (the sacculus): still a highly specific target for antibacterial agents that will last for a long time. Crit. Rev. Microbiol. 25:275-307. [DOI] [PubMed] [Google Scholar]
- 39.Koch, A. L. 2000. Penicillin binding proteins, β-lactams, and lactamases: offensives, attacks, and defensive counter measures. Crit. Rev. Microbiol. 26:1-35. [DOI] [PubMed] [Google Scholar]
- 40.Koch, A. L. 2000. Simulation of the conformation of the murein fabric. I. The oligoglycan, penta-muropeptide, and crosslinked nona-muropepetide. Arch. Microbiol. 174:429-439. [DOI] [PubMed] [Google Scholar]
- 41.Koch, A. L. 2001. Bacterial growth and form, 2nd ed. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 42.Koch, A. L. 2002. Why are rod-shaped bacteria rod shaped? Trends Microbiol. 10:452-455. [DOI] [PubMed] [Google Scholar]
- 43.Reference deleted.
- 44.Koch, A. L., and R. J. Doyle. 1985. Inside-to-outside growth and the turnover of the Gram-positive rod. J. Theor. Biol. 117:137-157. [DOI] [PubMed] [Google Scholar]
- 45.Koch, A. L., and R. J. Doyle. 1986. The growth strategy of the Gram-positive rod. FEMS Microbiol. Rev. 32:247-254. [Google Scholar]
- 46.Koch, A. L., M. L. Higgins, and R. J. Doyle. 1981. Surface tension-like forces determine bacterial shapes: Streptococcus faecium. J. Gen. Microbiol. 123:151-161. [DOI] [PubMed] [Google Scholar]
- 47.Koch, A. L., and M. F. S. Pinette. 1987. Nephelometric determination of osmotic pressure in growing gram-negative bacteria. J. Bacteriol. 169:3654-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koch, A. L., and T. M. Schmidt. 1991. The first cellular bioenergetic process: primitive generation of a protonmotive force. J. Mol. Evol. 33:297-304. [DOI] [PubMed] [Google Scholar]
- 49.Koch, A. L., and S. Silver. The first cell. FEMS Microbiol. Rev., in press.
- 50.Koch, A. L., and C. L. Woldringh. 1995. The metabolic inertness of the poles of a Gram-negative rod. J. Theor. Biol. 171:415-425. [Google Scholar]
- 51.Massova, I., and S. Mobashery. 1997. Molecular bases for interactions between β-lactams antibiotics and β-lactamases. Acc. Chem. Res. 30:162-168. [Google Scholar]
- 52.Massova, I., and S. Mobashery. 1999. Structural and mechanistic aspects of β-lactamases and penicillin-binding proteins. Curr. Pharm. Design 5:929-937. [PubMed] [Google Scholar]
- 53.Medeiros, A.A. 1997. Evolution and dissemination of β-lactamases, accelerated by generations of β-lactam antibiotics. Clin. Infect. Dis. 24(Suppl. 1):S19-S45. [DOI] [PubMed] [Google Scholar]
- 54.Merad, T., A. R. Archibald, I. C. Hancock, C. R. Harwood, and J. A. Hobot. 1989. Cell wall assembly in Bacillus subtilis: visualisation of old and new material by electron microscopic examination of samples selectively stained for teichoic acid and teichuronic acid. J. Gen. Microbiol. 135:645-655. [DOI] [PubMed] [Google Scholar]
- 55.Mobashery, S. 1997. Molecular bases for interactions between β-lactams antibiotics and β-lactamases. Acc. Chem. Res. 30:162-168. [Google Scholar]
- 56.Mobley, H. L. T., A. L. Koch, R. J. Doyle, and U. N. Streips. 1984. Insertion and fate of the cell wall in Bacillus subtilis. J. Bacteriol. 158:169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nanninga, N., C. L. Woldringh, and L. J. H. Koppes. 1982. Growth and division of Escherichia coli, p. 225-270. In C. Nicolini (ed.), Cell growth. Plenum Publishing Corp., New York, N.Y.
- 58.Nelson, D. E., and K. D. Young. 2001. Contributions of PBP 5 and DD-carboxypeptidase penicillin binding proteins to maintenance of cell shape in Escherichia coli. J. Bacteriol. 183:3055-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Obermann, W., and J.-V. Höltje. 1994. Alterations of murein structure and of penicillin-binding proteins in minicells from Escherichia coli. Microbiology 140:79-87. [DOI] [PubMed] [Google Scholar]
- 60.Oliveira, D. 2002. Secrets of success of a human pathogen: molecular evolution of MRSA. Lancet Infect. Dis. 2:180-189. [DOI] [PubMed] [Google Scholar]
- 61.Park, J. T. 1949. Uridine 5′ pyrophosphate derivative. I. Isolation from Staphylcoccus aureus. J. Biol. Chem. 194:877-884. [PubMed] [Google Scholar]
- 62.Pinette, M. F. S., and A. L. Koch. 1987. Variability of the turgor pressure of individual cells of a gram-negative heterotroph, Ancylobacter aquaticus. J. Bacteriol. 169:4737-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pinette, M. F. S., and A. L. Koch. 1988. Biophysics of ampicillin action on a gas vacuolated gram-negative rod, p. 157-163. In P. Actor, L. Daneo-Moore, M. L. Higgins, M. R. J. Salton, and G. D. Shockman (ed.), Antibiotic inhibition of bacterial surface assembly and function. American Society for Microbiology, Washington, D.C.
- 64.Pinette, M. F. S., and A. L. Koch. 1988. Turgor pressure responses of a gram-negative bacterium to antibiotic treatment measured by collapse of gas vesicles. J. Bacteriol. 170:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rammelkamp, C. H., and T. Maxon. 1942. Resistance of Staphylococcus aureus to the action of penicillin. Proc. Soc. Exp. Biol. Med. 51:386-389. [Google Scholar]
- 66.Ryter, A., Y. Hirota, and U. Schwarz. 1973. Process of cellular division in Escherichia coli. J. Mol. Biol. 12:283-290. [DOI] [PubMed] [Google Scholar]
- 67.Salton, M. R. J. 1994. The bacterial cell envelope—a historical perspective, p. 1-22.In J.-M. Ghuysen and R. Hakenbeck (ed.). Bacterial cell wall. Elsevier, Amsterdam, The Netherlands.
- 68.Schwarz, U., A. Ryter, A. Rambach, R. Hellio, and Y. Hirota. 1975. Process of cellular division in Escherichia coli: differentiation of growth zones in the sacculus. J. Mol. Biol. 98:749-759. [DOI] [PubMed] [Google Scholar]
- 69.Tipper, D. J., and J. L. Strominger. 1965. Mechanism of action of penicillin: a proposal based on their structural similarity to acyl-d-alanyl-d-alanine. Proc. Natl. Acad. Sci. USA 54:1133-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tipper, D. J., and A. Wright. 1979. The structure and biosynthesis of bacterial cell walls. p. 291-426. In J. R. Sokatch and L. N. Ornston (ed.), The Bacteria, vol. 7. Academic Press, Ltd., London, United Kingdom.
- 71.Weidel, W., and H. Pelzer. 1964. Bag-shaped macromolecules—a new outlook on bacterial cell walls. Adv. Enzymol. 26:193-232. [DOI] [PubMed] [Google Scholar]
- 72.Williams, B., and S. Epstein. 1963. Medicine from microbes. Julian Messner, New York, N.Y.
- 73.Woese, C. R. 1998. The universal ancestor. Proc. Natl. Acad. Sci. USA 98:6854-6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woese, C. R. 2000. Interpreting the universal phylogenetic tree. Proc. Natl. Acad. Sci. USA 97:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]