Abstract
Fungi may infect the cornea, orbit and other ocular structures. Species of Fusarium, Aspergillus, Candida, dematiaceous fungi, and Scedosporium predominate. Diagnosis is aided by recognition of typical clinical features and by direct microscopic detection of fungi in scrapes, biopsy specimens, and other samples. Culture confirms the diagnosis. Histopathological, immunohistochemical, or DNA-based tests may also be needed. Pathogenesis involves agent (invasiveness, toxigenicity) and host factors. Specific antifungal therapy is instituted as soon as the diagnosis is made. Amphotericin B by various routes is the mainstay of treatment for life-threatening and severe ophthalmic mycoses. Topical natamycin is usually the first choice for filamentous fungal keratitis, and topical amphotericin B is the first choice for yeast keratitis. Increasingly, the triazoles itraconazole and fluconazole are being evaluated as therapeutic options in ophthalmic mycoses. Medical therapy alone does not usually suffice for invasive fungal orbital infections, scleritis, and keratitis due to Fusarium spp., Lasiodiplodia theobromae, and Pythium insidiosum. Surgical debridement is essential in orbital infections, while various surgical procedures may be required for other infections not responding to medical therapy. Corticosteroids are contraindicated in most ophthalmic mycoses; therefore, other methods are being sought to control inflammatory tissue damage. Fungal infections following ophthalmic surgical procedures, in patients with AIDS, and due to use of various ocular biomaterials are unique subsets of ophthalmic mycoses. Future research needs to focus on the development of rapid, species-specific diagnostic aids, broad-spectrum fungicidal compounds that are active by various routes, and therapeutic modalities which curtail the harmful effects of fungus- and host tissue-derived factors.
INTRODUCTION
Ocular fungal infections, or ophthalmic mycoses, are being increasingly recognized as an important cause of morbidity and blindness; certain types of ophthalmic mycoses may even be life-threatening (213, 435). Keratitis (corneal infection) is the most frequent presentation (363), but the orbit, lids, lacrimal apparatus, conjunctiva, sclera, and intraocular structures may also be involved (Fig. 1). A comprehensive review of fungal diseases of the eye, in particular endogenous and exogenous fungal endophthalmitis, has recently been published (194). In the present article, emphasis is placed on mycotic keratitis and mycoses of the orbit and adjacent external ocular tissues; intraocular mycoses (excluding endophthalmitis) are briefly mentioned. Emphasis has been placed on literature published within the last 12 years, but prior noteworthy reviews and case reports are included.
FIG. 1.
Schematic representation of a cross-section of the human eyeball, depicting its parts.
Any review of the literature on ophthalmic fungal infections is hampered by several factors. The first is that there are few controlled or comparative studies on this subject, and much of the material is in the form of single case reports, reports of small numbers of patients, or papers dealing with a retrospective review of patient records. The second is that many fungal genera and species have been implicated in ocular infections, and it is difficult to give appropriate weight to the significance of these organisms. An important publication in 1998 listed some 105 species in 35 genera of fungi as causes of keratitis and other ophthalmic mycoses (424); however, the criteria by which these fungi were considered to be genuine ophthalmic pathogens, and not simply contaminants inadvertently introduced into specimens during or after collection (80a), were not clearly delineated. An evaluation made in 1980 (237) of more than 300 reports pertaining to human fungal infections published in the literature from the late 1940s to the beginning of 1979 encountered similar difficulties. That assessment included reports on 30 genera (60 species) of fungi isolated from ophthalmic infections, principally keratitis; only reports pertaining to 32 species in 19 genera of fungi satisfied strict criteria of acceptability (237).
A third problem is in assessing the accuracy of the genus or species identification of a fungal strain isolated in culture. For example, a fungal strain isolated from a patient with keratitis was initially identified as Arthrobotrys oligospora but later reidentified as Cephaliophora irregularis (128); C. irregularis was subsequently isolated from another patient with keratitis as well (235). Similarly, a filamentous fungus isolated from an intraocular lesion arising out of a retained contact lens was identified as Scedosporium prolificans (19); it now appears that this identification may have been erroneous (J. Guarro and J. Gené, Letter, J. Clin Microbiol. 40:3544, 2002).
To overcome these limitations, reports of single cases or small numbers of patients were considered acceptable for this review if they satisfied criteria similar to those described earlier (237): when an adequate clinical history was presented that suggested a mycotic infection; when the fungus was seen in the clinical specimens; and when the morphology of the fungus in the clinical specimens was consistent with the reported etiologic agent. Papers describing a series of patients with keratitis (120, 334) or other ophthalmic infection (313), many of which were based on retrospective analysis of patient records, were assessed differently since such publications rarely provided detailed descriptions of the fungi isolated from individual patients or of the appearance of the fungi in the specimens or tissues. The observations made in these papers were considered valid if definite criteria had been used to assess the significance of the fungi isolated; for example, the presence of clinical features suggesting a fungal infection, growth of the same fungus from repeated samples, growth of the same fungus on two or more solid media, or confluent growth at the site of inoculation in one solid medium with direct microscopic demonstration of fungal hyphae or yeast cells in the sample (85, 120, 208, 216, 364, 377).
A recent review of fungal infections of the eye (194) listed exceptions to the rule requiring isolation of the fungus from ocular tissue. The exceptions listed included entities such as endogenous endophthalmitis, in which fungi known to cause this disease had been isolated from blood culture and the clinical presentation was compatible with vascular dissemination of the fungus; histoplasmosis and coccidioidomycosis, which are commonly associated with characteristic chorioretinal lesions and in which isolation of the fungus from another anatomical site or measurement of titers of antibody to the fungus is usually deemed sufficient evidence to establish one of these fungi as the cause of the eye disease; and ophthalmic infections due to Cryptococcus neoformans, which usually occur in conjunction with meningoencephalitis and in which isolation of cryptococci from blood and/or cerebrospinal fluid is usually sufficient to explain the associated eye findings. Most of these exceptions pertain to reports of intraocular mycoses, whereas the present review highlights external ophthalmic infections.
In this review, fungal genera and species are cited as they have been reported in the literature. Unfortunately, in the majority of published reports, the strains have not been deposited in recognized culture collections to permit others to confirm the validity of the identifications; moreover, there is a need to apply modern molecular biological and other methods to the process of identification of fungi in the future (129; J. Guarro and J. Gené, Letter, J. Clin. Microbiol. 40: 3544, 2002). Hence, at present, only an uncritical compilation of the fungal genera and species as reported is possible.
ETIOLOGICAL AGENTS AND LABORATORY DIAGNOSIS OF OPHTHALMIC MYCOSES
Etiological Agents
Fungi are opportunistic in the eye, since they rarely infect healthy, intact ocular tissues. Even the trivial trauma of a dust particle falling on the cornea may disrupt the integrity of the corneal epithelium, predisposing to mycotic keratitis. In a compromised or immunosuppressed individual, serious sight-threatening and life-threatening infections such as rhinoorbitocerebral zygomycosis may supervene (435).
An overwhelming number of fungal genera and species have been implicated as causes of ophthalmic mycoses, and this number is steadily increasing. Species and genera of fungi implicated as genuine ophthalmic pathogens in the past 5 years include Chrysosporium parvum (415), Metarhizium anisopliae var. anisopliae (76), Phaeoisaria clematidis (131), and Sarcopodium oculorum (132). In this review, no attempt has been made to list every single fungal genus or species implicated in ophthalmic infection, given the limitations listed above. Instead, the salient features of the most important genera and species are highlighted, since it appears that only a relatively small number are repeatedly isolated in ophthalmic mycoses or have been isolated from more than one ocular site (Tables 1 to 5). For purposes of simplicity, the fungal genera and species have been grouped as hyaline filamentous fungi (Table 1), dematiaceous fungi (Table 2), yeasts and zygomycetes (Table 3), thermally dimorphic fungi (Table 4), and organisms of uncertain classification, namely, Pythium insidiosum, Rhinosporidium seeberi, and Pneumocystis carinii (Table 5). In Tables 1 to 5, brief descriptions and line drawings are included to highlight the salient microscopic morphological features of some ocular fungal pathogens which may be unfamiliar to most clinical microbiologists; more intricate details are provided in other papers and specialist mycology texts (50, 237, 238, 325, 329, 373).
TABLE 1.
Hyaline filamentous fungi implicated in ophthalmic infections
| Genus and species | Morphology | Ophthalmic infections in which implicated (references)a |
|---|---|---|
| Fusarium (F. solani, F. dimerum, F. oxysporum [keratitis usually due to F. solani or F. oxysporum]) | Microscopic morphology in ocular samples Septate, hyaline, branching hyphae, 2-4 μm wide, similar to other hyaline filamentous fungi. Adventitious sporulation may be seen (220); the conidia are larger than those of Paecilomyces spp. (220). Morphology in culture (glucose peptone agar, 30°C) (i) Macroscopic morphology. Colony is flat and floccose and attains a diameter of 30 mm (1 wk). Initially white, later acquires a buff coloration, followed by production of a variety of color pigments. (ii) Microscopic morphology. Crescent-shaped thick- or thin-walled macroconidia, each with 1-5 septa and definite foot cell. Small oval microconidia may be abundant (F. solani or F. oxysporum) or absent (F. dimerum). | Keratitis (120,334, 364, 377), scleritis (254), and intraocular infections (115) |
| Aspergillus (A. fumigatus, A. flavus, A. terreus) | Microscopic morphology in ocular samples Septate, hyaline branching hyphae, 3-6 μm wide, which exhibit parallel walls and radiate from a single point in tissues; smaller than hyphae of zygomycetes (220). Dichotomous (45°) branching may occur (301); this may not be pathognomonic in ocular infections (271) Morphology in culture (glucose peptone agar, 30°C) (i) Macroscopic morphology. Rapidly growing (60 mm in 1 wk), flat, floccose to granular colony. White in early stages, followed by production of various color pigments. (ii) Microscopic morphology. Conidiophore arises from a foot cell and terminates in a vesicle. Vesicle produces phialides in one or two series. Unicellular conidia (hyaline or colored blue-green, yellow, tan, etc.) are arranged in a chain with the youngest conidium at the proximal end near the phialide. | Keratitis following occupational trauma (85, 120, 398) or surgery (142,361), orbital lesions (172, 201,213), dacryocystitis (200, 213), scleritis (31), and endophthalmitis (367, 375) |
| Scedosporium (S. apiospermum [teleomorph Pseudallescheria boydii]; S. prolificans [formerly called Scedosporium inflatum]) | Microscopic morphology in ocular samples Septate, hyaline, branching hyphae, 2-4 μm wide, similar to other hyaline filamentous fungi. Morphology in culture (glucose peptone agar, 30°C) (i) Macroscopic morphology. Colony is flat to dome shaped, floccose or moist, and white to pale or dark gray or black, and attains a diameter of 20 mm (S. prolificans) to 40 mm (S. apiospermum) in 1 wk. (ii) Microscopic morphology. S. apiospermum conidiophores are long and slender, single or branched, and sometimes aggregated into bundles (Graphium state). Conidia (6-12 μm by 3.5-6 μm) are yellow to pale brown, oval with a scar at base, and usually abundant. S. prolificans conidiophores are short with inflated base and tapering tip; oval conidia (3-7 μm by 2.5 μm) frequently occur in groups. | S. apiospermum: keratitis (34, 79, 247, 360, 377, 430), scleritis (254, 379), endophthalmitis (239,298), and orbital infections (16, 176,264). S. prolificans: sclerokeratitis (19, 202, 370) Speciation of isolates reported to be S. prolificans may require confirmation by DNA sequencing (Guarro and Gené, letter) |
| Paecilomyces (P. lilacinus, P. variotii) | Microscopic morphology in ocular samples Septate, hyaline, branching hyphae, 2-4 μm wide, similar to other hyaline filamentous fungi. Abundant adventitious sporulation frequently occurs; reported in keratitis (220). Adventitious conidia subglobose to very short ellipsoidal. Morphology in culture (glucose peptone agar, 30°C) (i) Macroscopic morphology. Colony is flat to dome shaped, granular to loose or densely floccose, white to lilac (P. lilacinus) or olive brown (P. variotii); attains a diameter of 30 mm (P. lilacinus) to 50 mm (P. variotii) in 1 wk. (ii) Microscopic morphology. P. lilacinus phialide is flask shaped with swollen basal portion tapering in long distinct neck; conidia (2.5-3 mm by 2 μm) are ellipsoidal, smooth, and borne singly, in whorls or in penicillate heads. P. variotii phialide is flask shaped with long chains of large, ellipsoidal conidia (5-7 μm by 2.5-3 μm). | Keratitis (121, 197, 334,365), endophthalmitis (280), and intralenticular infection (80a) |
| Acremonium (A. kiliense, A. potronii) | Microscopic morphology in ocular samples | Keratitis (56, 93,248, 317, 399) and endophthalmitis (129) |
| Septate, hyaline, branching hyphae (2-4 μm wide); adventitious sporulation may occur (220). | ||
| Morphology in culture (glucose peptone agar, 30°C) | ||
| (i) Macroscopic morphology. Colony is flat, smooth, gray to orange, and rapidly growing (diameter of 50 mm in 1 wk). | ||
| (ii) Microscopic morphology. Conidiophore is long, straight, and slightly tapering; conidia (3-6 μm by 1.5 μm) are ellipsoidal and accumulated in slimy balls. |
Criteria for diagnosis of mycotic infection. (i) For isolates from keratitis: growth on at least two culture media; growth on one medium, and fungal hyphae seen by microscopy of corneal scrapes, biopsy specimens, or buttons. (ii) For isolates from other infections: growth in culture and fungal hyphae seen by microscopy of aspirates or necrotic material or by histopathological examination of tissue sections.
TABLE 5.
Ophthalmic lesions due to Pythium insidiosum, Rhinosporidium seeberi, and Pneumocystis carinii
TABLE 2.
Dematiaceous fungi frequently implicated in ophthalmic infections
| Genus and species | Morphology | Ophthalmic lesions in which implicated (references) |
|---|---|---|
| Bipolaris (B. spicifera, B. hawaiiensis), Curvularia (C. lunata, C. geniculata, C. senegalensis), Exophiala (E. jeanselmei var. jeanselmei, E. dermatitidis), Exserohilum (E. rostratum, E. longirostratum), Lecytophora (L. mutabilis, L. hoffmannii), and Phialophora verrucosa | Microscopic morphology in tissues Brown pigmented, septate, fungal hyphae Microscopic morphology in culture (glucose peptone agar, 30°C) Bipolaris spp. have a sympodial conidiophore with profuse sporulation. Conidia are oblong, ellipsoidal to fusoid (16-34 μm by 4-9 μm), basal cell of conidium is round, and hilum is continuous with conidial wall, slightly protruding and truncate; 3-7 pseudosepta present. Curvularia spp. have an erect, unbranched conidiophore. Conidia (18-37 μm by 18-14 μm) are smooth walled, olivaceous to dark brown (the end cell may be pale), 3 to 4 septate (the central or subterminal cell may be largest), and broadly ellipsoidal or obovoidal to distinctly curved. Exophiala spp. have a conidiophore that is brown, cylindrical to flask shaped, with a narrow apex with or without collarettes; apical or borne on the side of hyphae. Conidia (2.5-5.9 μm by 1-3 μm) are single celled, colorless to pale brown, and ellipsoidal; they may accumulate in clusters. Exserohilum spp. have a sympodial conidiophore with profuse sporulation. Conidia are ellipsoidal to fusoid (30-128 μm by 9-23 μm): the basal cell of the conidium is round to conical, and the hilum protrudes markedly and is truncate; 7-9 pseudosepta present. Lecytophora spp. have conidiogenous cells that emerge from the hyphal filament. Conidia (4-6 μm by 1.8-2.5 μm) are hyaline or subhyaline, smooth, thin walled, and subcylindrical to cylindrical. Phialophora spp. have a conidiophore that arises from the hyphal filament and is brown, cylindrical to flask shaped, with a very distinct flared, funnel-shaped, or cup-shaped collarette. Conidia (2.5-6 μm by 1-3 μm) are hyaline to pale brown, thick or thin walled, and oval to slightly kidney shaped; they may accumulate in slimy clusters. | Keratitis (40,111, 130, 212, 216, 228, 237, 238, 366; Ho et al., Letter); criteria for diagnosis include growth on multiple culture media or positive microscopy with growth in single culture medium. Orbital infections (44, 167, 233); criteria for diagnosis include growth in culture with positive microscopy. Intraocular infections (182); criteria for diagnosis include growth in culture with positive microscopy. |
| Lasiodiplodia theobromae | Microscopic morphology in tissues Septate, highly bulged, brown hyphae. Morphology in culture Rapid growth occurs (90 mm in 1 wk). Colony is floccose, gray to brown-black (Fig. 4); macroscopic fruiting bodies (pycnidia) are visible after 7-21 days. Conidia (20-30 μm by 10-15 μm) are initially colorless, ellipsoidal, nonseptate; later they are dark brown and septate, with longitudinal striations and truncate bases (50). | Severe keratitis (111,216, 305, 318, 392, 393); criteria for diagnosis include growth on multiple culture media or positive microscopy with growth on single medium. Endophthalmitis (37) and panophthalmitis (356); criteria for diagnosis include recovery from multiple ocular tissues and positive microscopy. |
TABLE 3.
Yeasts and zygomycetes implicated in ophthalmic infections
| Genus and species | Microscopic morphology | Ophthalmic infections in which implicated (references) |
|---|---|---|
| Yeasts Candida (C. albicans, C. parapsilosis, C. guilliermondii) in ocular samples | Morphology in ocular samples The presence of small (3-4-μm) budding yeast cells and pseudohyphae in corneal scrapes is almost diagnostic for Candida spp (269). The bud exhibits an off-axis position and a narrow base at the point of attachment; the yeast cell appears asymmetrical (301). | C. albicans and other Candida spp. implicated as causes of keratitis (334, 377), infectious crystalline keratopathy (419), and intraocular lesions (147, 165, 281). Criteria for diagnosis in keratitis include growth on multiple media or growth on single medium with positive microscopy. |
| Cryptococcus (C. neoformans var. neoformans, C. laurentii) | Morphology in ocular samples Typically 2-20 μm in diameter. The presence of teardrop-shaped, narrow-based budding of C. neoformans var. neoformans is a useful cytologic feature (301). | C. neoformans var. neoformans causes keratitis (216,377), blepharitis (66, 82), chorio retinitis (255), endophthalmitis (255), and solitary subretinal lesions (146). C. laurentii was recently implicated (with F. solani) in contact lens-associated keratitis (328). |
| Zygomycetes Rhizopus (R. arrhizus), Mucor (M. ramosissimus), Rhizomucor (R. pusillus), Absidia (A. corymbifera), Apophysomyces (A. elegans), Saksenaea (S. vasiformis) (87, 323,435) | Morphology in ocular samples Broad, aseptate, or sparsely septate hyphae with right-angled 90° branching; these neither possess parallel walls nor radiate from a single point in tissues. Hyphae stain poorly with PAS but stain well with hematoxylin-eosin and GMS stains. Cresyl fast violet stains zygomycete walls brick red and stains other fungi blue or purple (324). Seen in the midst of prominent inflammation, necrosis, and invasion of blood vessels. Morphology in culture (glucose peptone agar, 30°C) Asexual spores (sporangiospores) occur in a sac (sporangium); the sporangium is held aloft by a stalk (sporangiophore). The sporangium may be on a funnel-shaped base (Apophysomyces elegans) or may have an apical tubular extension (Saksenaea vasiformis). The stalk may arise from a branched root-like system of rhizoids (Rhizopus spp.) or from hyphae in between two aggregations of rhizoids (Absidia corymbifera). Pale or brownish sporangia arise from stalks lacking rhizoids in Mucor spp. The stalk may have a funnel-shaped top (A. corymbifera) or may have branches crowded near top of main stalk (Rhizomucor pusillus). | Various zygomycetes are reported to cause rhino-orbito-cerebral zygomycosis (15, 435). Criteria for diagnosis include suggestive clinical features; detection of the characteristic large, broad aseptate hyphae in necrotic material or tissue bits or sections; and growth on multiple culture media. A. corymbifera is reported to cause keratitis (231); the diagnosis is established by growth in culture and positive microscopy. Rhizopus spp. are reported as a cause of scleritis (221), but evidence is not convincing (fungus was not seen in tissues, only 1 colony grown in culture). |
TABLE 4.
Thermally dimorphic fungi implicated in ophthalmic infections
| Genus and species | Morphology | Ophthalmic lesions in which implicated (references) |
|---|---|---|
| Paracoccidioides brasiliensis | Spherical, yeast-like cells with multiple buds attached by narrow necks, also called “steering wheel forms,” seen in KOH mounts of material or in tissue sections and in culture at 37°C. | Reported to cause lesions of eyelids (46, 353), cornea (353), and bulbar conjunctiva (353); anterior uveitis (353); and granulomatous uveitis (75). Diagnosis by histopathological examination or direct microscopy of lesions (353); no photomicrographic evidence. Ophthalmic lesions are rare in the absence of lesions elsewhere in the body, unless entry is through a wound; usually unilateral. |
| Coccidioides immitis | Large, multinucleate, thick-walled cells (spherules) filled at maturity with spores; these escape by rupture of the cell wall. Spherules are usually found within giant cells (325). Spherules are seen on microscopic examination of KOH mounts of pus or necrotic material or by histopathological examination of infected ocular tissues (253). In culture at 30°C, barrel-shaped arthospores (2.5-4.5 μm by 3-8 μm) are seen. | Anterior-segment lesions (phlyctenular conjunctivitis, episcleritis, scleritis, and keratoconjunctivitis) reported in conjunction with underlying pulmonary infection; lid granulomata and inflammation reported in disseminated disease (331). Diagnostic criteria used unclear. Granulomatous uveitis and iris nodules noted in patients without systemic disease and 1 patient with previously treated pulmonary disease. Diagnosis established by the presence of spherules in various samples and by positive cultures (253). |
| Blastomyces dermatitidis | Spherical, multinucleate yeast-like cells (8-20 μm in diameter) with single broad-based bud and refractile double-contoured walls; generally larger than those of cryptococci (301). Seen in KOH mounts of necrotic material or in tissue sections, and generally extracellularly (215,338), and in culture at 37°C. | Lesions of eyelids (Barr and Gamel, letter; 26), cornea (332), conjunctiva (355), and orbit (215, 409), intraocular lesions (338), and endophthalmitis (215,338), reported. B. dermatitidis cultured from, and seen in, orbital lesions and endophthalmitis (215). Positive immunofluorescence test in corneal lesions of 2 patients (332). Detection of characteristic forms in tissues in others (338,355) |
| Sporothrix schenckii | Small, spherical, oval or elongated “cigar-shaped” budding yeast cells with irregularly stained cytoplasm, mostly located extracellularly (205). “Asteroid bodies,” which are central spherical or oval basophilic cells 3-5 μm in diameter surrounded by a thick, radiate eosinophilic substance, rarely occur (325). More important for identification is microscopic morphology in culture at 30°C (glucose peptone agar): hyaline hyphae, delicate conidiophores bearing an apical rosette of minute conidia (3-10 μm by 1-3 μm). | Endophthalmitis (52, 205,427), scleritis (Brunette and Stulting, letter), uveitis (410), and orbital lesions (369). In most reports, diagnosis by detection of characteristic forms in affected tissues. In two reports (205, 369), positive culture and histopathology findings. |
| Histoplasma capsulatum (H. capsulatum var. capsulatum, H. capsulatum var. duboisii) | Organisms may be missed in wet mounts, hence stained smears should be examined (301). H. capsulatum var. capsulatum has thin-walled oval yeast cells (2-3 μm by 3-4 μm), free or phagocytized within cells; there may be associated infiltrate of lymphocytes and histiocytes (357). H. capsulatum var. duboisii has larger yeast cells (8-15 μm) than those of H. capsulatum var. capsulatum; the cell wall is thicker, and the isthmus and bud scar are more prominent (5, 373). In culture at 30°C (glucose peptone agar), large tuberculate globose macroconidia (6-15 μm) are seen. | Endogenous (118) and exogenous (303) endophthalmitis; choroiditis, retinitis and optic neuritis in patients with AIDS (224, 357, 433); anterior segment lesions are rare (89). |
Hyaline filamentous fungi.
Species of Fusarium (Table 1) are widespread saprobic fungi that cause important diseases of plants, particularly major crop plants (71), and of humans, particularly immunocompromised patients (263). They have long been regarded as important pathogens in eye infections, especially keratitis (263, 384).
Aspergillus spp. abound in the environment worldwide, thriving on a variety of substrates such as corn, decaying vegetation, and soil. These fungi are also common contaminants in hospital air (367) and have been implicated in a recent outbreak of endophthalmitis following cataract surgery that was traced to ongoing hospital construction (375); they are also implicated in other types of ophthalmic mycoses.
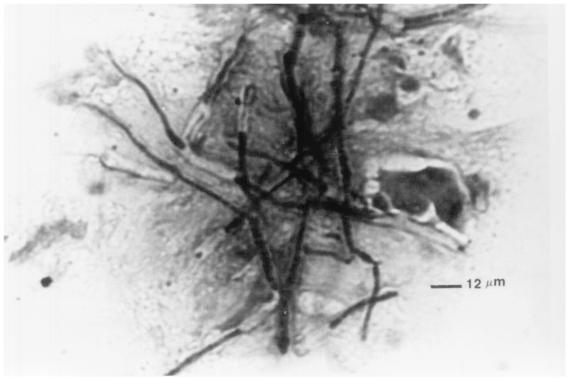
Scedosporium apiospermum (teleomorph Pseudallescheria boydii) (Fig. 2) has been isolated from soil, sewage, and polluted water and from the manure of farm animals (373). It has been reported to cause severe ocular infection following trauma by plant material, contact with polluted water, and immunosuppression (211, 325, 379, 430). The fungus Scedosporium prolificans, which was first described as a human pathogen in 1984, has been reported as a cause of sclerokeratitis (202, 370).
FIG. 2.
Hyaline filamentous fungi S. apiospermum, Paecilomyces, and Acremonium.
Species of Paecilomyces (Fig. 2), which are found worldwide as saprobes in soil and decaying vegetation, may also contaminate sterile solutions and culture media, since they are resistant to most of the common sterilizing procedures. Many documented ocular infections by Paecilomyces spp. have followed surgical procedures (121, 197, 280).
Species of Acremonium (Fig. 2) are widespread, occurring in soil, decaying plant material, and the air (129). Several cases of keratitis (93, 237, 315, 317) and occasional cases of endophthalmitis (93) due to Acremonium spp. have been reported in the literature.
Dematiaceous (phaeoid) fungi.
The primary factor unifying the dematiaceous fungi (Table 2; Fig. 3) is the dark pigmentation of their hyphae (238). At least 20 species of fungi belonging to 11 different genera have been implicated as causes of keratitis (the most frequently reported ones are listed in Table 2). Dematiaceous fungi have been reported to be the third most frequent cause of mycotic keratitis (behind Aspergillus and Fusarium) (111, 120, 208, 288, 364, 383) and may also cause infections of the orbit (164, 167, 233 W. J. Chang, C. L. Shields, J. A. Shields, P. V. De Potter, R. Schiffman, R. C. Eagle, Jr., and L. B. Nelson, Letter, Arch. Ophthalmol. 114: 767-768, 1996) or intraocular infections (182). These fungi exhibit a brown-to-olive-to-black color in the cell walls of their vegetative cells, conidia or both, colonies thus appear olive to black.
FIG. 3.
Dematiaceous fungi Bipolaris, Curvularia, Exophiala, Exserohilum, Lecytophora, Phialophora, and L. theobromae.
Lasiodiplodia theobromae (Table 2; Fig. 4) is an important cause of rot in corn, yams, citrus, bananas, and other plants, mainly in tropical regions (266, 373). This organism was initially reported as a cause of human keratitis in two patients in India (305). Subsequently, reports from the southern United States, other parts of India, Sri Lanka, and other countries have confirmed that this fungus is pathogenic in the human cornea (37, 117, 216, 318, 356, 392, 393); brown, highly bulged, septate hyphae are seen in infected corneal tissue. This fungus causes severe keratitis in experimental animals (305, 318) and in humans (37, 318, 392, 393).
FIG. 4.
A 5-day growth of L. theobromae on Sabouraud glucose-neopeptone agar, Emmons' modification. Growth has reached the edge of the petri dish (90 mm in diameter), indicating rapid growth. The colony is floccose and grey to brown-black. Macroscopic fruiting bodies (pycnidia) have not yet appeared.
Yeasts and zygomycetous fungi.
Most episodes of yeast infections in corneal ulcers and other ocular infections are due to various Candida species, predominantly Candida albicans (Table 3), and usually occur in the presence of systemic illness (diabetes mellitus or immunocompromise) or ocular disease (lid abnormalities or dry eyes) or in patients receiving prolonged topical medications or topical corticosteroids (334, 377). Species of Cryptococcus (see Table 3) may also cause ocular lesions (146, 185, 255, 328, 377).
Ocular infections by the zygomycetes (Table 3; Fig. 5) include rhino-orbitocerebral zygomycosis (435) and keratitis (231). Although Rhizopus spp., especially Rhizopus arrhizus, are most frequently involved, other genera of the order Mucorales may also cause ocular disease (87, 323, 435). The detection of fungi belonging to the Mucorales by direct microscopy in clinical material or tissue sections (Table 3) is more significant than their isolation in culture (323, 324).
FIG. 5.
Zygomycetes Rhizopus, Mucor, R. pusillus, A. corymbifera, A. elegans, and S. vasiformis.
Thermally dimorphic fungi.
Paracoccidioides brasiliensis (Table 4; Fig. 6), which has been recovered from soil and decaying vegetation in zones of endemic infection (southern Mexico and Central and South America), causes a severe, usually chronic disease with involvement of the skin, lungs, and lymphoid organs. Ocular involvement usually represents reactivated disease and commonly manifests as a chronic papular or ulcerating lesion of the eyelid in a man older than 30 years engaged in agriculture and coming from regions of endemic infection (353).
FIG. 6.
Thermally dimorphic fungi P. brasiliensis, C. immitis, B. dermatitidis, and S. schenckii.
Coccidioides immitis (Fig. 6) is found in the alkaline soil of warm, dry regions where infection is endemic (southwestern United States, northern Mexico, and localized areas in Central and South America) (373). Disease ranges from self-limited primary pulmonary coccidioidomycosis to disseminated disease; ocular lesions (72, 222, 331) have also been reported.
Blastomyces dermatitidis (Fig. 6), which has been isolated from moist soil with high organic content, is known to cause pulmonary, cutaneous, osteoarticular, and genitourinary disease (373). Ocular infections include eyelid lesions (26, 355; G. C. Barr and J. W. Gamel, Letter, Arch. Ophthalmol. 104:96-97, 1986), orbital disease (215, 409), keratitis (332), and endophthalmitis (215, 338).
Histoplasmosis is classically caused by Histoplasma capsulatum var. capsulatum, while a variant form, known as African histoplasmosis or large-celled histoplasmosis, is caused by H. capsulatum var. duboisii. The disease is most prevalent in the central region of North America, in Central and South America, in the tropics, and in certain river valleys in temperate regions (373). H. capsulatum var. capsulatum has been implicated in the “presumed ocular histoplasmosis syndrome” and in several other ophthalmic infections, mostly of intraocular structures (118, 180, 224, 303, 424); H. capsulatum var. duboisii has been reported to cause orbital disease (5).
Sporothrix schenckii (Fig. 6), which has been isolated from soil and decaying plant material worldwide, generally causes nodular lesions in the cutaneous and subcutaneous tissues, which ultimately suppurate, ulcerate, and drain. This fungus has been reported to cause lesions of the orbit (369), sclera (I. Brunette and R. D. Stulting, Letter, Am. J. Ophthalmol 114:370-371, 1992), and intraocular structures (205).
Organisms of uncertain taxonomic classification.
Pythium insidiosum (Table 5), a cosmopolitan fungus-like aquatic organism, is found predominantly in swampy environments, where water lilies, various vegetables, and especially certain grasses support the asexual phase of its life cycle; motile zoospores, which appear to be chemotactically attracted to plant leaves or human and horse hairs, are the likely infective particles (244). This organism, originally considered to be an oomycete in the kingdom Fungi and later a member of the kingdom Protoctista (244, 373), is now placed in the kingdom Stramenopila, containing organisms that are related to algae (373). P. insidiosum has been implicated in diseases of plants and animals (horses, cattle, dogs, cats, or fish), particularly in tropical and subtropical parts of the world (22, 155, 260, 381). In Thailand, this organism causes subcutaneous lesions and chronic inflammation and occlusion of blood vessels (especially of the lower extremities) in thalassemic and nonthalassemic patients (381). Keratitis due to P. insidiosum has been noted in tropical (22, 155, 244, 411) and temperate (260) regions. Two particularly aggressive cases of orbital cellulitis with deep facial tissue involvement have occurred in the United States (244).
Rhinosporidium seeberi (Table 5; Fig. 7) an endosporulating microorganism which causesrhinosporidiosis, has traditionally been considered a fungus but is now of uncertain taxonomic classification (295). Lesions of rhinosporidiosis manifest as polypoid or papillomatous, very friable, proliferative outgrowths principally in the nasal cavity; ocular lesions may account for 13% of all lesions, with the ratio of nasal to ocular lesions being 1.4:1 (284).
FIG. 7.
Photomicrograph showing presence of sporangia (cysts) of R. seeberi in stroma of the lacrimal sac. The cysts are of all sizes, with a sharply defined, chitinous-appearing wall. The largest sporangium reveals maturing spores (endospores). The smaller cysts represent “trophic” stages of the organism. Hematoxylin-eosin stain; magnification, ×400.
Pneumocystis carinii (Table 5) was originally considered to be a protozoon, based on its morphology and response to antiparasitic drugs, but has now been reclassified as a member of the kingdom Fungi subsequent to analysis of its nucleic acids (48). It has been implicated as a cause of choroiditis (83, 104, 350) and orbital infection (D. N. Friedberg, F. A. Warren, M. H. Lee, C. Vallejo, and R. C. Melton, Letter, Am. J. Ophthalmol. 113: 595-596, 1992) in patients with AIDS.
Laboratory Diagnosis
Laboratory investigation of a suspected ophthalmic mycosis begins with the collection of an appropriate specimen (Table 6); these samples are subjected to direct microscopic examination (Table 7), culture, histologic testing, or other investigations.
TABLE 6.
Specimens used for diagnosis of ocular fungal infectiona
| Orbital lesions |
| Biopsy specimens from necrotic tissue, and necrotic material from the nose, paranasal sinuses, and oropharynx for HPEb and culture (324) |
| Purulent material aspirated with a sterile syringe and needle |
| Serum for serological investigations (181) |
| Blepharitis and eyelid lesions |
| Cotton or calcium alginate swabs, moistened with TSB,b used to scrub anterior lid margins and any ulcerated area |
| Lid biopsy specimen may be indicated for certain lesions, e.g., those due to B. dermatitidis or P. brasiliensis (353) |
| Dacryoadenitis |
| Lacrimal gland surgically removed in toto and bisected for HPE |
| Lacrimal gland surgically removed, bisected, ground, suspended in sterile buffered saline, and used for culture |
| Dacryocanaliculitis |
| Lid and canaliculus are compressed to express purulent material, which is carefully collected on a sterile spatula |
| If concretions are present within the canaliculus, they are scraped off with a sterile spud or small chalazion curette; concretions are crushed on slide prior to staining |
| Dacryocystitis |
| Material drained from lacrimal sac with a sterile syringe and needle |
| If lacrimal sac is removed by surgery, it is bisected for HPE and cultured as for the lacrimal gland (177, 199) |
| If pressure on lacrimal sac expresses material into conjunctival sac, it is collected as for the conjunctival swab (36) |
| Conjunctivitis |
| For suspected rhinosporidiosis, the lesion is surgically excised for HPE (321) |
| Inferior tarsal conjunctiva and fornices are vigorously scrubbed with calcium alginate/cotton-tipped swabs, which are moistened in TSB if lesions are dry; local anesthetic should not be used |
| Inferior tarsal conjunctiva is scraped by sterile spatula; local anesthetic is used |
| Conjunctival biopsy specimen for HPE and culture indicated if above specimens do not yield results |
| Keratitis |
| Sterile cotton-tipped or calcium alginate swabs used to collect material from ipsilateral and contralateral lid and conjunctiva |
| Corneal scrapes collected with a Kimura spatula, Bard-Parker knife, sterile razor, surgical blade, or spatula; local anesthetic is used (158, 163,216) |
| Calcium alginate swabs moistened with TSB have been used to collect corneal material; good results have been reported by some (163) |
| If the above samples do not yield results, corneal tissue is collected by epithelial biopsy or superficial keratectomy for HPE, culture, and other tests (8, 196); if penetrating keratoplasty is done, the corneal button is bisected and used for HPE and culture (334) |
| Corneal material is inoculated in the form of “C” streaks on culture plates; only growth on the streaks is considered significant (Fig. 8) |
| Scleritis |
| Material may be obtained as for conjunctivitis or keratitis |
| If intact scleral abscess is present, material is carefully aspirated with a sterile syringe and needle; if the abscess has burst, it is carefully collected with a sterile swab or spatula |
| In nodular, diffuse, or necrotizing scleritis, or where the above-mentioned samples do not yield results, scleral biopsy is performed (31); it is necessary to exercise caution |
| Endophthalmitis |
| Conjunctival swab (only if leaking filtering bleb or wound is present) |
| Vitreous or aqueous aspirate collected via sterile syringe |
| Vitreous biopsy specimen |
| Vitreous wash material either concentrated by centrifugation before inoculation onto culture media or passed through a membrane filter which is cut into pieces for culture |
| Choroiditis and retinitis |
| Diagnosis is usually based on the presence of characteristic clinical features in the choroid and retina, with recovery of fungi from blood or other body lesions, or demonstration of high titers of fungal antigen in blood or other body fluids |
| Rarely, material is collected from the lesion itself by surgery |
General guidelines. Whenever possible, culture media should be brought to the operation theater or sample collection room so that ocular samples can be directly inoculated onto the plates of appropriate culture media immediately after collection. This is essential for corneal samples. Conjunctival samples may be collected on swabs and transported in appropriate containers. Samples of fluids or aspirates may be inoculated into sterile screw-cap tubes. After inoculation, culture plates should be transported to the laboratory in ≤15 min at room temperature, while swab specimens or fluids and aspirates in screw-cap tubes should be transported to the laboratory in ≤2 h at room temperature. If these transport times cannot be adhered to, samples may be stored at room temperature for ≤24 h (246a). Culture plates (dishes) are preferred to culture tubes for recovery of ocular fungal pathogens since they provide better aeration of cultures, a large surface area for better isolation of colonies, and greater ease of handling by technologists; dehydration of the agar in such culture plates can be minimized by adding at least 40 ml of agar and by placing the culture plates in an incubator that contains a pan of water to achieve a relative humidity of 40 to 50% (329). Cultures should be incubated at room temperature or, preferably, 30°C for at least 30 days; culture plates should be opened and examined only within a certified biological safety cabinet (329).
HPE, histopathological examination; TSB, tryptone soy broth.
TABLE 7.
Important direct microscopic techniques in ophthalmic mycoses
| Method and specimens | Features | Reported drawbacks |
|---|---|---|
| Potassium-hydroxide (KOH) wet mounts KOH only Ink-KOH KOH-dimethyl sulfoxide digestion and counterstaining with PAS or acridine orange; used for corneal scrapes, aqueous and vitreous aspirates, pus, necrotic material, biopsy, and tissue bits | Rapid (1 or 2 steps), inexpensive, easy to perform. KOH ensures good digestion of thick samples. Use of ink, PAS, or acridine orange as the counterstain facilitates the detection of fungal structures. Sensitivities of 75-91% for KOH mounts in culture-proven mycotic keratitis in India (55, 56,120). | Artifacts common. Corneal cells may not swell sufficiently to produce transparent preparations. Optimal viewing time for ink-KOH mounts is 12-18 hs. Ink-KOH has a short shelf life (ink precipitates out). Fluorescence microscope fitted with appropriate filters needed if acridine orange counterstain is used (24,55, 56, 120, 158, 160, 175, 271, 314, 324, 334). If no dye or ink is added, there is no contrast to facilitate the detection of usually colorless fungus against a colorless background. |
| Gram staining Sensitivity of 55-98% in culture-proven keratitis (85,216); used for corneal scrapes, aqueous and vitreous aspirates, pus, necrotic material, and tissue sections | Stains yeast cells and fungal hyphae (Fig. 9) equally well, and bacteria in the preparation can be differentiated. Takes only 5 min to perform. | Fungal hyphae may stain irregularly or not at all. Less useful in thick preparations. False-positive artifacts common. Crystal violet precipitates may cause confusion (120, 174, 175, 216, 314). |
| Giemsa staining Sensitivity of 66-85% in culture-proven mycotic keratitis (120, 216); used for corneal scrapes, aqueous and vitreous aspirates, pus, and necrotic material | Stains yeast cells and fungal hyphae (216). Acanthamoeba cysts, P. carinii, and cytological details can be noted (314). | Staining time of 60 min. Staining of nuclei and cytoplasmic granules of tissue cells may cause opacities in smear. False-positive artifacts may occur. Sensitivity of only 27% in culture-proven mycotic keratitis reported by some (334). |
| Lactophenol cotton blue (LPCB) staining Sensitivity of 70-80% in culture-proven mycotic keratitis (351,387); used for corneal scrapes and aqueous and vitreous aspirates | Rapid, simple, inexpensive one-step method which detects all common ocular fungi and Acanthamoeba cysts. Stain commercially available, with long shelf-life; stained mounts of corneal scrapes can be kept for years (the mounts must be sealed correctly or they will dehydrate). | No tissue digestion; hence, thick preparations may pose problems. Contrast between fungi and background may be insufficient. Unusual fungi may escape detection (24, 351,387). |
| Modified GMS staining Sensitivity of 86% in culture-proven mycotic keratitis (216); used for corneal scrapes, necrotic material, and tissue sections | Fungal cell walls and septa clearly delineated against a pale green background. Acanthamoeba cysts and Pneumocystis carinii can also be detected. | False-positive results occur due to staining of cellular debris and melanin. The multistep procedure requires 60 min. Reagents and procedures need standardization (175,216). Sensitivity of only 56% in culture-proven mycotic keratitis reported by some (398). |
| Calcofluor white Sensitivity of 80-90% in culture-proven mycotic keratitis (55, 56, 120); used for corneal scrapes, aqueous and vitreous aspirates, and tissue bits | Fungal hyphae and yeast cells clearly delineated against a dark background; clearly seen even in thick preparations. Acanthamoeba cysts and P. carinii also seen. Rapid two-step method. | Fresh reagents needed, or false-positive artifacts occur. Fluorescence microscope fitted with appropriate filters is needed. Reagents and procedures need standardization. The viewer should be protected against the hazards of UV light. |
| Hematoxylin-eosin stain Used to detect hyaline and dematiaceous fungi in necrotic material and tissue sections (57) | Host reaction, nuclei of yeast-like cells (especially B. dermatitidis) and hyphae of hematoxylinophilic fungi (aspergilli and zygomycetes) can be visualized (57, 215, 229, 314, 338). The melanin (brown pigment) of dematiaceous fungi and agents of chromoblastomycosis can be detected. | May not be possible to distinguish poorly stained fungi from tissue components. Preparation of stained sections requires time and standardization (57). |
Direct microscopic detection of fungi in ocular samples.
Identification of the fungal genus by direct examination (Table 7) is generally not considered possible (175, 271). However, the occurrence of adventitious sporulation (the presence of conidial structures) in tissue samples, including corneal material, has been reported to aid the differentiation of genera of hyaline filamentous fungi, such as Acremonium, Fusarium (Fig. 8), and Paecilomyces (220).
FIG. 8.
A 24-h growth of F. solani on Sabouraud glucose-neopeptone agar, Emmons' modification, that had been inoculated with corneal scrapes. Note that growth has occurred on the “C” streaks, representing the sites of inoculation. Only growth on C streaks is considered significant.
The potassium hydroxide (KOH) wet mount and its modifications (Table 7) are widely used for the rapid detection of fungal hyphae in necrotic tissue samples from patients with infections of the orbit (324) and other ocular structures (175). Several limitations have been reported when such mounts are used for corneal scrapes, including low sensitivity, frequent misinterpretation, presence of artifacts, and lack of detection of Candida and other yeasts (271, 314, 334). Moreover, if no dye or ink is added, the microscopist is looking for a usually colorless fungus against a colorless background; that is, there is no contrast to facilitate the detection of the fungal organisms. This may explain why American ophthalmologists currently seem to prefer other techniques for detection of fungal elements in corneal scrapes. However, elsewhere, relatively good sensitivities have been reported in the diagnosis of culture-proven mycotic keratitis (120, 288, 351, 429, 431).
The ability to detect and differentiate gram-positive and gram-negative bacteria within 3 min in an ocular sample is the most important function of the Gram stain (329) (Table 7); an additional advantage is that fungi (Fig. 9), filamentous bacteria, and cysts of the protozoon Acanthamoeba can also be detected (314, 329). Identification of the fungal genus by direct examination is generally not possible (175, 271). Direct microscopy of corneal scrapes stained by a fluorescent Gram stain technique permitted a rapid presumptive diagnosis of mycotic keratitis in five patients (335); culture confirmed the diagnosis in all five (three infections were due to F. solani, and one each was due to A. flavus and C. albicans). This stain also detected fungi in the vitreous biopsy specimen of one patient with culture-proven endophthalmitis due to A. flavus (335). Advantages of this fluorescence technique over the conventional method need to be assessed by experiments with samples from more patients.
FIG. 9.
Photomicrograph showing branching, septate fungal hyphae in corneal scrapes. The cell walls and cross-walls are unstained, but the protoplasm has taken up the stain, permitting easy visualization of the fungus. Gram stain; magnification, ×400.
The Giemsa stain can be used to detect fungal hyphae and yeast cells in ocular tissue; this technique has been reported to have a sensitivity of 55 to 85% in diagnosing culture- proven mycotic keratitis (120, 216, 271), although others have obtained poor results (334). This stain can also detect other organisms (Table 7).
Lactophenol cotton blue is a mounting medium commonly used in microbiology laboratories for preparing mounts of fungal cultures. This mounting medium has been recommended for the preparation of clinical samples, including corneal scrapes and aqueous and vitreous aspirates, for direct microscopic examination (24). Although lactophenol cotton blue mounts of ocular samples can be stored for long periods, they must be sealed properly to prevent dehydration.
The Gomori methenamine silver (GMS) and the periodic acid-Schiff (PAS) stains are special stains for detection of fungi in tissue. A modified GMS staining technique has been used for this purpose in corneal scrapes (216), in paraffin-embedded tissue sections (406), and in other ocular samples (Table 7). The entire procedure comprises nine steps and takes about 1 h. This stain can also detect filamentous bacteria such as Nocardia and cysts of Acanthamoeba (175). Although widely available, the PAS technique has been infrequently used as a stain for smears from ophthalmic specimens; the reason for this is not known. PAS stains fungal elements well, and hyphae and yeast cells can be readily distinguished; fungal structures were detected in 91% of the PAS-stained sections of corneal buttons which were positive by culture (431).
In recent years, nonspecific fluorochromatic stains have become popular for the detection of fungi in ocular samples. Calcofluor white appears to be the most widely used of these stains (56, 120, 351, 372) since it can detect fungi in 50% of smears previously considered negative by Gram and Giemsa staining methods (372). Calcofluor white is more sensitive than KOH wet mounts in detecting the common ocular fungi F. solani, A. fumigatus, and C. albicans in corneal scrapes (55, 120, 351). A fluorescence microscope fitted with appropriate filters is needed to view mounts of ocular samples that have been stained with calcofluor white. Blankophor and Uvitex 2B, while similar to calcofluor white in many respects, have certain other advantages for detecting fungi in specimens (337, 414) but have apparently not been used widely for the diagnosis of ophthalmic mycoses; the reasons for this are not known.
Several recent studies of small numbers of patients (126, 179) have confirmed that the acridine orange stain is useful to detect fungal hyphae in corneal scrapes. However, the sensitivity of this method in diagnosing culture-proven mycotic keratitis and its specificity when used for patients with ulcerative keratitis need to be assessed in a large series of patients. A fluorescence microscope fitted with appropriate filters is needed for this technique.
Lectins are ubiquitous proteins, which are particularly common in plant seeds that bind specifically to carbohydrates. Fluorescein-conjugated concanavalin A was found to provide consistently bright staining of the fungal structures in corneal scrapes from 18 patients with culture-proven mycotic keratitis (330) and was thought to be a promising first-line fluorochromatic stain to visualize fungi in ocular samples. Again, this technique does not appear to be used as widely as calcofluor white, perhaps because of the cost involved in preparing the necessary reagents.
Garcia et al. (110) have recently described a peroxidase-labeled wheat germ agglutinin staining technique for diagnosis of experimental mycotic keratitis due to C. albicans, A. fumigatus, and F. solani. In addition to excellent sensitivities and specificities for detecting these infections, there was a high degree of test-retest and inter-rater concordance between two independent observers for all three fungi tested. This technique needs to be assessed in the clinical setting, since the use of the peroxidase label for the lectins would eliminate the need for expensive fluorescence microscopes fitted with appropriate filters. One potential disadvantage of this technique is that tissue sections of corneal biopsy material are required, whereas ophthalmologists and patients would probably feel more comfortable if corneal scrapes could be used as the samples.
When fungi such as Candida or Aspergillus are stained with eosin, they fluoresce under UV illumination; this facilitates their detection. Mucin and vegetable fibers do not interfere with this fluorescence (314). Fluorescence microscopy of a tissue section stained with hematoxylin-eosin revealed the presence of yeast cells of B. dermatitidis in periocular cutaneous lesions that had initially been misdiagnosed as squamous cell carcinoma (229).
Because of their size, polysaccharide content, and morphologic diversity, most mycotic agents can be satisfactorily stained and studied in tissue sections by light microscopy. Sections stained with hematoxylin-eosin have many advantages (Table 7), but species of Fusarium or Candida may not be stained at all. Similarly, fungal structures can be easily detected in sections of corneal tissue stained with the GMS or PAS stains (406), but little else can be visualized. Hence, a replicate tissue section stained with hematoxylin-eosin should always be examined before special stains for fungi are used; alternatively, a section stained with GMS can be counterstained with hematoxylin-eosin for simultaneous demonstration of a mycotic agent and the evoked tissue response (57).
Direct immunofluorescence of fungi in formalin-fixed, paraffin-embedded ocular tissue sections has been used to confirm presumptive histologic diagnoses of ocular infection due to B. dermatitidis, H. capsulatum var. capsulatum, S. schenckii, P. insidiosum, and a zygomycete (98, 224, 244, 283, 409). Other dimorphic fungi and hyaline filamentous fungi can also be detected by this technique (57). Factors that have possibly prevented the routine use of immunofluorescence for diagnosis of ophthalmic mycoses include the need for a fluorescence microscope fitted with appropriate filters, antibodies of good quality, and the standardization of reagents and procedures. This technique is especially helpful when atypical forms of an agent are encountered or when infectious elements are sparse. Moreover, for retrospective studies, tissue sections previously stained by the hematoxylin-eosin, Giemsa, and modified Gram procedures can be decolorized in acid-alcohol and then restained with the specific reagents used for immunofluorescence; however, this is not possible with sections previously stained with GMS or PAS (57).
Culture.
Even with the advent of many new techniques, culture remains the cornerstone of the diagnosis of most ophthalmic mycoses, except for rhinosporidiosis (since Rhinosporidium seeberi cannot be cultivated) and perhaps rhino-orbito-cerebral zygomycosis, where direct microscopic examination of necrotic material or biopsy samples yields more reliable results (324). Commonly used culture media include Sabouraud glucose neopeptone agar (Emmons' modification, neutral pH) incubated at 25°C, blood agar (preferably sheep blood agar) incubated at 25 and37°C, brain heart infusion broth incubated at 25°C, and thioglycolate broth incubated at 25 to 30°C (271). These media were found to be sufficient to permit the isolation of different types of ocular fungi (216, 334). Using these different media, growth of fungi was identified within 2 days in 54%, within 3 days in 83%, and within 1 week in 97% of patients with mycotic keratitis; a positive initial culture was observed in 90% of scrapings (334).
Other media that have been found useful for primary isolation of ocular fungi include chocolate agar (334), cystine tryptone agar (384) and rose bengal agar (P. A. Thomas, unpublished observations). Since many of these media also support bacterial growth, antibacterial antibiotics, such as chloramphenicol (40 μg/ml) or a penicillin-streptomycin combination, are usually incorporated to suppress bacterial growth and permit the isolation of fungi alone. However, cycloheximide must never be used in culture media meant for the isolation of ocular fungi, since most of the fungi implicated in ocular infections are suppressed by this chemical (271). Wherever possible, it is best to use more than one medium, preferably a combination of appropriate solid and liquid media, and to incubate these at 37°C and at 25 to 30°C for the optimal recovery of ocular fungi; the use of liquid-shake cultures may facilitate the recovery of ocular fungi (398). However, some workers feel that since liquid cultures are prone to contamination by environmental fungi, they should not be used in the microbiological workup of patients with mycotic keratitis, to avoid erroneous results (364, 398). Uninoculated culture media should be incubated for a long period to ensure the sterility of the media used; frequent sterility checks are needed.
Sensitivity testing of fungi isolated from ophthalmic lesions.
The clinical relevance of antifungal susceptibility testing is thought to lie in guiding the clinician in the selection of an appropriate antifungal compound. Such tests have been reported to help in the selection of the appropriate antifungal in different ophthalmic mycoses (161, 173, 233, 234). Unfortunately, many of these reports have not provided details of the test procedures used, the criteria by which MICs were deemed significant, details of the severity of the clinical lesions, or the criteria used for authentic diagnosis of mycotic infection. The use of reproducible tests conforming to rigorous standards, such as the approved document (M27A) of the National Committee for Clinical Laboratory Standards (NCCLS) for sensitivity testing of yeasts (261), and a standard method for susceptibility testing of filamentous fungi, especially Aspergillus spp., may clarify in the future whether antifungal susceptibility testing is at all useful in guiding the therapy of ophthalmic mycoses. Interestingly, when the in vitro antifungal susceptibilities of nine isolates of filamentous fungi were determined by the NCCLS method in 11 different laboratories and compared to antifungal treatment outcomes in animal infection models, only a limited association between MIC and treatment outcome was seen, due to drawbacks in the models used (278). Curvularia senegalensis was isolated from a patient with mycotic keratitis, and the MIC of itraconazole for this isolate was found (by a broth microdilution method performed as described by NCCLS guidelines for filamentous fungi) to be 0.25 μg/ml; however, the patient did not respond to antifungal therapy with natamycin or itraconazole (130). Above all, the relationship between in vitro susceptibility data and clinical response to topical antifungal medication needs to be clarified; hitherto, no studies have been performed in this important area.
PCR.
Since the revolutionary molecular biology technique of PCR involves enzymatic amplification of even minute quantities of a specific sequence of DNA (Table 8), it is of great benefit in rapidly detecting the presence of organisms which are difficult to culture. Ocular samples which can be submitted for PCR include intraocular fluid (aqueous or vitreous), tears, any fresh ocular tissue, formalin-fixed or paraffin-embedded tissue, and even stained or unstained cytology slides or tissue sections from which DNA can be extracted. Minute samples (1 to 10 μl) of aqueous, vitreous, or tear fluids generally suffice (311). Table 8 summarizes the salient observations of studies employing PCR in the diagnosis of ophthalmic mycoses. The results of all these studies suggest that PCR is more sensitive than culture as a diagnostic aid in ophthalmic mycoses. However, concern persists regarding the specificity of this technique and the problems that may arise from the production of false-positive results. In most of these studies, insufficient detail has been provided to permit an independent assessment of the adequacy of the techniques used for culture. In the diagnosis of ophthalmic mycoses, PCR would probably be most valuable in providing a positive result in a shorter period than that required for culture (91, 92) and in identification of a fungal isolate which does not sporulate (22). Although PCR is more advantageous than the estimation of antibodies in serum or ocular fluids because of its extreme sensitivity and specificity, it cannot be used (unlike serological tests, for which serial antibody titers can be studied) to monitor the patient's response to treatment. PCR does not distinguish viable from nonviable organisms; it may therefore be difficult to assess the relevance of a positive PCR result in a healing corneal ulcer, where culture is negative (7), or in locations such as the conjunctival sac, where fungi may be found as transient commensals (112). A few culture media will suffice to detect and grow the common ocular pathogens, but PCR must be multiplexed for each microorganism that is suspected; the use of panfungal primers may alleviate this problem. Finally, PCR can detect only fungi for which the DNA sequence is known and primers are available; it also does not provide details of cellular morphology or localization (311).
TABLE 8.
Use of PCR for diagnosis of ophthalmic mycosesa
| Lesion and patients (reference) | Criteria for diagnosis of fungal infection | Correlation of PCR and fungal culture results | Response to antifungals | Characteristics of PCR
assay
|
Comments | |
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | |||||
| Suspected Candida endophthalmitis in 4 patients (147) | Suggestive clinical symptoms, signs,b and risk factors of Candida endophthalmitis | Both PCR and culture positive in 2 patients (C. albicans in culture) PCR positive, culture negative in 2 patients | Yes Yes | 100 cells of C. albicans | Primers did not amplify DNA of other Candida spp., other fungi, or bacteria | PCR assay and culture on vitreous and aqueous; too few samples tested to draw conclusions. |
| Suspected Candida endophthalmitis in 4 patients (281) | Suggestive clinical symptoms, signs,b and risk factors of Candida endophthalmitis | PCR and culture positive in 2 patients, PCR positive, culture negative in 1 patient PCR and culture negative in 1 patient (responded to antibacterials) | Yes (all three) Not given | 10 fg of C. albicans DNA (1 copy of gene) | Primers did not amplify DNA of other Candida spp., other fungi, bacteria, or white cells | Too few samples tested to draw conclusions. |
| Panophthalmitis in a 52-yr-old male with acute myeloid leukemia and disseminated fusariosis (6) | F. solani isolated from blood, and skin nodule before death Filamentous fungi in postmortem tissue sections and samples (no culture) | Tissue sections and samples of normal eye: PCR negative Tissue sections and samples (retina, choroid, sclera, and cornea) collected postmortem from affected eye: PCR positive (no culture) | Study done on postmortem samples | Details not provided Primers targeted Fusarium cutinase gene | Primers did not amplify DNA of other fungi, viruses, or bacteria | Fusarium-like fungi seen by microscopy in antemortem and postmortem ocular samples; no immunofluorescence or culture tests; hence, diagnosis of Fusarium infection only presumptive. |
| Suspected Candida endophthalmitis in 3 patients (165) | Suggestive risk factors and clinical featuresb of fungal endophthalmitis | PCR and culture positive in 1 patient (C. albicans in culture); PCR positive, culture negative in 1 patient PCR and culture negative in 1 patient (responded to antibacterials); PCR primers targeted 18S ribosome | Yes (both) Not given | 50 pg of C. albicans DNA (1-2 C. albicans cells); 100 fg of A. fumigatus and F. solani DNA | Primers did not amplify human or bacterial DNA; species specificity of primers confirmed by sequencing | Primers developed for A. fumigatus and F. solani DNA not tested in samples from patients with clinical diseases. Sample size too small to draw conclusions. |
| Experimental Fusarium keratitis in rabbits (3 test eyes, 1 control eye) (7) | Culture positivity assumed in all eyes at all times since experimental inoculation done (“gold standard”) | In test corneas, 25 (89%) of 28 samples were PCR positive; 3 (21%) of 14 samples were culture positive In control corneas, 7 (88%) of 8 samples were PCR negative; 100% of samples were culture negative; in relation to “gold standard”, PCR sensitivity was 89% and PCR specificity was 88% | 10-10,000 Fusarium conidia; primers targeted Fusarium cutinase gene | Primers did not amplify human, bacterial, or other DNA; they amplified F. oxysporum DNA | Very low sensitivity of culture techniques (possibly inadequate). Some test eyes at 4 wk postinoculation were still PCR positive, although ulcers were almost healed and were culture negative. Relevance of positive PCR results in healed or healing ulcers not addressed. | |
| Presumed microbial keratitis in 30 patients (16 fungus culture positive)c (112) | Growth on ≥2 media; microscopy positive and growth on 1 medium (growth consistent with KOH mounts, Gram, Giemsa, and Calcofluor white smear results) | PCR and culture positive in 15 patients; PCR positive, culture negative in 7 patients (2 smear positive); PCR negative, culture positive in 1 patient; PCR and culture negative in 7 patients (1 smear positive) Taking culture as “gold standard,” PCR sensitivity was 94% and PCR specificity was 50% | Details not provided | 38 C. albicans cells, 10 F. solani cells, 5 A. fumigatus cells (PCR primers targeted 18S ribosome) | Filamentous fungi differentiated from yeasts and bacteria without further taxonomic specificity | PCR was highly sensitive but of low specificity compared to culture. Fungal DNA detected in the conjunctival swabs of healthy fellow eyes in 5 of 30 patients; suggests low specificity of PCR. Relevance of positive PCR results in healthy eyes not addressed. |
| Presumed endophthalmitis and keratitis (nonherpetic) in 11 samples from 10 patients (3 corneal scrapes, 6 aqueous taps, 2 vitreous taps) (91, 92) | Growth of fungi in culture (from 5 samples): C. parapsilosis from 2 samples, and A. fumigatus, A. niger, and Alternaria alternata from 1 sample each (criteria for significance of these isolates?) | PCR, culture and microscopy, positive in 1 corneal scrape; PCR and culture positive, microscopy negative in 2 corneal scrapes and 1 vitreous tap; PCR and culture positive in 1 aqueous tap | Details not provided | 1 fg of C. albicans (1 C. albicans cell) 10 fg of A. fumigatus (1-10 microorganisms) | Negative controls (human and bacterial DNA) did not amplify by PCR | PCR results essentially duplicated fungus culture results, but in a shorter time. Not clear how significance was assigned to the fungal isolates (only 1 sample was positive for fungi by microscopy). |
PCR tests were developed by investigators in-house and are not commercially available.
Suggestive clinical signs of Candida endophthalmitis included fluffy vitreous infiltrate, white retinal lesions, and yellow-white choroidal lesions.
16 fungus culture positive (Fusarium spp. in 5, Aspergillus spp. in 2, not identified in 9).
PATHOGENESIS
Ocular fungal infections probably occur due to an interaction between various agent (fungus), host (tissue and immunological mechanisms), and other factors. Since such factors have been fairly extensively studied in mycotic keratitis, they are reviewed here. The virulence factors of A. fumigatus (207) and zygomycetes (323) in human disease have been extensively described elsewhere.
Putative Agent Factors in the Pathogenesis of Mycotic Keratitis
The ideal test to identify a virulence factor is to compare the infectivity of the fungus in the absence and the presence of the factor, by using naturally occurring mutants or those obtained by UV or chemical means; however, such methods may result in the mutant strains being deficient in more than just one factor (207). Molecular biological techniques can overcome such problems by detecting the gene encoding for the presumed virulence factor being studied; such techniques have not been applied to a great extent to study fungal pathogens in the setting of ocular disease. Therefore, the putative virulence mechanisms reviewed here require confirmation in the future.
The key agent factors thought to be involved in pathogenesis of mycotic infections include adherence, invasiveness, morphogenesis, and toxigenicity (Table 9). There is a paucity of data relating to the role of fungal adhesins in pathogenesis of mycotic keratitis.
TABLE 9.
Putative agent factors contributing to pathogenesis of mycotic keratitis
| Factor | Observations made | Significance | Comment |
|---|---|---|---|
| Adhesins | Outer fibrillar layer of yeast and filamentous fungal cell wall composed of mannan or mannoprotein (151) | Fungal adhesins may be important in pathogenesis of mycotic keratitis since corneal tissue possesses potential binding sites (laminin, fibronectin, collagen) | Exact relevance in pathogenesis of mycotic keratitis still undefined and unexplained |
| Carbohydrate and protein molecules on conidial surface of A. fumigatus bind to host proteins in specific and saturable manner (39,207) | |||
| Invasiveness in infected corneal tissue | Certain strains of Fusarium spp. invade entire corneal thickness and enter anterior chamber, forming lens-iris-fungal mass at pupillary area; tissue invasion demonstrated histopathologically and by isolation of fungi from various ocular tissues (173,204) | Normal drainage of aqueous humor affected, raised intraocular pressure results, causing fungal malignant glaucoma (keratomycotic malignant glaucoma) | Further studies needed to clarify if this occurs only in Fusarium keratitis and to find factors determining which fungal isolates invade the anterior chamber |
| Fungal load and extent of tissue invasion in corneal buttons of patients undergoing penetrating keratoplasty found inversely related to intensity of inflammatory response (i.e., the heavier the fungal load and extent of tissue invasion, the less intense the inflammatory response) (406) | In early mycotic keratitis, a heavy fungal load and extensive tissue invasion may overwhelm the inflammatory response in corneal tissue or the inflammatory response may be mild at this stage; fungi multiply and invade tissue, and lesions progress | This putative sequence of events needs confirmation in experimental studies, documenting the histological changes early and late in the disease | |
| Fungal morphogenesis in infected corneal tissue | “Intrahyphal hyphae” and thickened fungal cell walls detected by electron microscopy of corneal tissue in L. theobromae keratitis (392) and in corneas of dexamethasone-treated rabbits with experimental F. solani keratitis (190); when the same strain of L. theobromae is grown in presence of antifungal, the same morphological changes are seen (393) | Fungal morphogenesis permits opportunistic fungi invading corneal tissue to survive in a restrictive unnatural environment and possibly to resist antifungal therapy (392) | Intrahyphal hypae still considered to occur only in vitro; significance in infected ocular tissue requires elucidation |
| Secretion of toxins and enzymes in infected corneal tissue | Fusarium isolates from mycotic keratitis (all resistant to antifungals) all had the same C29 and C31 sterol content; 72% produced nivalinol, and 50% produced T-2 toxin in vitro (316) Fusaric acid contributes to vascular wilt in Fusarium-infected plants (383) | Neither sterol content nor toxin production appeared related to severity or outcome of keratitis in 18 patients from whom the fungi were isolated No corneal lesions produced after intracorneal inoculation of 1,000 μg of fusaric acid in rabbits | Demonstration of fungal toxins in human corneal tissue infected by Fusarium spp. needed to confirm possible contribution to pathogenesis |
| F. solani from keratitis patients in Colombia produced UV light-absorbing extracellular substance (71) | This substance elicited an erythematous reaction in a rabbit eye after topical instillation | ||
| A. flavus isolated from a patient with severe keratitis secreted metalloproteinase only, or a mixture of proteinases, when grown in vitro (438) | These proteinases thought to have contributed to the patient's severe keratitis. | Fungal proteinase should be detected in human corneal tissue infected by fungi to confirm possible contribution to pathogenesis of mycotic keratitis | |
| Ocular isolates of A. flavus and F. solani when grown in vitro, found to secrete predominantly serine proteinase activity, with little metalloproteinase activity; corneal homogenate of rabbit eyes inoculated with the same fungal strains revealed predominantly metalloproteinase activity and little serine proteinase activity (119) | Although these fungi can secrete proteinases in vitro, they may secrete negligible amounts in infected corneal tissue; the proteolytic activity in such infected corneal tissue may arise from host cells or infiltrating polymorphonuclear leukocytes. |
Invasiveness.
Fungi causing keratitis, in particular Fusarium spp., sometimes invade the anterior chamber and form a lens-iris-fungus mass at the pupillary area, thereby interfering with the normal drainage of the aqueous humor and leading to a rise in the intraocular pressure (173, 204). At present, little is known about what determines the occurrence of this condition, how frequently it complicates the course of mycotic keratitis, and whether such a complication is unique to keratitis due to Fusarium spp. A recent study has sought to answer some of these questions by performing a histologic evaluation of corneal buttons obtained from patients with mycotic keratitis who underwent penetrating keratoplasty (406); the fungi involved were principally Fusarium spp. and Aspergillus spp. In corneal buttons exhibiting fungal hyphae, an inverse correlation was noted between the quantum and distribution of these hyphae and the degree and distribution of inflammatory cells; that is, the larger the number of hyphae seen, the smaller the number of inflammatory cells seen. Corneal buttons from patients on whom keratoplasty had been performed relatively early in the course of the disease tended to exhibit many fungal hyphae, along with marked penetration into the depth of the corneal tissue and a relatively minimal inflammatory cell response; when keratoplasty had been performed several weeks after diagnosis, there were relatively fewer hyphae and a more marked inflammatory cell infiltration. These authors speculated that in the early stages of mycotic keratitis, both agent factors (heavy fungal load with deep penetration) and host factors (insufficient inflammatory response) influenced the progression of the disease. Again, the question of which factors influence these responses remains unanswered. Studies with an experimental animal model, with samples collected at frequent intervals, may provide valuable data.
Morphogenesis and phenotypic switching permit fungi to adapt to live in different microenvironments and to survive in the infected host (341). The presence of “intrahyphal hyphae” or “hypha-in-hypha,” and thickened fungal cell walls (Table 9) may reflect such morphogenesis occurring in fungi invading corneal tissue; these morphological alterations may constitute a barrier against antifungal drugs or host defenses (392, 393) or may be a virulence factor for fungi in corneas where the defense mechanisms have been compromised by the application of corticosteroids (190). Rigorous experimental and other studies are required to elucidate these aspects. Interestingly, in the study referred to earlier (406), there was no mention of the occurrence of such morphological changes in the fungi seen in corneal tissue.
Toxigenicity.
Fusarium spp. are known to cause myelosuppression through toxin production (263), but little is known about whether Fusarium toxins such as nivalenol, T-2 toxin, deoxynivalenol, diacetoxyscirpenol and fusaric acid contribute to the pathogenesis of mycotic keratitis (Table 9). The results of two studies (316, 383) suggest that these factors do not make any such contribution, but further investigation is required.
Some other studies have examined the possible role of fungal proteinases in the pathogenesis of mycotic keratitis (Table 9). Clearly, isolates of F. solani and A. flavus from patients with keratitis possess the ability to secrete proteinases (71, 119, 438). What is not clear, however, is whether these fungi actually secrete these proteinases when infecting corneal tissue and whether such proteinases appreciably influence the outcome of such infections. A recent study attempted to correlate the presence of fungal proteinases in vitro and in an experimental animal system (119). When corneal isolates of A. flavus and F. solani were grown in vitro, the fungal cultures were found to contain predominantly serine proteinase activity, and, to a lesser extent, metalloproteinase activity. However, homogenates of rabbit corneas that had been infected with the same strains of A. flavus and F. solani exhibited metalloproteinase activity alone, and no serine proteinase activity; this suggests that although the fungal strains could secrete proteinases in vitro, they did not do so while infecting corneal tissue. None of the available evidence conclusively establishes or refutes the contribution of fungal proteinases to the pathogenesis of mycotic keratitis. This requires the demonstration of fungal toxins and enzymes in situ in fungus-infected tissues (320) in humans. Similarly, the disease produced in experimental animals by fungal strains secreting a particular proteinase or toxin should be more severe than that produced by a mutant not secreting these products. With the rapid strides made in molecular biological techniques, it should be possible, in the coming years, to investigate these aspects.
Putative Host Factors in the Pathogenesis of Mycotic Keratitis
Defects in local ocular defense mechanisms, such as epithelial or stromal ulceration due to antecedent herpes simplex keratitis or contact lens-associated corneal abrasions (334, 377, 423), as well as lid notches, lagophthalmos (seen frequently in patients with leprosy), impaired tear secretion, defective secretion of immunoglobulin A in tears, and defective positioning of the lids and mechanisms of lid closure (174, 271) are risk factors for mycotic keratitis, especially that caused by yeasts and less virulent filamentous fungi. Systemic diseases, such as diabetes mellitus, and conditions of general immunosuppression may also be contributory factors. Spontaneous fungal corneal ulceration has been reported in a patient with AIDS (291).
A transient commensal fungal flora is present in a variable percentage of healthy eyes (363). Fungal conidia from the environment which colonize the conjunctival sac as innocuous commensals possibly turn pathogenic after ocular trauma or corticosteroid use, after which they invade corneal tissue through minute breaks in the corneal epithelium (268). This hypothesis needs to be tested in a suitable experimental model.
In some cases of mycotic keratitis which are responding well to antifungal therapy, a sudden deterioration accompanied by renewed tissue destruction (in the absence of a demonstrable microbial cause) has been noted; this phenomenon is thought to occur because dying fungal hyphae may elicit a type of hypersensitivity reaction (100). This hypothesis also needs testing in a suitable experimental model; if substantiated, it may result in modifications to conventional therapeutic protocols for mycotic keratitis.
Polymorphonuclear leukocytes are known to be pivotal in preventing fungal infections since they phagocytize and subsequently destroy fungal structures by oxygen-dependent mechanisms; the presence of disease or the use of corticosteroids, tetracycline, doxycycline, or certain other drugs may interfere with these mechanisms and hence lower the host resistance to fungal infection (366). Polymorphonuclear leukocytes, other acute inflammatory cells, the corneal epithelium, and keratocytes appear to also play a key role in sterile corneal ulceration (184); however, their role in stromal matrix degradation is not clear. When amidated glucose oxidase was inoculated into rabbit corneas, an initial corneal opacification and a later corneal melting were observed; the initial lesions were thought to arise due to the effects of hydroxyl radicals derived from hydrogen peroxide-generated glucose oxidase, with the later lesions occurring after the release of collagenases and lysosomal hydrolases from invading phagocytic cells (53). In another study, the basal proteolytic activity (65 kDa) detected in uninfected rabbit corneas was shown to reside in matrix metalloproteinase 2 (MMP-2) (119). When rabbit corneas were experimentally infected with A. flavus or F. solani, additional proteolytic activity (92 and 200 kDa) was detected, with the 92-kDa activity being identified as MMP-9. The expression of 92- and 200-kDa gelatinases correlated positively with the number of polymorphonuclear leukocytes in infected corneas. These authors contended that activated corneal cells or inflammatory cells (polymorphonuclear leukocytes) were responsible for the increased proteolytic activities seen in fungus-infected corneas.
Lesions simulating keratitis were produced in rabbit eyes by applying lipid mediators, such as prostaglandins, leukotriene, and platelet-activating factor (395). The urokinase-plasminogen activator system plays an important role in the regulation of collagen synthesis, secretion, and activation during wound remodeling and stromal ulceration (30). MMP-2 and MMP-9, derived from corneal stromal keratocytes, have also been shown to contribute to the degradation of corneal stroma and epithelial basement membrane, respectively (94). It is not known to what extent these various factors contribute to the progression of stromal ulceration in a case of mycotic keratitis, but they certainly need to be considered when dealing with a patient whose keratitis is refractory to antifungal therapy alone.
There is compelling experimental (276) and clinical (366, 394, 428) evidence to suggest that the administration of corticosteroids may predispose humans to mycotic keratitis. This may occur because corticosteroids suppress ocular immune mechanisms by inhibiting chemotaxis and ingestion by phagocytes, by blocking degranulation, and by reducing the production of phagocytes (366). They may also cause changes in the infecting fungal strain itself, the reasons for which are not clear (394).
Traditional eye remedies are routinely used for the “therapy” of eye ailments in many agricultural communities in the developing world. In India, traditional remedies described include extracts of green leaves, the juice of the banyan tree, coconut and castor oil, goat and human breast milk, and chicken blood (120, 388). Fungi contaminating such concoctions could conceivably be carried into the deeper corneal layers when applied to a traumatized cornea. The use of certain oils may be associated with excessive corneal irritation, thus predisposing to mycotic keratitis. Experimental studies may help to clarify the validity of such hypotheses.
ANTIFUNGAL AGENTS USED TO TREAT OPHTHALMIC MYCOSES
In treating ophthalmic mycoses, the ultimate aim is to preserve vision, and this depends on rapid diagnosis and efficient administration of appropriate antifungal therapy (227). There are three main chemical groups of drugs with antifungal activity for use in therapy of ophthalmic mycoses, namely, the polyenes, azoles (imidazoles and triazoles), and flucytosine (5-fluorocytosine). The clinical use of additional classes of antifungals, such as the allylamines and candins, is not widespread. In this section, the salient characteristics of antifungal agents currently used for therapy of ophthalmic mycoses are highlighted (Table 10); the efficacies of these antifungals in specific ophthalmic mycoses are discussed later (see “Clinical features, predisposing factors, and management of specific ophthalmic mycoses”). Antifungals such as clotrimazole, econazole, flucytosine, and nystatin were widely used in the 1970s and early 1980s for treatment of ophthalmic mycoses. Unfortunately, the data pertaining to these drugs were derived from case reports or uncontrolled studies. Moreover, these drugs have generally poor pharmacokinetics or poor therapeutic profiles in the eye or are obsolete. Hence, they are not included in this review. While the reported in vitro spectrum of antifungal activity is mentioned in certain instances, it should not be taken to imply that there is necessarily a correlation between in vitro antifungal susceptibility data and efficacy in the clinical setting of ophthalmic mycoses.
TABLE 10.
Antifungal drugs currently used to treat ophthalmic mycoses
| Drug | Features and advantages | Drawbacks |
|---|---|---|
| Natamycin (pimaricin) | Polyene; mol mass, 665.75 Da (28) Commercially available as topical 5% suspension for ophthalmic use in some countries, where it constitutes the first-line therapy for mycotic keratitis Ophthalmic preparation is well tolerated and stable and can be sterilized by heat (269) Relatively high levels reportedly achieved in cornea after topical application (274) | Not commercially available as an ophthalmic preparation in many regions Effective only when applied topically May not be effective when keratitis is associated with deep stromal lesions (287) Only about 2% of the total drug in corneal tissue is bioavailable (269) |
| Amphotericin B | Macrocyclic polyene; mol mass, 924.10 Da (28) Good in vitro activity against Aspergillus spp. and Candida spp.; emergence of resistant mutants rare Can be administered by topical (0.15-0.30% solution), intracameral (7.5-30 μg/0.1 ml), intravenous (0.5-1 mg/kg/day), or intravitreal (1-5 μg/0.1 ml) routes in ophthalmic mycoses (15, 64, 183, 334, 377, 416, 429,435) Collagen shields soaked in 0.5% amphotericin B have been found useful in experimental mycotic keratitis (347) Penetrates deep corneal stroma after topical application; bioavailability sufficient for susceptible fungi (269) Exerts direct fungicidal effect and exhibits immunoadjuvant properties (432) | Intravenous administration frequently associated with renal tubular damage, due to use of deoxycholate as vehicle (187) Subconjunctival injection causes marked tissue necrosis at the site of injection (269) Topical application of concn >5.0 mg/ml may cause ocular irritation (solutions of 1.5-3.0 mg/ml are better tolerated) Not commercially available as topical ophthalmic preparation; needs to be reconstituted from powder or intravenous preparation Poor intraocular penetration after intravenous administration |
| Miconazole | Synthetic phenylethyl imidazole; mol mass, 416.12 Da (28) Reported routes of administration in mycotic keratitis: topical (1%), subconjunctival (10 mg/0.5 ml), intravenous (600-1,200 mg/day); topical and subconjunctival administration generally well tolerated (16, 96, 101, 161,264) Found to penetrate rabbit corneas after subconjunctival and topical administration; high concentrations in tissue achieved (103) | Use of intravenous preparation occasionally associated with toxicity due to the vehicle used (16) Undetectable concn of drug in rabbit corneas and vitreous after intravenous administration (103) Anomalous effects after subconjunctival administration in experimental C. albicans keratitis (275) Generally considered useful in S. apiospermum ocular infections, but treatment failures have occurred (34,430) |
| Ketoconazole | Substituted imidazole; mol mass, 531.44 Da (28) Given by oral (200-400 mg/day) or topical (1-2% suspension) routes in ophthalmic mycoses (111,309, 396)) Well absorbed with good tissue distribution after oral administration (267); peak concn in serum of 2-3 μg/ml 2-3 h after a 200-mg oral dose (223) Concn of 44.0 ± 10.1 μg/g in undebrided rabbit corneas and 1,391.5 ± 130.0 μg/g in debrided rabbit corneas after topical or subconjunctival application of 1% solution (145) | Oral doses of >400 mg/day may cause transient rise in concn of transaminases in serum (157) Acidic pH required for absorption Prolonged administration of high doses may cause impotence, gynecomastia or alopecia (267), or papilledema (282) No commercially available solution of ketoconazole for topical or subconjunctival administration in ophthalmic mycoses |
| Itraconazole | Synthetic dioxolane triazole; mol mass, 705.64 Da (28) Given by oral (200-400 mg/day) or topical (1% suspension) routes in ophthalmic mycoses (233, 234,390) Oral solution and intravenous formulation recently developed (139); no reports of use in ophthalmic mycoses Peak concn of 0.3 μg/ml in serum after single oral dose of 200 mg (401); increased to 3.5 μg/ml after 200 mg/day orally for 14 days (51) Concn of 200-250 μg/g attained in rabbit corneas after topical application of itraconazole in balanced salt solution polyvinyl alcohol, boric acid, or olive oil (136) Detectable concn found to persist in rabbit corneas after subconjunctival administration (193) | Commercially available capsule (100 mg) should be taken with meal; difficult to give in infants and children (124) May be poorly absorbed after oral administration in certain groups of patients (139); caution needed when given to patients with previous hepatic disease Absorption after oral dosing affected by antacids and H2 receptor antagonists; may interact with other drugs Poor penetration into rabbit ocular tissue compared to fluconazole and ketoconazole, after oral dosing (342) Intravitreal injection (>10 μg) causes focal retinal necrosis in rabbits (344) No commercially available solution for topical or subconjunctival administration. |
| Fluconazole | Synthetic bistriazole; mol mass, 306.3 Da (28) Soluble in water, hence excreted via kidneys; 10-20% protein bound in serum; long half-life (28,342) Given by oral (50-100 mg/day), topical (0.2-2% solution), or intravenous routes in ophthalmic mycoses (2, 72, 219, 222,315, 380) High bioavailability, low toxicity, good stability Biodegradable polymeric scleral implant containing fluconazole is promising for intravitreal delivery (249) Commercially available for oral and intravenous use | May interact with cisapride, oral antidiabetic drugs, and phenytoin after oral administration. Less active against C. glabrata and C. krusei than against C. albicans (267) May not be effective in treatment of filamentous fungal keratitis (315) |
General Considerations
The clinical efficacy of an antifungal agent in an ophthalmic mycosis depends to a great extent on the concentration achieved in the target ocular tissue. This, in turn, depends on a number of factors including the molecular mass and concentration of the drug and the route by which it has been administered, the duration of contact with the target ocular tissue, and the ability of the compound to penetrate the eye (28, 227). Compounds with a molecular mass exceeding 500 Da, such as amphotericin B (924.10 Da), natamycin (665.75 Da), or ketoconazole (531.44 Da) barely penetrate an intact corneal epithelium because the force of friction increasingly reduces diffusion (227). Solubility in lipid-rich tissue is another determinant of diffusion. The ocular penetration of molecules with intermediate molecular masses, such as miconazole (416.12 Da) or fluconazole (306.30 Da) is probably determined by both factors. Lipophilic compounds, such as itraconazole, easily cross the lipid-rich epithelial and endothelial cell membranes and the blood-aqueous barrier; hydrophilic compounds more easily cross the corneal stroma; and biphasic compounds (which possess both lipid and water solubility) penetrate all corneal layers (107). The exact relevance of these considerations in the use of topical antifungals for therapy of mycotic keratitis, where the integrity of the corneal epithelium and superficial stroma is usually breached by the disease process itself, needs to be addressed.
The concentration of the drug applied to the eye may be increased by the preparation of fortified eye drops (107), but this is not generally done for antifungals. Frequent topical application of drops is a useful means of achieving therapeutic levels in the eye, but this is laborious and may cause irritation. Ointments and subconjunctival injections may prolong the contact time between the antifungal and the corneal and conjunctival tissue. Only amphotericin B and miconazole are available as ophthalmic ointments. Subconjunctival injections can be painful for the patient and inconvenient for the physician (107) and can cause damage to the ocular tissue at the site of injection (236).
Collagen shields, iontophoresis, and pumps have all been used in an attempt to enhance drug delivery to the eye. The use of iontophoresis and pumps has not gained acceptance, and these techniques should now probably be considered obsolete. However, the collagen shield, which is shaped like a contact lens and is packaged in a dehydrated form and rehydrated before use, has been used to promote corneal epithelial healing and deliver drugs. The source of the collagen may be porcine sclera or bovine corium (107). The collagen shield has been found useful to deliver drugs to the eye since therapeutic levels of medication are delivered reliably with a minimum number of applications. Drug delivery depends on absorption and subsequent release of the medication by the shield. When a solution containing a water-soluble drug is used for rehydration, the drug becomes trapped in the interstices of the collagen matrix; the drug is released as the shield dissolves (107). Shields soaked in water-soluble drugs have been found to produce corneal and aqueous levels comparable to those obtained with frequent topical therapy. The prolonged exposure time of medication to the cornea provided by a presoaked shield may produce higher levels in tissue than a single drop that is rapidly carried away by the tears (107). Currently, the only antifungal to be used in collagen shields is amphotericin B (267, 299, 347). Clearly, the potential of this technique for delivery of antifungals to the eye should be explored further.
Polyenes
Polyenes continue to be an important component of the ocular antifungal armamentarium. These bond directly to ergosterol, a sterol unique to fungal cytoplasmic membranes; the integrity of these membranes is disrupted, resulting in leakage of essential intracellular constituents (267). The extent of damage to fungal membranes is dose related; however, it is not possible to increase the drug dosage beyond a certain concentration, since the cytoplasmic membrane of human cells may then be affected (toxicity of polyenes). Natamycin (pimaricin) and amphotericin B are the polyenes in current use for treatment of ophthalmic mycoses.
Natamycin.
Natamycin was the first antifungal specifically developed for topical ophthalmic use (Table 10) and is currently the only topical ophthalmic antifungal compound approved by the Food and Drug Administration of the United States (267). It is reported to have a broad spectrum of activity against various fungi, including species of Fusarium, Aspergillus, Acremonium, Penicillium, Lasiodiplodia, and Candida (236, 267, 271), but the validity of the methods used to derive these data, as well as the relevance of these data to the clinical use of natamycin, which is given only topically, is a contentious issue.
Natamycin is poorly soluble in water. It is stable in a 5% suspension and, in this form, adheres well to the cornea for clinically useful periods (236). The 5% topical ophthalmic suspension, although viscous, is well tolerated and causes no pain or secondary corneal damage (236). Punctate keratitis is sometimes encountered (170). It was initially thought that natamycin penetrated the cornea and conjunctiva poorly after topical application, that effective drug levels were not achieved in either the cornea or aqueous, and that it was therefore useful only in the treatment of superficial mycotic keratitis (236). However, radiolabeling studies suggest that it actually penetrates the cornea well after topical application (274). Thirteen topical applications every 5 min resulted in a drug concentration of approximately 2.5 mg/g cornea in rabbit corneas debrided of epithelium; levels peaked at approximately 10 min after administration. Far lower levels (7.0 μg/g) were attained in corneas where the epithelium was left intact (274). It is unclear whether these levels are actually achieved during therapy of clinical mycotic keratitis.
Natamycin is the drug of choice for therapy of mycotic keratitis in many countries (235, 288, 328, 334), particularly for keratitis due to filamentous fungi. It has also been used in association with other treatment modalities for therapy of mycotic scleritis (370), conjunctivitis, and endophthalmitis (267); controlled clinical trials are needed to confirm the efficacy of natamycin for these indications.
Amphotericin B.
Amphotericin B (Table 10) is variably fungistatic and occasionally fungicidal, depending on the concentration achieved in serum (187) and the susceptibility of the pathogens; maximum activity is seen at a pH range from 6.0 to 7.5. Amphotericin B has been administered by the intravenous, topical, intravitreal, and intracameral routes for therapy of ophthalmic mycoses (236, 267).
For intravenous infusion of amphotericin B, a solution of 0.1 mg/ml in a 5% solution of dextrose is used (saline cannot be used since the drug may precipitate out). Unused solutions should be discarded after 24 h. Amphotericin B is both heat labile and light sensitive; hence, the dry powder should be refrigerated and protected from light (236). The recommended dosage is usually 1 mg/kg of body weight/day; smaller doses may be relatively ineffective (236). However, since tolerance to amphotericin B varies greatly among patients, the dosage must be individually adjusted; the safest approach is to initially give low test doses and to gradually increase the dose (236). Treatment needs to be given only once daily, or on alternate days once clinical improvement is noted; alternate-day therapy is advised for at least 2 months for many infections, with administration of a total dose of at least 3.0 g of amphotericin B (236). Renal toxicity is estimated to occur in almost 80% of patients receiving intravenous amphotericin B (187); this should be zealously guarded against by frequent monitoring of the blood urea nitrogen and other tests of kidney function. Headaches, chills, fever, and anorexia are common with systemic use; other adverse side- effects include moderate anaemia, nausea, vomiting, gastrointestinal cramps and diarrhea, and local thrombophlebitis at the infusion site (236). In view of these toxic effects, treatment should be reserved for patients in whom a diagnosis of mycotic infection is reasonably well substantiated; patients receiving systemic amphotericin B for ophthalmic reasons should be comanaged with an internist, who will monitor the patient for toxicity (236).
Intravenous amphotericin B continues to be the treatment of choice for invasive fungal infections of the orbit (164, 213, 323, 349); it has also been used in the treatment of endophthalmitis due to dimorphic fungi (205, 215, 224, 253, 338, 357) and lesions of the eyelids, conjunctiva, and cornea caused by P. brasiliensis (353). The efficacy of intravenous amphotericin B in endophthalmitis due to dimorphic fungi is difficult to evaluate since, in many of the reports cited, posttreatment cultures were negative but the affected eyeball had to be enucleated due to other complications.
Lipid formulations of amphotericin B have been evaluated because of the renal and systemic toxicity of conventional amphotericin B, especially when high doses are required, as in the treatment of zygomycosis (416). These formulations include amphotericin B-lipid complex, which consists of amphotericin B complexed with two phospholipids, dimyristoylphosphatidylcholine and dimyristoylphosphatidylglycerol (340, 368); and amphotericin B colloidal dispersion, which combines cholesteryl sulfate and amphotericin B in a 1:1 molar ratio, forming a novel lipid delivery system in a disk-like array (diameters range from 120 to 140 nm), which is dispensed in a lyophilized form (257). Local nebulized amphotericin B (308) is reported to be a useful adjunct to conventional therapy in rhinocerebral zygomycosis. However, controlled trials are needed to assess the efficacy of these lipid formulations of amphotericin B and of conventional amphotericin B administered by these different routes in the therapy of ophthalmic mycoses.
For topical administration, a solution (0.15 to 0.3%) may be freshly prepared with sterile water (amphotericin B precipitates in saline); the preparation must be refrigerated in a dark bottle to reduce the speed of disintegration (236). Drops may be instilled every 30 to 60 min. The corneal penetration of amphotericin B is reduced in the presence of an intact corneal epithelium (273, 274). One persistent concern in the topical application of amphotericin B is the problem of possible corneal toxicity (102). Fortunately, the 0.15% solution of amphotericin B in sterile water used in clinical practice appears to be well tolerated (377, 429). Topical application of 0.5% ointment may cause some conjunctival irritation (236), although a 2% ointment was reported to be well tolerated in therapy of mycotic keratitis (148). Subconjunctival injection has been reported to lead to severe toxic effects and is no longer recommended. Amphotericin B in solution or as an ointment has been used topically to treat conjunctivitis, scleritis, and keratitis (31, 148, 334, 377, 429); it is the treatment of choice for keratitis due to Candida spp. (see below).
Delivery of amphotericin B by a collagen shield may improve compliance and ensures a more constant rate of drug delivery in mycotic keratitis (236). In one study, collagen shields soaked in amphotericin B were found to achieve corneal amphotericin B levels comparable to those achieved by hourly topical administration of drops (347). In another study, collagen shields presoaked with 0.5% amphotericin B and applied for 1 h/day were found to be as effective as topical applications of 0.15% amphotericin B every hour for 8 h/day in reducing fungal colony counts in experimental C. albicans keratitis (299). Peak levels with collagen shield delivery were found to occur at 1 h and then to fall to achieve a steady state between 3 to 6 h; however, even at 6 h, corneal amphotericin B levels obtained with the collagen shield were still within the therapeutic range (347). These observations require validation in controlled clinical studies. The use of collagen shields may make it difficult for the clinician to perform frequent clinical examination of the affected eye; improper use may also lead to increased toxicity (347).
Intravitreal injections of amphotericin B (in amounts of 1 to 5 μg) have been recommended for the treatment of mycotic endophthalmitis. This mode of administration can be highly destructive, leading to retinal necrosis and detachment, if the injection is not made slowly exactly in the center of the vitreous, as far as possible from the retina (236). Intracameral administration (7.5 to 10 μg in 0.1 ml) has been used to treat intraocular mycoses, including endophthalmitis (348) and three patients with keratitis and hypopyon due to A. flavus (183), with minimal toxicity being reported. Again, the efficacy of these modes of administration is difficult to evaluate in the absence of evidence from controlled clinical trials.
Azoles
Azoles bind to a cytochrome P-450 fungal enzyme involved in the 14α demethylation of either lanosterol or 25-methylenedihydrolanosterol, resulting in a decrease in ergosterol synthesis and an accumulation of 14-α-methylated sterols; this leads to increased permeability of the fungal cell membrane, alteration of membrane enzymes, inhibition of growth, and ultimate death of the fungal cell. All azoles, except fluconazole, appear to decrease the function of immune system cells, especially lymphocytes; this may lessen the degree of tissue damage occurring with the inflammatory reaction but also affects the efficacy of the azoles in vivo (432). Since azoles, with the exception of fluconazole, achieve only limited concentrations in the eye, they are to be considered as fungistatic when used in ocular fungal infections (170).
Miconazole.
Miconazole (Table 10) is available as a solution for intravenous administration in some countries; it can be used for topical administration as a 1% (10-mg/ml) solution (101) or for subconjunctival administration (5 to 10 mg) (96). Topical administration of 1% miconazole nitrate was not found to retard the closure of 8.5-mm corneal epithelial defects in a rabbit model (102). In the clinical setting, topical miconazole therapy is sometimes associated with reversible superficial punctate keratitis (101).
In an experimental rabbit model, aqueous levels of 8 μg/ml were noted 1 h after intravenous administration of miconazole (30 mg/kg), levels of 10 μg/ml were noted after subconjunctival injection (10 mg), and levels of 4.5 μg/ml were noted after topical administration (1% solution) every 15 min for eight doses to corneas with the epithelium debrided (103). Corneal miconazole levels were not attained by intravenous injection, but following subconjunctival injection, levels of 35 μg/g were noted in corneas where the epithelium had been debrided; after topical administration of miconazole, concentrations of 10 μg/g (in undebrided corneas) and 93 μg/g (in debrided corneas) were achieved (103). These results suggested that miconazole administered topically and, to a lesser extent, subconjunctivally was a potentially effective means of treating mycotic keratitis.
In the 1980s, miconazole was reported to be useful for therapy of mycotic keratitis in two series of patients. In the first series (101), topical and subconjunctival miconazole therapy resulted in resolution of all lesions in seven patients with mycotic keratitis (four cases due to C. albicans, two due to A. fumigatus, and one due to A. flavus); four of these patients had had deep lesions (endothelial plaque in three and descemetocele in one). In the other series (96), miconazole (applied by the topical and subconjunctival routes) was used with ketoconazole (administered orally) to treat 20 patients with mycotic keratitis (eight cases due to Fusarium spp., and four each due to Curvularia spp. and Candida spp.); this regimen resulted in healing in 13 patients. However, the severity of the keratitis in the patients was not clearly defined in the second series. Topical miconazole administration has been reported to be useful in therapy of superficial keratitis due to S. apiospermum (P. boydii) (77, 336). Intravenous administration of miconazole (600 to 3,600 mg/day) was reported to result in successful outcomes in patients with S. apiospermum orbital infection (16, 264), as well as in a few patients with mycotic keratitis (161, 173; Y. Ishibashi and Y. Matsumoto, Letter, Am. J. Ophthalmol. 97:646-647, 1984). It is difficult to derive conclusions based on the small number of patients evaluated; moreover, the intravenous use of miconazole is known to be associated with significant toxic reactions.
Ketoconazole.
Ketoconazole (Table 10), the first successful orally absorbable broad-spectrum antifungal azole, is currently available as an oral preparation (200 mg) worldwide. Formulations for topical or subconjunctival administration are not available, which is unfortunate since experimental studies suggest that concentrations as high as 1,391.5 ± 130.0 μg/g can be achieved, particularly after topical administration and to a lesser extent after subconjunctival injection, if the corneal epithelium has been debrided (145). Topical application is not associated with significant corneal toxicity (102). Another experimental study suggested that a single intravitreal dose of ketoconazole (≤540 μg) in dimethyl sulfoxide could be safely used for fungal endophthalmitis (436), although this finding has not been verified in patients. Since the absorption of ketoconazole is heavily dependent on the gastric pH, cimetidine or other antacids that inhibit gastric secretion or alter the pH should not be given concurrently with ketconazole (Table 10). Oral administration of ketoconazole may lead to various reversible side effects (Table 10). Ketoconazole-induced papilledema was reported in a patient who received 800 mg/day of ketoconazole over a 4-month period (282).
Ishibashi (157) reported that oral ketoconazole therapy (300 mg/day) was effective in two patients with mycotic keratitis, one case due to F. solani (therapy for 3 weeks), and the other due to an unidentified fungus (therapy for 8 weeks). The use of oral ketoconazole in therapy of keratitis due to Fusarium spp., Aspergillus spp., and Curvularia spp. and in therapy of mycotic blepharitis and other ophthalmic mycoses is discussed below. In addition, long-term oral ketoconazole therapy has been credited with improvement in a woman suffering from the keratitis-ichthyosis-deafness syndrome (141) and, in association with cyclosporin, has been shown to be effective in controlling and preventing reactivation of endogenous uveitis as well as in treating chronic uveitis affecting the posterior pole of the eye (312).
Itraconazole.
The synthetic dioxolane triazole itraconazole is well absorbed after oral administration (Table 10). It is larger than fluconazole, very hydrophobic, and more than 90% bound to protein in serum (342). It is highly concentrated in lipid-rich tissue and poorly soluble in aqueous solution but well absorbed orally, especially when given with a meal or formulated in polyethylene glycol (401). Itraconazole is generally well tolerated after oral administration; the most common complaint is gastrointestinal upset (310, 390). Less frequently observed side effects include hypertriglyceridemia, hypokalemia, edema, decreased libido, and gynecomastia (267).
The major drawback of using itraconazole by the oral route for therapy of ocular fungal infections is its poor penetration into the cornea, aqueous humor, and vitreous compared to that of fluconazole and ketoconazole. This was the case in a rabbit model of Candida endophthalmitis, even when itraconazole was given at a dose of 80 mg/kg orally (342). However, when treatment was started 24 h postinoculation, itraconazole was at least as effective as fluconazole or ketoconazole (342). Itraconazole was found to be effective in experimental keratitis due to Aspergillus spp. (402). In a recent study, prophylactic administration of an itraconazole oral solution, at a dose of 2.5 mg/kg body weight twice daily, was found to significantly reduce superficial fungal infections in patients with hematological malignancies and neutropenia (139). The ocular pharmacokinetics of this itraconazole oral solution need to be defined.
Attempts have been made to administer itraconazole topically to the eye. In one study, topical 1% itraconazole cream was found to be effective only in nonsevere mycotic keratitis (310). In another study, a 1% suspension of itraconazole, prepared in a commercial isotonic eye drop formulation containing methylcellulose, borax, boric acid, sodium chloride, and potassium chloride, was found to be well tolerated when used for therapy of mycotic keratitis; however, it was also not very effective in treating severe mycotic keratitis, perhaps due to insufficient corneal penetration (385). The vehicle used to prepare the solution or suspension of itraconazole or ketoconazole may influence corneal penetration. Bioassay of rabbit corneas which received topical applications of itraconazole in different vehicles (balanced salt solution, polyvinyl alcohol, boric acid, or olive oil) demonstrated approximate itraconazole concentrations of 200 to 250 μg/g of tissue (136). In an experimental animal model, itraconazole (2.5 mg/ml) that had been administered subconjunctivally was found to persist for at least 24 h in normal and debrided corneas, in contrast to amphotericin B, miconazole, fluconazole, and ketoconazole, which did not persist beyond 4 to 8 h (193). However, intravitreal injection of itraconazole appears to cause focal areas of retinal necrosis when doses exceeding 10 μg are used (344). There are no reports of itraconazole being administered subconjunctivally or intravitreally in a clinical setting.
Fluconazole.
The synthetic bistriazole antifungal compound fluconazole exhibits outstanding physical and pharmacokinetic properties (Table 10). Orally administered fluconazole was found to readily penetrate all ocular tissues and fluids of Dutch-belted rabbits; there was no difference between phakic and aphakic eyes (272). After a single oral dose of 20 mg/kg, the levels achieved were 13.3 ± 1.4 μg/g (cornea), 7.4 ± 0.3 mg/liter (aqueous), 9.8 ± 0.9 mg/liter (vitreous), and 5.2 ± 0.4 μg/g (choroid and retina); the concentrations in the cornea correlated highly with those in serum. A steady accumulation in both normal corneas and those infected with C. albicans was noted when fluconazole was given in a twice-daily divided dose; the presence of inflammation induced by fungal infection did not influence corneal uptake (272).
Since fluconazole is a stable, water-soluble, bis-triazole antifungal with low molecular weight, high bioavailability, and low toxicity, it is potentially useful as a topical ocular agent. The penetration of 0.2% fluconazole into corneas (with or without epithelial debridement) and the aqueous humors of New Zealand White rabbits was assayed by gas-liquid chromatography (434). Peak levels of 8.2 ± 1.2 μg/g (debrided corneas) and 1.6 ± 0.6 μg/g (nondebrided corneas) in corneas were noted after 5 min, and levels of 9.4 ± 2.3 and 1.6 ± 0.6 μg/ml, respectively, in aqueous humor were noted after 15 min; the half-life of fluconazole in debrided eyes was 15 min, and that in nondebrided eyes was 30 min. A loading dose of a 20-μl drop per min for 5 min resulted in levels of 59.9 ± 11.3 μg/g in debrided corneas and 32.4 ± 1.9 μg/ml in the corresponding aqueous; this loading dose, followed by 1 drop (20 μl) every 1 or 6 h, resulted in lower levels (434). This confirms that relatively high drug concentrations are achieved in the cornea after topical application of a loading dose of fluconazole, especially if the epithelium has been debrided.
Intravenous administration of fluconazole (5 or 25 mg/kg) in albino rats resulted in aqueous, vitreous, and serum drug levels (1 h after administration) of 2.87, 1.72, and 4.6 μg/ml (5 mg/kg) and 14.9, 7.05, and 20.6 μg/ml (25 mg/kg), respectively; the intraocular penetration was moderately enhanced by vitrectomy (250). In addition, in vitro electroretinograms remained unchanged after perfusion with fluconazole (20 μg/ml) while the in vivo electroretinogram and visual evoked potentials were unchanged after daily fluconazole (25 mg/kg) for 8 days, suggesting a good safety profile. Following intravenous inoculation of 20 mg of fluconazole per kg as a single dose or 20 mg/kg every 12 h for four doses in nonpigmented rabbits, fluconazole concentrations in the aqueous, vitreous, cerebrospinal fluid, and serum were determined by a microbiological assay; the penetration of fluconazole into all the anatomical compartments was found to be >70% of that in serum (246). Since the cerebrospinal fluid and ocular pharmacokinetic parameters closely resemble each other, either could be used as a surrogate for the other (246).
A biodegradable polymeric scleral implant containing fluconazole was reported to be a promising intravitreal drug delivery system to treat fungal endophthalmitis (249). Scleral implants loaded with 10, 20, and 30% doses gradually released fluconazole over 4 weeks in vitro, while those with 50% doses released most of the drug in 1 week; implants with 30% fluconazole that were studied in pigmented rabbits resulted in vitreous concentrations of fluconazole (sustained for 3 weeks) sufficient to inhibit C. albicans. In another study (345), intravitreal injection of up to 100 μg of fluconazole per 0.1 ml of vitreous did not produce biomicroscopic, ophthalmoscopic, electroretinographic, or light microscopic evidence of intraocular toxicity, even 8 days after inoculation.
Oral fluconazole therapy has been used with success in treating one patient with mycotic keratitis (380), one with multifocal choroiditis due to coccidioidomycosis (72), one with chorioretinitis and iridocyclitis complicating disseminated coccidioidomycosis (222), and four with endogenous Candida endophthalmitis (222). Oral fluconazole therapy for 8 weeks also resulted in a remarkable improvement in retinitis following disseminated cryptococcosis in a renal allograft recipient (2). These promising results require confirmation in a larger number of patients and in controlled clinical trials.
Miscellaneous Compounds
Polyhexamethylene biguanide.
Polyhexamethylene biguanide (PHMB) is a general environmental biocide that is believed to act on the cytoplasmic membrane of microorganisms, causing leakage of cellular components and inhibition of the respiratory enzymes that are necessary for survival; this molecule exhibits good in vitro activity against bacteria, fungi, and Acanthamoeba (95). It has been used as a swimming pool disinfectant, sanitizer, and preservative in topical ophthalmic preparations. Since PHMB is water soluble, a 0.02% solution can be prepared by dilution of the 20% concentrate with sterile distilled water. PHMB has been used for the treatment of Acanthamoeba keratitis at concentrations of 0.02 to 0.053% without causing adverse effects (84). PHMB at 0.02% was found to be effective in significantly reducing fungal growth in a New Zealand white rabbit model of F. solani keratitis; no growth was obtained in 58% of PHMB-treated eyes and in only 17% of placebo-treated eyes (95). These preliminary results need to be corroborated in other experimental studies using other fungal species that cause mycotic keratitis and in controlled clinical trials.
Chlorhexidine.
The cationic antiseptic bis-biguanide chlorhexidine (PHMB is a polyhexamethylene biguanide) inhibits microbial function by affecting the functioning of the cell membrane, therein leading to a leak of cell electrolytes. The bactericidal and amoebicidal effects of chlorhexidine gluconate are well known (348). Attempts have been made to evaluate the efficacy of chlorhexidine in the treatment of mycotic keratitis. In a study conducted in Bangladesh (319), 0.2% chlorhexidine gluconate was compared with 2.5% natamycin therapy in the treatment of 71 patients with suspected mycotic keratitis (2.5% natamycin was used since this formulation was commercially available in Bangladesh); 22 patients had keratitis due to Aspergillus spp., and another 22 had keratitis due to Fusarium spp. None of the severe ulcers was fully healed at 21 days, but three of those treated with chlorhexidine eventually healed in times up to 60 days. Of the nonsevere ulcers, 66.7 and 36% were healed at 21 days by treatment with chlorhexidine and natamycin, respectively (319). When 5% natamycin was used instead of the 2.5% preparation, better results were obtained. The results obtained in this study are misleading, since the 2.5% natamycin formulation used apparently delivered subtherapeutic concentrations of natamycin to infected corneas, resulting in less than optimal outcomes. It might be erroneously infered from this study that natamycin per se is less effective than chlorhexidine against Aspergillus species and other filamentous fungi, whereas in fact an effective formulation of natamycin was not used. Moreover, before performing this study, the pharmacokinetics and antifungal activity of this 2.5% natamycin formulation should have been compared to that of the 5% natamycin formulation that is used worldwide. Recent attempts to use chlorhexidine in the treatment of mycotic keratitis in two locations in Africa have not had encouraging results (171).
Silver sulfadiazine.
Silver sulfadiazine derives synergistic benefits from sulfonamides and heavy metals; it functions as an organic base-heavy metal release system. Silver is liberated and binds to microbial DNA, preventing unzipping of the helix and thereby inhibiting the replication of microorganisms without interfering with epithelial cell regeneration (251). The efficacy of a 1% silver sulfadiazine ointment was compared with that of 1% miconazole in therapy of clinical mycotic keratitis in a prospective, controlled, randomized, double-blind clinical study (251). Overall, a higher success rate was achieved with silver sulfadiazine (80%) than with miconazole (55%), although the response of Aspergillus keratitis was comparable in the two groups. Miconazole was totally ineffective in patients with Fusarium keratitis; however, all four patients who received silver sulfadiazine as primary therapy, as well as three other patients who had not responded to initial miconazole therapy and who subsequently received silver sulfadiazine, responded to treatment. The absence of significant ocular or systemic adverse effects, coupled with the efficacy of the compounds, led these workers to suggest that silver sulfadiazine was a safe and effective broad-spectrum antifungal agent for use in mycotic keratitis (251). Unfortunately, details of the severity of the keratitis in the patients who responded to silver sulfadiazine, and in those who did not respond to miconazole were not clearly provided in this paper. Also, since the publication of this report in 1988, there has been no confirmation by others of the results obtained. This compound was not found effective in therapy of culture-proven mycotic keratitis in one study in southern India (Thomas, unpublished).
CLINICAL FEATURES, PREDISPOSING FACTORS, AND MANAGEMENT OF SPECIFIC OPHTHALMIC MYCOSES
Fungal Infections of the Orbit
Fungal infections of the orbit rarely occur spontaneously. Fungi may gain access to the orbital space by direct extension from adjacent tissues (sinuses, teeth, lacrimal sac, lids), by traumatic implantation of foreign bodies contaminated with fungi, or by hematogenous seeding from a distant focus. Spread of infection from the sinuses to the orbit is thought to occur in 67 to 85% of orbital infections (15, 290) due to the proximity of the sinuses to the orbit (194). Thus, virulent fungal pathogens causing sinusitis can devastate orbital structures by contiguous spread (233). The variable presentations of orbital fungal infections parallel the presentations of paranasal sinus mycoses (192, 233), the salient features of which are listed in Table 11. Just as differences between these types of paranasal sinus mycoses are not always clear, due to closely associated patterns of clinical behavior and pathological reactions (78, 176, 213), differences between presentations of orbital fungal infections, especially the chronic varieties, may not always be distinct.
TABLE 11.
Clinical presentations of fungal sinusitis with orbital involvement
| Type and clinical course | Immune status | Fungi involved | Key findings with orbital involvement | Histopathology | Treatment |
|---|---|---|---|---|---|
| Acute/fulminant Rapid, invasive clinical course; orbit involved in 84% (15) | Immunocompromised; risk factors mainly diabetes and diabetic ketoacidosis (435) | Mucorales,aAspergillus spp., S. apiospermum, Bipolaris spp | Mucosal necrosis, facial edema, visual loss, ophthalmoplegia, proptosis (435) | Angioinvasion, tissue necrosis | Radical surgical debridement and systemic antifungals (amphotericin B); correction of predisposing factors |
| Chronic/indolent Slow, invasive clinical course | Usually nonatopic and immunocompetent, sometimes immunocompromised (138) | Aspergillus spp., various dematiaceous fungi, Mucorales (occasional), S. schenckii (rare), C. albicans (rare), R. seeberi (rare) | Proptosis, ophthalmoplegia, visual loss, mucosal necrosis, retrobulbar pain | Granulomatous inflammation, tissue invasion | Surgical debridement, systemic antifungals (amphotericin B, rarely azoles); correction of predisposing factors |
| Fungus ball (in exenterated orbit, orbital prosthesis) | Immunocompetent, nonatopic | Aspergillus spp. | Local pain and tenderness | Noninvasive, tightly packed fungal hyphae | Surgical debridement |
| Slow course, no tissue invasion | |||||
| Allergic Slow course, no tissue invasion; orbit involved in 17% (192) | Immunocompetent, atopic | Aspergillus spp., C. lunata, Bipolaris spp. | Young adults with recurrent sinusitis, nasal obstruction, local pain, rhinorrhoea; multiple sinuses involved; proptosis, diplopia, epiphora; visual loss rare | Fungal hyphae (small no.), eosinophils (many) in mucin; peripheral blood eosinophilia, increased IgG and IgE levels | Debridement; systemic and/or topical corticosteroids; role of antifungals uncertain |
Several genera and species implicated.
Acute rhinocerebral (rhino-orbito-cerebral) zygomycosis.
Acute rhinocerebral zygomycosis represents the prototype of the acute/fulminant variety of invasive fungal orbital infection, usually running an acute course in an immunocompromised host (rarely in nonimmunocompromised individuals), with angioinvasion and marked tissue necrosis being key features. The vast majority of all reported cases of rhinocerebral zygomycosis are caused by Rhizopus arrhizus (synonym, R. oryzae) (323); less common causes are Absidia corymbifera, Apophysomyces elegans (43, 87, 306), and Saksenaea vasiformis (181). Infection is presumably contracted by inhalation of fungal conidia from environmental sources (323, 435).
The Mucorales are generally considered to be opportunistic pathogens. Neutrophil dysfunction induced by diabetic ketoacidosis underlies most cases of human zygomycosis (323); juvenile diabetics are not spared (1). Neutropenia induced by bone marrow suppression during chemotherapy or immunosuppression induced following transplantation is also thought to be an important risk factor; however, zygomycosis was observed in only 13 (0.9%) of 1,500 consecutive patients who underwent bone marrow transplantation (256). Thus, zygomycosis may occur in both neutropenic and nonneutropenic patients. The use of corticosteroids may be another risk factor, acting by suppressing the normal inflammatory cell response and by inducing a diabetic state.
Patients undergoing hemodialysis and receiving deferoxamine/desferrioxamine for iron or aluminium overload are thought to be at special risk (35, 323). A review of case records of 25 patients who developed zygomycosis while taking deferoxamine for iron overload (74) revealed 7 patients with rhinocerebral zygomycosis Four received no treatment and died, one died in spite of surgery, and one died in spite of surgery and amphotericin B therapy; only one survived after treatment with surgery and amphotericin B. This association between deferoxamine therapy and the occurrence of zygomycosis was confirmed in an alloxan-induced diabetic, immunocompromised murine model of zygomycosis; deferoxamine iron chelation caused rhinocerebral zygomycosis in animals that were challenged intraethmoidally with Rhizopus spores (15). This is thought to occur because feroxamine, which is the iron chelate form of deferoxamine, provides the iron that is an essential growth factor for fungi of the order Mucorales (152).
Other putative risk factors for rhinocerebral zygomycosis include protein-calorie malnutrition and iron overload (with or without the concomitant use of deferoxamine in patients undergoing hemodialysis), intravenous drug abuse, leukemia, aplastic anemia, myelodysplastic syndrome, burns, and treatment with the immunosuppressive medications necessary to maintain liver and other solid organ transplants (74, 265, 308, 323). When disease occurs in nonimmunocompromised individuals, which is rare, there is usually some associated antibiotic use or a breakdown in the mucocutaneous barrier (32, 292, 340); such patients may fare better than immunocompromised patients.
In recent years, the thermophilic fungus A. elegans has emerged as a cause of rhinocerebral zygomycosis (43, 87, 306) in patients without well-recognized immunologic or metabolic abnormalities following traumatic inoculation and/or soil contamination. Such cases have occurred in countries with warm climates (43, 87, 306), which again differs from the pattern of the disease observed with the more “traditional” genera.
Fever, nasal ulceration or actual necrosis, periorbital or facial edema, decreased vision, ophthalmoplegia, sinusitis, and headache have been reported as the most frequently observed clinical features of rhinocerebral zygomycosis and occur in 25 to 44% of patients; facial pain, decreased mental status, leukocytosis, nasal discharge, nasal stuffiness, corneal anesthesia, orbital cellulitis, and proptosis are less frequent manifestations (435). In contrast, another set of investigators (265) opined that a susceptible patient classically presents with unilateral severe headache and facial pain, nasal stuffiness with granular or purulent discharge, facial or eyelid edema, fever, and leukocytosis.
Orbital findings occur due to ischemic necrosis of the intraorbital contents and cranial nerves, while bony involvement is uncommon because of the angioinvasive nature of the fungus. In addition to the usual manifestations listed above, rhino-orbito-cerebral zygomycosis sometimes manifests as a painless orbital apex syndrome without any sign of orbital cellulitis or acute systemic disease (23), which may have a good outcome with medical therapy; orbital infarction syndrome (38); bilateral cavernous sinus thrombosis (13); isolated pontine infarction (49); palatal ulcer (292, 403); sudden blindness (209); fever with right-sided hemiparesis, and dysarthria (1); and numbness and loss of sensation over the temporal region, with loss of vision and proptosis on one side of the face (21). Other conditions which can mimic these manifestations include sinusitis, viral infections, diabetic ketoacidosis, cavernous sinus thrombosis, bacterial orbital cellulitis, fulminant orbital aspergillosis, and pseudallescheriosis. Early visual loss and retinal artery occlusion would favor a diagnosis of rhinocerebral zygomycosis over bacterial cavernous sinus thrombosis, in which blindness occurs much later (87). When the fungus infecting the orbital cavity actually invades the eyeball, the prognosis is particularly poor (359).
Magnetic resonance imaging (MRI) and computerised tomography (CT) can help to establish an anatomical, if not pathological, diagnosis in suspected rhinocerebral fungal infections (213, 252, 302). Findings of diagnostic significance (in descending order of occurrence) include soft tissue opacification of sinuses with hyperdense material, nodular mucosal thickening, and an absence of fluid levels in different sinuses. Sinus contents have a variety of MR signal characteristic, including T2 hyperintensity or marked hypointensity on all sequences. There is often soft tissue infiltration of the deep face and obliteration of the normal fat planes. Typically, proptosis occurs because of enhancing soft tissue masses crowding the orbital apex and the cavernous sinuses; thickening and lateral displacement of the medial rectus muscle are characteristic features indicating orbital invasion from disease in the adjacent ethmoid sinuses. These techniques may have certain limitations in establishing the diagnosis of both cerebral zygomycosis and cavernous sinus thrombosis, which can be overcome by performing sequential CT and MRI studies on a patient suspected to have rhino-orbito-cerebral zygomycosis (252, 323). Whether any specific radiological findings exist for rhinocerebral zygomycosis is a contentious point, although CT nonenhancement of the superior ophthalmic artery and vein, which is related to vasculitis and thrombosis, may represent one such specific sign (109). A combination of MRI and pathology helped to document the perineural spread of rhinocerebral zygomycosis, following the trigeminal nerve to the pons (241). While CT and MRI scans aid in making the diagnosis and in defining the extent of bone and soft tissue destruction, they are more useful in planning surgical intervention (324). MRI scans may be preferred for diabetic patients, for whom CT contrast agents may be contraindicated (324).
A prompt and accurate diagnosis of rhinocerebral zygomycosis necessitates a high level of clinical suspicion, as well as good coordination between the clinical and laboratory staff. Specimens that should be collected to establish a microbiological diagnosis of rhinocerebral zygomycosis have been outlined in Table 6. Swabs are not satisfactory. Instead, abscesses should be aspirated, and lesions on the mucous membranes should be irrigated or scraped; multiple biopsy specimens should be taken (324). Zygomycetes may be found not in the center of the necrotic tissue but, rather, at the edge of or proximal to it (324). Once collected, samples should be transported immediately to the laboratory due to the fragility of zygomycetes, which do not survive more than a few hours at refrigerator temperature; if overnight storage is required, it is recommended that samples be kept in Stuart's transport medium and left at room temperature. Tissue samples should be minced and not ground in order to avoid the destruction of any viable fungal elements that are present.
The microscopic demonstration of zygomycetes in KOH mounts or stained smears of clinical material taken from necrotic lesions (Tables 3 and 7) is more significant than their isolation in culture (323, 324). Although invasion of intact tissue by nonseptate hyphae is good evidence of a zygomycetous infection, failure to observe such elements does not exclude the diagnosis. In tissue stained with hematoxylin-eosin (Table 7), abundant large, irregularly branching hyphal elements can be seen. If cultures are deemed necessary for accurate identification of the fungus involved, nasal, palatal, and sputum cultures can be done, although these are seldom helpful. However, isolation of Mucorales from sputum, material aspirated from sinuses, or bronchial washings taken from diabetic or immunocompromised patients should not be ignored (324). Although zygomycetes are not especially fastidious, they frequently do not growth out in cultures of necrotic tissue, although direct microscopy is positive; therefore, culture media should be inoculated with as much material as possible. Sabouraud glucose neopeptone agar (with an antibacterial such as chloramphenicol or polymyxin B, but no cycloheximide) is adequate (324). Growth is usually rapid (2 to 5 days) and fills the petri dish or tube.
An enzyme-linked immunosorbent assay was used to demonstrate antibodies to S. vasiformis in the serum of a patient with rhinocerebral zygomycosis due to this fungus in whom conventional methods helped to establish the diagnosis (181). Although demonstration of antibodies to Mucorales by this assay may be a rapid yet specific technique for identification of the etiological agent in rhinocerebral zygomycosis, this method does not seem to have attained widespread use, perhaps due to the inherent limitations of applying a serological technique to the diagnosis of so rapidly fulminant an infection as rhinocerebral zygomycosis.
General principles in the treatment of acute invasive rhinocerebral zygomycosis and other acute invasive orbital mycoses include (i) control of diabetic ketoacidosis or other systemic underlying diseases, along with elimination of predisposing factors; (ii) surgical debridement and restoration of sinus drainage; and (iii) intravenous amphotericin B. Table 12 summarizes the salient features of recent series of patients treated on the basis of these principles.
TABLE 12.
Management of invasive mycoses with orbital involvementa
| Reference | No. of patients and underlying disease | Final diagnosis and criteria for diagnosis | Surgical management | Medical treatmentf | Outcomef | Comment |
|---|---|---|---|---|---|---|
| 15 | 6 patients (3 males, 3 females) aged 12-53 yr; diabetes mellitus in 4, multiple myeloma and chemotherapy in 1, Sideroblastic anemia in 1 | Rhino-orbito-cerebral zygomycosis (4 had intracranial lesions) diagnosed by CT,b MRI,c and HPEd | Debridement in all patients (average 2.3/patient), orbital exenteration and intracranial debridement in 2 patients | IV AB only in 2 patients IV AB and Rif in 1 patient IV AB and HBO in 1 patient IV AB and Rif and HBO in 2 patients | 1 patient recovered, 1 patient died Recovered Recovered 1 patient recovered, 1 patient died | 4 (67%) of 6 survived |
| 435 | 6 patients; diabetic ketoacidosis in 2, diabetes mellitus in 4 | Rhino-orbito-cerebral zygomycosis diagnosed by CT, direct microscopye 2 patients, and culture in 4 patients (all 4 grew Mucorales) | Therapeutic surgery in 5 patients (3-22 days after onset); other details not provided | IV AB in all 6 patients (details not provided) 1-12 days after onset of symptoms | 4 patients survived (all had surgery and AB), 2 patients died (1 did not have surgery) | 4 (67%) of 6 survived |
| 313 | 10 patients (7 males, 3 females) aged 27-80 yr; diabetic ketoacidosis in 3, diabetes mellitus in 7 | Rhino-orbito-cerebral zygomycosis diagnosed by direct microscopy and HPE in 5 patients, HPE and culture (Mucor) in 3 patients, and direct microscopy and culture (Mucor) in 2 patients | Orbital exenteration in 6 patients, debridement in 2 patients, turbinectomy in 1 patient, enucleation in 1 patient | IV AB (500-3,000 mg) or IV AB (3,000 mg) and Flu | All patients survived | Survival 100%; not clear whether intracranial lesions occurred in all 10 patients |
| 32 | 24-yr-old female; apparently no disease | Rhino-orbital zygomycosis diagnosed by CTb and HPE (from palatal ulcers) | Extensive debridement, including orbital exenteration; repeated | IV AB (2 courses) | Recovered (repeat CT and HPE negative) | No intracranial lesion |
| 23 | 60-yr-old female; diabetes mellitus | Painless orbital apex syndrome due to rhino-orbital zygomycosis diagnosed by MRI and HPE | None | IV AB only | Recovered (follow-up imaging negative) | No intracranial lesion |
| 376 | 14 patients (8 males, 6 females) aged 22-75 yr; acute leukemia | Invasive Aspergillus rhinosinusitis diagnosed by nasal histology and culture in 7 patients (A. flavus), sinus histology and culture in 2 patients (A. flavus), gingival histology and culture in 1 patient (A. flavus); another 4 only culture positive | All patients received IV AB; 3 patients also received Rif and WBC; 3 patients also received WBC; 3 patients also received Flu; 2 patients also received Rif | Overall 3 patients cured, 4 patients stable, 7 patients progressed | Extent of orbital involvement not clear; overall, 50% were cured or in a stable condition | |
| 64 | 13 patients (4 males, 9 females) aged 21-78 yr; all apparently immunocompetent | Invasive sinus aspergillosis with orbital involvement diagnosed by HPE and culture | None in 2 patients, orbital exenteration in 1 patient, other surgeries in 10 patients | None in 1 patient (only surgery) Details not provided for 1 patient IV AB in 7 patients IV AB plus KC or Flu in 3 patients IC in 1 patient | Cured Progressed 2 patients cured; 2 patients relapsed or progressed, 3 patients died 1 patient cured; 1 patient stable, 1 patient died Stable | 38% of patients were cured or in a stable condition |
Based on papers published since 1991.
Evidence of sinusitis, bony erosion, and increased soft tissue density within the orbit on CT.
Evidence of sinusitis, bony erosion, and increased soft tissue density within the orbit on MRI.
HPE, characteristic fungal elements seen by microscopy of tissues or biopsy specimens (histopathological examination).
Characteristic fungal elements seen by microscopy of necrotic material.
AB, amphotericin B; IV, intravenous; Rif, rifampicin; WBC, white blood cell transfusion; Flu, flucytosine; HBO, hyperbaric oxygen; KC, ketoconazole; IC, itraconazole.
Management of infection in diabetic patients should consist of prompt correction of acidosis and other metabolic abnormalities and elimination of predisposing factors.
(i) Surgical debridement and restoration of sinus drainage.
Surgical debridement of all necrotic tissue is crucial and often quite mutilating; it usually requires multiple operations. Wide local excision and debridement of all involved and devitalized oral, nasal, sinus, and orbital tissue is required, while establishing adequate sinus and orbital drainage. Wherever possible, all necrotic tissue should be removed until normal bleeding is encountered, since infected tissue typically bleeds little due to the vaso-occlusion caused by the Mucorales; however, this may not be possible in the setting of extensive infections which can extend to the dura or beyond (435). Serial radiological imaging identifies the extent of disease and the response to treatment. A frozen-section-guided surgical debridement technique for biopsy-proven rhinocerebral zygomycosis has recently been described (206) (Table 13), although it may not be possible to use this technique when there are extensive lesions extending to the dura. Reoperation to debride areas of progressive disease should be planned if the morbidity of the often mutilating surgery does not outweigh its potential benefits (35). The importance of prompt and extensive surgical debridement in the management of rhinocerebral zygomycosis cannot be overstated. A review of evaluable patients with this condition reported in the literature between 1970 and 1994 (435) revealed that 81% of patients survived when the interval between onset of symptoms and surgery was 1 to 6 days, compared to 52% when the interval was 7 to 12 days and 42% when the interval was 13 to 30 days. Interestingly, the percentage of diabetic patients who survived was higher than that of nondiabetics in each group.
TABLE 13.
Alternative therapeutic regimens reported for mycoses of the orbita
| Reference | Therapeutic regimen | Patients and risk factors | Diagnosis | Criteria for diagnosisd | Outcome | Comments |
|---|---|---|---|---|---|---|
| 339 | Debridement, intravenous fluconazole (10 mg/kg), G-CSFb (8-10 μg/kg) (plus intravenous liposomal amphotericin B in 2 of the 4 patients) | 4 patients (2 males, 2 females) aged 19-30 yr; relapsed acute leukemia | ROCZc | CF, CT, HPE; culture not done | All 4 survived; in repeat CT, lesions appeared resolved | Extent of lesions not clearly defined; apparently focal lesions. All patients relatively young. No follow-up HPE. |
| 292 | Oral itraconazole (200 mg/day) + oral antidiabetics for 3 mo | Male aged 45 yr; dental extraction | ROCZ | CF, CT, HPE; culture not done | Survived; marked resolution of all lesions (except palatal ulcer) | No follow up HPE or examination of patient after stopping therapy. No mention of debridement. Dose of itraconazole given appears low in relation to the diagnosis. |
| 368 | Intravenous amphotericin B (560 mg); intravenous amphotericin B-lipid complex (125 g in 18 wk); no exenteration or debridement | Female aged 60 yr; diabetic with rheumatoid arthritis | ROCZ | CF, CT, HPE; culture negative | Survived; in repeat HPE and CT, infection appeared eradicated | First report of medical cure of ROCZ (no surgery). Enucleation finally done (ulceration after exposure keratitis). No histological evidence of zygomycosis in eye. |
| 340 | Surgery; liposomal amphotericin B (5 g in 2 mo) | Female aged 46 yr; diabetic | ROCZ | CF, CT, HPE; culture not done | Survived; repeat CT (7 mo) revealed no lesions | Extent of lesions not clearly defined; no follow-up HPE. |
| Male aged 76 yr | ROCZ | CF, CT, HPE; culture not done | Survived; repeat CT (7 mo) revealed no lesions | Follow-up period not stated. Extent of lesions not clearly defined. No follow-up HPE. | ||
| 257 | Thorough debridement; intravenous amphotericin B colloidal dispersion (12.3 g in 8 wk) and 2 g of intravenous amphotericin B | Female aged 44 yr; diabetic ketoacidosis | ROCZ | CF, CT, HPE; Rhizopus sp. grown in culture | Survived; lesions resolved; no recurrence (3 yrs) on MRId | No follow-up HPE. Good response to amphotericin B colloidal dispersion possibly because of prior thorough debridement. |
| 257 | Initially only intravenous amphotericin B colloidal dispersion; then debridement and intravenous amphotericin B colloidal dispersion (6 wk); finally conventional and liposomal amphotericin B | Male aged 62 yr; myelodysplastic syndrome | ROCZ | CF, CT, HPE; Rhizopus sp. grown in culture. | Survived ROCZ; partial response to colloidal dispersion; resolved when given liposomal form | Eventually died of myelodysplastic syndrome. Debridement improved response to amphotericin B colloidal dispersion. Resolution in response to liposomal formulation perhaps helped by the prior therapy. |
| Ericsson et al.e | Surgical debridement and liposomal amphotericin B for 8 mo | Diabetic | ROCZ | CF, CT, HPE | Survived; lesions resolved | Good outcome possibly due to debridement and slow evolution of disease. Possibly a case of chronic progressive ROCZ. |
| 234 | Repeated debridement, intravenous amphotericin B (two courses), oral itraconazole 200 mg twice daily | Female aged 40 yr; recurrent proptosis and sinusitis | Sino-orbital aspergillosis | CF, CT, HPE; A. fumigatus grown in culture | Survived; lesions appeared resolved on repeat CT (10 mo) | Repeated debridement and prior amphotericin B possibly reduced fungal load, permitting action of itraconazole and resulting in resolution of lesions. Probably limited tissue invasion. |
| 206 | Repeated resection of necrotic tissue till frozen sections did not reveal fungus; packing of resected area with amphotericin B-soaked gauze; repeat biopsies after 4 days; finally intravenous amphotericin B | Female aged 43 yr; diabetic ketoacidosis) | ROCZ | CF, CT, HPE; culture not done | Survived; lesions resolved; no recurrence (34 mo) | Extent of invasive disease unclear. Good outcome possibly due to focal disease and recent onset of diabetes. Not clear whether follow-up CT or HPE was done. |
| 176 | Intravenous amphotericin B (1 mg/kg/day) and oral itraconazole (400 mg/day); no debridement | Male aged 68 yr; leukemia | Orbital abscess | CF, CT, DM (aspirate); S. apiospermum grown in culture of aspirate | Survived; lesions resolved (patient died after 3 mo due to leukemia) | Therapeutic decompression by the fine-needle aspiration done for diagnosis, as well as the focal nature of lesions, possibly aided resolution without debridement. |
| Reference | Therapeutic regimen | Patients and risk factors | Diagnosis | Criteria for diagnosisd | Outcome | Comments |
| 403 | Initial ketoconazole and metronidazole; finally intravenous amphotericin B | female aged 79 yr; diabetic ketoacidosis | ROZc | CF, HPE (palatal biopsy) | Died (after 4 wk) | Intravenous amphotericin B instituted late in the course of disease. No debridement. |
| 64 | Initial intravenous amphotericin B (13 wk); then amphotericin B-lipid complex (5 mg/kg/day) and surgery, flucytosine (37.5 mg/kg/6 h) and itraconazole (300 mg twice daily), and gamma interferon (0.5 mg/mm2 three times a wk) | female aged 70 yr | Invasive sinus aspergillosis with extension to orbit | CT, HPE; culture of biopsy sample grew A. fumigatus | Survived; no recurrence (24 mo) on MRI | Lesions progressed in spite of surgery (orbital exenteration, debulking of mass) and intravenous amphotericin B, but fungal load may have been sufficiently reduced to permit response to the other therapies given. |
Based on papers published since 1989.
G-CSF, granulocyte colony-stimulating factor.
ROCZ, rhino-orbito-cerebral zygomycosis; ROZ, rhino-orbital zygomycosis
CF, clinical features suggestive of fungal infection; CT, Evidence of sinusitis, bony erosion and increased soft tissue density within the orbit on computed tomography; HPE, Histopathological examination of tissue samples revealed fungal structures; MRI, Evidence of sinusitis, bony erosion and increased soft tissue density within the orbit on magnetic resonance imaging; DM, fungal hyphae seen by direct microscopy of necrotic material.
M. Ericsson, M. Anniko, H. Gustafsson, C.-Å. Hjalt, P. Angus, and M. L. Grayson, Letter, Clin. Infect. Dis. 16:585-586, 1993.
Orbital exenteration entails removal of the eye, together with its extraocular muscles and other soft tissues of the orbit; this procedure could be life-saving in patients with rhinocerebral zygomycosis and is usually considered only in an acutely infected orbit with a blind immobile eye (435). Unless extensive fungal invasion is demonstrable, exenteration may not be indicated if a seeing eye is present (195). A series of individuals who survived rhinocerebral zygomycosis with unaltered visual acuity and in whom exenteration was not performed was reported in 1985 (195); this favorable outcome may have been helped by early diagnosis and by management of focal areas of fungal infection, but this is speculative since these workers did not adequately describe the severity of the lesions in their patients.
Traditionally, an external or transantral approach has been the classic method to perform surgical debridement. Recently, endoscopic sinus surgery has been tried on several occasions to reach the goal of radical resection, with survival of 89% of the patients (168). It has been suggested that when endoscopic sinus surgery is used to treat rhinocerebral zygomycosis, alone or in combination with the traditional surgical procedures, there is lower morbidity and greater accuracy during surgery. However, this needs to be confirmed in studies of a larger number of patients.
(ii) Intravenous amphotericin B.
Although treatment modalities have not undergone clinical trials, the combination of aggressive surgical debridement and intravenous amphotericin B therapy is still considered appropriate for treatment of rhinocerebral zygomycosis (Table 12). Intravenous amphotericin B treatment should be rapidly instituted in order to be effective; when this is done within 1 to 6 days of onset of symptoms, 76% of patients have been reported to survive, compared to 36% when the interval is 7 to 30 days (435). In spite of the emergence of new antifungals in therapy of invasive fungal disease (Tables 10 and 13), amphotericin B is still regarded as the antifungal of choice for treating rhinocerebral zygomycosis (Table 12).
In an attempt to improve the outcome of rhinocerebral zygomycosis, efforts have been made to deliver amphotericin B directly to the infected tissue. When daily irrigation and packing of the involved orbit and sinuses with amphotericin B (1 mg/ml) were incorporated into the standard therapeutic regimen for rhinocerebral zygomycosis, excellent results were obtained in a small series of patients (195); the focal nature of the lesions may have contributed to the success of this treatment modality. Rhinocerebral zygomycosis in a juvenile diabetic was successfully treated by using intravenous, intracavitary/interstitial, and cerebrospinal fluid (intraventricular) amphotericin B (1). Local irrigation via a percutaneous catheter, in addition to intravenous amphotericin B, has also been tried, with a fair degree of success (265).
Several novel formulations of amphotericin B, including amphotericin B colloidal dispersion, liposomomal amphotericin B, and amphotericin B-lipid complex, have recently been developed (187, 416). These formulations have been used in small numbers of patients with rhinocerebral zygomycosis (257, 308, 340, 368), with favorable outcomes being reported (Table 13). These preliminary results require corroboration in controlled clinical trials on larger numbers of patients.
(iii) Other therapeutic options.
Other therapeutic options for treating rhinocerebral zygomycosis include the use of itraconazole (292), fluconazole (339), and granulocyte colony-stimulating factor (339) (Table 13). The results of these studies require careful interpretation since either long-term follow-up details were not provided (292) or the lesions described seemed to have been focal and to have occurred in relatively young patients (339).
Experimental studies have demonstrated that 100% hyperbaric oxygen, at 1 to 3 atms, exerts a fungistatic effect (90). Hyperbaric oxygen may also decrease tissue hypoxia, enhance oxygen-dependent cidal mechanisms, and decrease tissue acidosis. Treatments have consisted of exposure to 100% oxygen at 2 to 2.5 atm for 90 to 120 min every 12 to 24 h (90, 109). Adverse effects of hyperbaric oxygen therapy include decompression sickness and aeroembolism; there is also the ever-present fire hazard and the expensive and cumbersome equipment (233). The exact role of this therapeutic modality in the therapy of rhinocerebral zygomycosis is uncertain. A review of the literature indicated that 22% of patients with bilateral rhinocerebral zygomycosis who received standard therapy survived while 83% of patients who received standard therapy plus adjunctive hyperbaric oxygen survived; it was therefore suggested that hyperbaric oxygen should be considered part of the initial therapy for rhinocerebral zygomycosis, and should be continued until evidence of disease regression is observed (435). Others, however, do not think that there is sufficient evidence to support the view that the use of adjunctive hyperbaric oxygen therapy has changed the prognosis of this infection (35).
Over the past 20 years, the prognosis for patients with rhinocerebral zygomycosis has improved. Factors contributing to a lower survival rate appear to include delayed diagnosis and treatment, hemiparesis or hemiplegia, bilateral sinus involvement, leukemia, renal disease, and treatment with deferoxamine (435). The presence of facial necrosis (435), intraocular invasion by the fungus (359), and cerebral lesions (15) also appear to carry a poor prognosis. The mortality of rhinocerebral zygomycosis caused specifically by A. elegans is currently unknown because of the rarity of diagnosed cases, but it would seem to fall at the more favorable end of the spectrum (87).
Chronic rhinocerebral zygomycosis.
Chronic rhinocerebral zygomycosis is indolent and slowly progressive, often evolving over weeks to months. A review of the case records of 18 patients with this presentation revealed that the median time from onset of symptoms to diagnosis was 7 months; the most common presenting features were ophthalmologic, including ptosis, proptosis, visual loss, and ophthalmoplegia; this seemed to occur in those with diabetes and ketoacidosis; and the overall survival rate for the chronic disease was 83%, even though the incidence of internal carotid artery and cavernous sinus thrombosis was higher than in patients with the acute disease (138). Chronic rhinocerebral zygomycosis is clinically distinct from chronic infection due to the Entomophthorales (principally due to Conidiobolus coronatus and Basidiobolus ranarum). In chronic rhinocerebral zygomycosis, ophthalmologic features predominate (sinusitis predominates for C. coronatus, and subcutaneous mycosis predominates for B. ranarum), angioinvasion is an important feature (most Entomophthorales infections are localized, with no angioinvasion), and surgical resection of necrotic tissue is an important component of disease management (surgical resection may actually hasten the spread of infection due to B. ranarum, while the surgical approach is not always optimal in infection due to C. coronatus) (138, 323, 324).
Treatment of fulminant infections caused by non-Mucorales fungi.
Treatment would probably be along the lines of the treatment outlined above; however, antifungals other than amphotericin B may play a role in such infections. A devastating bilateral optic neuropathy due to Bipolaris hawaiiensis (repeatedly culture positive) did not respond to 3,700 mg of amphotericin B but ultimately responded to oral itraconazole therapy (233); it is possible that the initial amphotericin B therapy may have reduced the fungal load sufficiently to permit itraconazole to exert a therapeutic effect. A child with acute orbital infection and brain abscess due to S. apiospermum (P. boydii), who did not respond to initial intravenous amphotericin B therapy, ultimately responded to intravenous miconazole and multiple surgical debridements, although it was not clear whether the surgery or the miconazole was more important (16). Surgical debridement of the orbit and a 6-week course of intravenous miconazole led to a reduction of S. apiospermum orbital infection in another patient as well (264). However, firm conclusions cannot be derived based on the results of these few individual case reports.
Orbital aspergillosis.
Aspergillus species have been implicated in a wide variety of primary ocular orbital conditions, characterized by rapid, uncontrollable progression and sometimes death (201, 374). Some presentations of orbital aspergillosis, such as optic nerve involvement, may lead to use of systemic corticosteroids, which delays the diagnosis and may potentiate the infectious process (213). Levin et al. (213) described four patients who represented the spectrum of orbital aspergillosis, namely, infection of an exenteration socket, a complex dacryocystitis, a nerve tumor, and postoperative periorbital swelling; they cautioned that in neutropenic or otherwise immunocompromised patients, a high index of suspicion should be maintained to forestall the emergence of fulminant aspergillosis.
The key presenting complaints of sino-orbital aspergillosis appear to be abrupt onset of proptosis, ophthalmoplegia, and blepharoptosis with precipitous visual loss; debilitating periorbital pain or headache, without inflammatory signs, may also be noted (213). Predisposing factors include alcoholism, high-dose corticosteroid therapy, and insulin-dependent diabetes mellitus (367). Invasive Aspergillus rhinosinusitis occurring as a potentially lethal complication of chemotherapy-induced neutropenia in patients with acute leukemia has also been described (376); the majority of cases are due to A. flavus. Such patients may develop symptoms of orbital or cavernous sinus disease.
Aspiration cytology and immunohistochemistry have been described for diagnosis of orbital aspergilloma (367).
In a recent study, it was observed that even with limited surgical debridement and local and systemic amphotericin B in patients with sino-orbital fungal infections, all patients retained their preoperative visual acuities and only one patient underwent an orbital exenteration for progressive orbital fungal infection (349). Thus, conservative orbital debridement with local amphotericin B irrigation may be an effective adjunct in the control of sino-orbital infections, especially in patients with reversible immunosuppression and good preoperative visual activities. Massry et al. (234) reported successful resolution of sino-orbital aspergillosis following initiation of itraconazole treatment, without recurrence at 10 months follow-up, in an immunocompetent patient; notably, traditional therapeutic modalities (surgical debridement and amphotericin B therapy) had not resulted in resolution. Oral itraconazole could be considered as a treatment option in orbital aspergillosis occurring in immunocompetent patients who have recurrent or recalcitrant disease or in those who cannot tolerate amphotericin B (234), but this requires confirmation in studies of a larger number of patients.
Mycotic Infections of the Eyelids
Although infections of the eyelids are caused almost exclusively by bacteria (particularly Staphylococcus), fungi may also cause superficial or deep eyelid lesions (Table 14).
TABLE 14.
Mycotic blepharitis (mycoses of the eyelids), dacryocystitis and conjunctivitis
| Reference | Patients | Clinical presentation of ocular lesion | Criteria for diagnosis of fungal infectionb | Fungus isolated | Treatment | Outcome of ocular lesions | Comments |
|---|---|---|---|---|---|---|---|
| Barr and Gamel, letter | Male aged 84 yr; verrucous lesions on forehead, scalp, and forearm | Verrucous lesion on lower eyelid, progressive entropion | HPE, culture (excised lesion) | B. dermatitidis | Excision, potassium iodide, intravenous amphotericin B (410 mg) | No recurrence of eyelid lesion | Lesion resolved with surgery and antifungal |
| 353 | 6 patients, all male, aged 33-57 yr; lesions at other sites also | Papules of upper and lower eyelids in 3 patients, eyelid papules and conjunctival lesions or anterior uveitis in 2 patients; eyelid papules and conjunctival and corneal lesions in 1 patient | HPE of papules in 5 patients; positive microscopy of lid scrapings in 2 patients | Presumed P. brasiliensis (culture not done) | All patients received intravenous amphotericin B; 1 patient also received oral ketoconazole | Presumed resolved in 5 patients who survived (received 1,985-2,050 mg of amphotericin B) | Lesions presumed resolved with amphotericin B only (no surgery) |
| 262 | 40 patients (double-blind placebo-controlled trial) | Symptomatic seborrheic or mixed seborrheic and staphylococcal blepharitis | CF, positive microscopy (Pityrosporum yeasts) in lid scrapings of 39 patients; positive cultures in some patients | Pityrosporum ovalec and P. orbicularec | Topical 2% ketoconazole cream in 20 patients; topical placebo cream in 20 patients; lid hygiene practiced by all 40 pts; measures performed for 5 wk | Lids of 69% of ketoconazole users and 42% of placebo users were normal or markedly better at 5 wk; no. of Pityrosporum yeasts in microscopy significantly reduced in both groups | Lid hygiene and use of cream apparently sufficiently reduced the no. of yeasts and relieved symptoms; antifungals may be of some use. |
| 66 | Male aged 37 yr; AIDS | Papular lesion on eyelid border | HPE of excised lesion; cryptococcal antigen in serum and CSF | Culture not done | Excision; intravenous amphotericin B and fluconazole; disseminated lesions appeared after 5 wk | Eventually controlled (22 mo); occasional recurrences | Eyelid lesions a “sentinel” lesion of disseminated cryptococcosis |
| 405 | Male aged 10 yr | Preseptal cellulitis and vesicles on eyelids | Scrapings of skin lesions | Trichophyton spp. | Oral itraconazole (2 courses) | Resolved | Microscopy findings not mentioned |
| 304 | Female aged 62 yr | Fluctuant mass over lacrimal sac; obstructed NLDa | Culture of lacrimal sac; pus | C. albicans | Surgery | Resolved | Microscopy findings not mentioned |
| 304 | Female aged 89 yr; previous vitrectomy | Corneal ulcer and pus on syringing | Positive microscopy and culture | C. albicans (from corneal scrapes and lacrimal sac) | Surgery, miconazole, natamycin | Resolved | Lesion resolved with surgery and antifungals |
| 225 | Female aged 65 yr | Conjunctival nodule and necrotizing granuloma | Positive microscopy and culture of lung lesions | C. immitis (presumed to have caused ocular lesions) | Aggressive debridement, topical amphotericin B and oral fluconazole | Resolved | Lesions resolved with surgery and prolonged administration of antifungals |
| 200 | Female aged 35 yr | Dacryocystitis, epiphora, and lacrimal sac tenderness | CT of lacrimal sac; HPE and culture of lacrimal sac material | A. fumigatus | Mass removed in toto from lacrimal sac; no antifungals | Symptoms and signs completely resolved; no recurrence | Lesions resolved with surgery alone |
| 114 | 86 eyes (66 patients) | Congenital dacryocystitis | Culture of lacrimal sac discharge; repeated where possible | Aspergillus spp. in 9; C. albicans in 8; Rhizopus spp. in 4; fungi recovered from 32 eyes (26 patients) | Antibacterials and topical amphotericin B for 7 eyes | Good response in 5 of 7 eyes | Microscopy findings not noted; reporting of Rhizopus spp. without microscopy or HPE results suggests contamination |
| Antibacterials, probing, and syringing for 6 eyes | Good response in 6 of 6 eyes | ||||||
| Probing, syringing, and antifungal for 1 eye | Good response | ||||||
| 177 | 65 eyes (65 patients) | Chronic dacryocystitis | Culture of material taken from interior of incised lacrimal sac | Fungi recovered from 6 eyes (A. flavus in 4; Fusarium spp. in 2) | Dacryocystectomy for all 6 patients | Symptoms and signs of chronic dacryocystitis resolved with surgery alone; no antifungals administered | Microscopy findings not noted; fungi isolated from interior of lacrimal sac |
| 36 | 102 eyes (102 patients) | Acute conjunctivitis | Microscopy and culture of conjunctival swabs | Fungi from 34 eyes (Aspergillus spp. in 18) | Details not provided | Details not provided | Microscopy done, but no details of results provided |
NLD, nasolacrimal duct.
HPE, fungal elements seen on histopathological examination; CF, clinical features suggestive of fungal infection; CSF, cerebrospinal fluid; CT, computed tomography findings suggestive of space-occupying lesion.
Now designated Malassezia spp. (20).
Eyelid lesions due to Cryptococcus spp. are usually ulcerative. However, necrotizing fasciitis of the eyelids and periorbital area due to C. neoformans was reported in a young man after trivial trauma by a wood splinter (82), while an eyelid nodule was reported to be a sentinel lesion of disseminated cryptococcosis in a patient with AIDS (66). Rhinosporidiosis of the lid margins is a rare occurrence, since the conjunctiva is the most common site of ocular rhinosporidiosis (226).
An eyelid lesion due to Candida spp. usually suggests spread from a focus, with the use of broad-spectrum antibacterials or immunosuppressive agents predisposing to the infection (14). Ulceration begins at the base of an eyelash; small granulomas are present at its edge, and vesicles and pustules may be present. In a study of 407 patients with chronic severe ulcerative blepharitis, 47 (12%) had positive cultures for Candida spp.; most of these patients also had atopic dermatitis (154).
Lesions caused by the dermatophytes begin as erythematous scaly papules that slowly enlarge; healing occurs simultaneously in the central, paler area. Induration of the lid margin and fracture of the cilia may occur (424). A kerion celsi reaction in the eyebrow due to Trichophyton rubrum has recently been described (149).
Although the lid is generally considered to be the most common ocular tissue affected by B. dermatitidis infection (338, 355; Barr and Gamel, letter), Bartley (26) reported that only 1 of 79 patients with systemic blastomycosis seen by him had such lesions. The lesions may arise due to contiguous spread from facial lesions or due to hematogenous dissemination from a pulmonary lesion. Small abscesses may be visible around the eyelashes; these later form granulomatous ulcers with thick crusts and an underlying purplish discoloration of the skin. Healing of the lesions may lead to severe cicatrization and ectropion (14, 355). The evolution of eyelid lesions due to the dimorphic fungi C. immitis and S. schenckii is similar to that seen in B. dermatitidis infections (14).
Eyelid lesions, alone or in association with corneal and conjunctival lesions, occur in more than 50% of reported cases of ocular paracoccidioidomycosis (46, 353). Males, particularly those older than 30 years who are engaged in agriculture and come from an area of endemic infection seem to be at greatest risk (353). The palpebral lesion starts as a papule, usually close to the lid border, and grows and ulcerates in the center. The base of the ulceration reveals fine hemorrhagic, punctate, elevated, thickened, and hardened borders. The lesions evolve toward palpebral coloboma, with loss of the eyelashes. Ocular lesions due to P. brasiliensis need to be differentiated, particularly in the initial stages, from hordeolum, bacterial blepharitis, trachoma, leishmaniasis, sporotrichosis, lupus erythematosus, tuberculosis, and secondary syphilis.
Malassezia furfur (formerly Pityrosporum orbiculare and Pityrosporum ovale) is a cause of pityriasis versicolor, a chronic mild skin infection sometimes found around the eyebrows and eyelids, and may be associated with seborrheic blepharitis (20, 373). Lid scrapes from 40 symptomatic patients with seborrheic or mixed seborrheic and staphylococcal blepharitis were subjected to direct microscopy and culture; yeast and hyphal forms suggestive of Malassezia spp. were detected in scrapes from 39 of the 40 patients; fungi were isolated from the scrapes from about half the patients (262).
The hyphae or yeast cells of fungi causing eyelid lesions can be easily demonstrated by examination of a 10% KOH wet preparation or a Gram-stained smear of lid scrapes.
Many therapeutic regimens for mycoses of the eyelids have been recommended (14, 28), but the basis for these suggestions is not clear. Table 14 lists some of the therapies and outcomes described in recent reports of various eyelid mycoses, which are briefly summarised below. Eyelid lesions due to rhinosporidiosis require excision (226).
A double-blind, placebo-controlled clinical trial of topical 2% ketoconazole cream with lid hygiene was conducted to treat seborrheic and mixed seborrheic and staphylococcal blepharitis (262). Although ketoconazole was no better than placebo at improving the symptoms of blepharitis, more ketoconazole-treated patients had normal, or markedly improved, lids after treatment than did the placebo group (262). Two doses of ketoconazole (100 mg per day) with topical miconazole ointment for 6 weeks has been recommended for treatment of mycotic blepharitis due to Candida spp. (154).
Preseptal cellulitis due to Trichophyton spp. was recently described in a 10-year-old boy (405). The cellulitis did not respond to antibacterials, prompting microbiological studies. Trichophyton spp. were recovered from two skin scrapings taken on two separate occasions. The lesions rapidly resolved after administration of oral itraconazole at 100 mg daily for 6 weeks but recurred 15 weeks after therapy was stopped. Therapy was restarted, the lesions were completely resolved, and there was no further recurrence. In another study, lesions of the eyebrow (kerion celsi) due to T. rubrum were found to disappear almost entirely after 3 weeks of oral itraconazole therapy (149).
Verrucous lesions of the eyelid due to B. dermatitidis were reported to resolve after treatment with a combination of antifungals (potassium iodide and intravenous amphotericin B) and surgery (Barr and Gamel, letter). Six patients with papular lesions of the eyelids due to P. brasiliensis (some patients also had conjunctival, corneal, and anterior uveal lesions) were treated with intravenous amphotericin B (one patient also received oral ketoconazole); all five patients for whom outcome data were evaluable responded to medical therapy alone (no surgery was required). Lesions of the eyelid were recently described in one patient who had disseminated cryptococcosis (66); the infection was controlled (with occasional recurrences) by a combination of surgical excision and intravenous amphotericin B and fluconazole.
Mycotic Dacryocanaliculitis
Gram-positive filamentous bacteria are frequent causes of dacryocanaliculitis (28). Fungi such as Alternaria spp., A. fumigatus and other Aspergillus spp., Candida spp., dermatophytes, Fusarium spp., Penicillium spp., Scopulariopsis spp., and Sporothrix schenckii have been reported as causes of canaliculitis (28, 213), although the significance of some of these isolates is doubtful since they do not appear to satisfy the criteria described previously (237). Most of the clinical features described and the surgical procedures recommended are for dacryocanaliculitis in general and are not necessarily specific for mycotic infection.
Usually, only one canaliculus on one side is affected; there is no good evidence that the inferior canaliculus is affected more frequently than the superior (28). Persistent unilateral epiphora (watering) with an itching sensation is the frequent presentation, and there may be unilateral mucopurulent conjunctivitis. Clinical features include a red, swollen eyelid in the area of the affected canaliculus, a unilateral conjunctivitis (conjunctival follicles may be present), reddening and swelling of the canaliculus itself (the opening is dilated and the edges are elevated and inflamed), and a mucopurulent discharge; white, yellow, or brown concretions (dacryoliths) may be visible in the lacrimal punctum or may be extruded after applying pressure to the canaliculus (28, 404). The remainder of the lacrimal passage is patent, and there is no preauricular lymphadenopathy.
Local environmental factors in the canaliculus, such as stasis arising out of congenital diverticula, may promote the growth of anaerobic bacteria and hence lead to local infections; however, most cases do not have an identifiable predisposing factor (28). Accumulated bacterial growth, cellular debris, and products of inflammation cause a progressive expansion and ectasia of the canalicular lumen, producing the characteristic fusiform swelling noted externally. Mixed aerobic and anaerobic bacterial infections may also be present. Rubbery concretions occur in the presence of infections due to species of Candida, whereas brown or black debris may be seen in infections due to A. niger (28).
Pavilack and Frueh (294) emphasized the importance of thorough curettage as the most effective treatment for chronic canaliculitis. Although their recommendations were not based on the study of specific actinomycotic or fungal causes of canaliculitis, the same principles can be applied for therapy of mycoses of the canaliculi. In essence, the punctum is dilated after topical and local anesthesia and a small curette is introduced. If there is extensive ectasia and retention of concretions in diverticuli, a canaliculotomy may be necessary (28). Following curettage (294), the canaliculus is thoroughly irrigated to remove any remaining fragments, to identify unrecognized pockets of retained debris, and to ensure the patency of the distal drainage system.
Mycotic dacryocanaliculitis is reported to respond satisfactorily to topical administration of 5% natamycin or to topical application and local syringing of the canaliculi and sac with amphotericin B (1.5 to 8 mg/ml) or nystatin (25,000 to 100,000 units/ml) solutions (28, 424); however, again, the basis of these recommendations is not clear. If medical management fails, surgery (canaliculotomy) is performed. All the material removed is used to prepare smears and to inoculate various culture media; the canaliculus is then syringed with the medications. Silicone intubation may be required for reconstruction of the canaliculus (28).
In a study of 40 patients with canaliculitis (404), only 10% were cured by medical treatment alone, while 40% showed a recurrence; 80% of individuals who underwent canaliculotomy in addition to receiving medical therapy were cured. Epiphora was a side effect of the surgery in a few patients. These results suggest that surgical treatment of canaliculitis in combination with medical therapy yields better results than those obtained by medical therapy only.
Mycotic Dacryocystitis
Dacryocystitis refers to an infection of the lacrimal sac (41). This is the most common infection of the entire lacrimal apparatus and generally arises due to the stasis resulting from obstruction of the nasolacrimal duct. There are several possible causes of this obstruction (28). Infection may be acute or chronic; fungi usually do not cause acute dacryocystitis. Chronic dacryocystitis is usually due to a single site of partial or complete obstruction within the lacrimal sac or within the nasolacrimal duct. With partial or complete obstruction of the nasolacrimal duct, a laminated concretion (dacryolith) may develop in the lacrimal sac and is often associated with bacterial and fungal infections.
Bacteria are the etiological agents in 95% of patients with acquired dacryocystitis, with aerobic and facultative anerobic bacteria predominating; fungi were found to account for only 5% of infections in two studies (41, 177). However, fungi may account for almost 14% of cases of congenital dacryocystitis (114).
Although several fungi have been implicated as causes of dacryocystitis, including Acremonium spp., Aspergillus spp., Candida spp., Paecilomyces spp., R. seeberi, dermatophytes, and S. schenckii (114, 177, 213, 226, 304, 424), the significance of some of these isolates is doubtful, on the basis of the criteria described previously (237). Infections due to S. schenckii and Acremonium spp. are reported to generally manifest as chronic suppurative dacryocystitis; there may be preauricular and submaxillary lymphadenitis, and an abscess may develop which ruptures outside, resulting in an indolent ulcer (28). Aspergillus spp., Candida spp., Paecilomyces spp., R. seeberi, and dermatophytes may cause chronic granulomatous dacryocystitis (178, 199, 200, 213, 226, 304).
Kristinsson and Sigurdsson (200) described a patient in whom A. fumigatus caused plugging of the lacrimal sac, leading to epiphora, extreme tenderness of the sac, and discharge from the lacrimal punctum. Two patients with dacryocystitis due to C. albicans have also been described (304).
A microbiological evaluation of congenital dacryocystitis in 86 eyes of 66 patients was undertaken (114). Fungi (principally Aspergillus spp. and C. albicans) were isolated from 32 eyes of 26 patients, but these results must be viewed with caution since there was no mention of direct microscoopy findings, and four isolates of Rhizopus spp. were reported. In another study, 65 eyes of 65 patients with chronic dacryocystitis were subjected to microbiological investigations (177); fungi (principally A. flavus) were recovered from 6 eyes. This report also made no mention of direct microscopy observations, although the fungi isolated appear to have been significant since they were recovered from material taken from the interior of the lacrimal sacs incised at surgery.
Krishnan et al. (199) described the occurrence of a diverticulum of the lacrimal sac in association with rhinosporidiosis, while Kalavathy et al. (178) recently described rhinosporidiosis of the lacrimal sac in two patients; the etiology of the lesions was confirmed by histopathological examination of the excised lacrimal sacs.
While epiphora is frequently the only clinical finding in patients with chronic dacryocystitis, there may also be lid edema, conjunctival injection, and swelling in the medial canthus; pressure over the area usually results in a purulent discharge through the lower punctum (177). In rhinosporidiosis of the lacrimal sac, blood-stained epiphora is a frequent complaint, due to the extreme fragility of the lesion (178).
Table 14 lists some of the therapies and outcomes described in recent reports of mycotic dacryocystitis, which are briefly summarized below.
Mycotic dacryocystitis is managed by dacryocystectomy (for rhinosporidiosis), where the lacrimal sac is removed in toto, or dacryocystorhinostomy, where the patency of the nasolacrimal duct is restored. Dacryoliths, if present, are surgically removed, and dacryocystorhinostomy is then performed. Dacryocystitis following plugging of the lacrimal sac by A. fumigatus was reported to have been relieved after the plug was removed by opening the lacrimal sac; dacryocystorhinostomy did not have to be performed, and the patient was symptom free 1 year after the procedure (200). Dacryocystitis due to C. albicans resolved with surgery alone in one patient and with surgery and topical miconazole and natamycin therapy in another (304). Some patients with congenital dacryocystitis were reported to respond satisfactorily to topical antifungals, probing, and syringing (114), although complete details of the therapy were not provided in the report. Chronic mycotic dacryocystitis in six patients was found to resolve completely following dacryocystectomy (177). Recently, endoscopic and laser technologies for minimally invasive transnasal dacryocystorhinostomy have been introduced. Postoperative infection following surgery on the lacrimal sac could probably be reduced by intraoperative or postoperative antifungal therapy.
Mycotic Dacryoadenitis
Acute fungal infections of the lacrimal gland are rare. Zygomycetes are reported to be capable of causing acute necrotizing dacryoadenitis following contiguous spread from a rhinoorbital lesion, while chronic granulomatous dacryoadenitis can be caused by certain filamentous fungi (28, 424). Treatment is by systemic antifungal agents.
Mycotic Conjunctivitis
Fungal infection in patients with acute conjunctivitis appears to be uncommon. In a microbiological study of 102 patients with clinically diagnosed acute conjunctivitis, fungi were isolated from only 14 samples (36); no mention was made about the results of direct microscopic observations or about the criteria used to define significant growth in culture (Table 14).
Although the clinical manifestations of mycotic conjunctivitis are described as being dependent on the fungi involved (28, 424), the basis for these observations is unclear. Infections due to Candida spp. present as purulent, acute or subacute superficial epithelial lesions; Malassezia spp. may cause a catarrhal conjunctivitis. The dimorphic fungi B. dermatitidis, C. immitis and P. brasiliensis have been reported to cause conjunctival lesions (225, 353, 355). Although conjunctival lesions in patients with blastomycosis may occur due to contiguous spread from eyelid lesions, they may also occur as separate entities (355). Severe, necrotizing granulomatous conjunctivitis due to C. immitis has been described in a patient who had received treatment with corticosteroids by various routes (225).
Treatment of mycotic conjunctivitis can be difficult. Topical antifungal therapy may suffice for superficial conjunctivitis, while deeper lesions may require systemic antifungal therapy. Necrotizing granulomatous conjunctivitis due to C. immitis required aggressive debridement of the affected area and months of topical amphotericin B and oral fluconazole therapy (225).
Conjunctival rhinosporidiosis.
Rhinosporidioisis appears to be endemic in the Indian subcontinent (54, 199, 258, 352), but significant numbers of cases have also been reported from Malawi and Kenya (295), northern Serbia (413), Zaire (397), Kuwait (371), and the United States of America (108, 169, 321). The prevalence of this condition in a south Indian village was reported to be 470 per 100,000 population (61). Children and young adults (up to 30 years of age) living in rural areas, who work in rice fields or bathe in stagnant water, appear to be the most severely affected (61, 258, 284, 295, 352, 413). Most studies have reported a male preponderance, but one study reported a female preponderance (61), while yet another study did not report a predominance of either sex (258). The mode of transmission is not definitely known but is thought to involve frequent exposure to contaminated water (258); this hypothesis is strengthened by a paper describing an unusual outbreak of rhinosporidiosis in the Balkans, where most patients reported having bathed in the same accumulation of stagnant water (the Silver Lake) just prior to the onset of symptoms (413). The causative organism possibly spends some or all of its life cycle in water; the organism may also be airborne (226). Conjunctival rhinosporidiosis may follow accidental injury to the eye by possible contaminated soil dust (169).
Most reported ocular lesions due to rhinosporidiosis have occurred in hot, dry climatic regions, with the occasional case being reported from temperate zones (321). Nasal lesions are thought to predominate in areas of endemic infection, while ocular lesions reportedly predominate during an epidemic (413). Ocular lesions are supposedly more frequent in Sri Lanka than in India, especially in Sinhalese women (226); however, no explanation has been given for this observation. Ocular rhinosporidiosis most frequently involves the palpebral conjunctiva; conjunctival growths are pink or red, granular, or lobulated (occasionally flattened); they may be sessile or stalked and are attached to the upper or lower fornix or tarsal conjunctiva (108, 295, 321, 352, 397). Conjunctival rhinosporidiosis with associated scleral melting and staphyloma formation, a rare occurrence, has recently been found in three Indian patients (54); the lesions presented as grey-white spherules without polyps. Other sites of ocular rhinosporidiosis are the lacrimal sac (178, 199, 352), lid margins, canaliculus, and sclera (226). Most infections of the eye are unilateral, and a solitary lesion develops. These lesions usually cause no discomfort to the patient; however, there may be increased lacrimation, discharge, tenderness of the lids, and photophobia.
A clinical diagnosis of rhinosporidiosis is suggested by the presence of lesions in other parts of the body, the extreme friability of the lesion, and the presence of small, white dot-like structures against a red background, i.e., the sporangia embedded in the vascular tissue bed (Fig. 10). However, other causes of a focal lesion on the conjunctiva, eyelid, or sclera, such as a cystic inclusion or adenoma of the various glandular structures, pterygium, pedunculated granuloma due to retained foreign body, or end-stage chalazion need to be excluded.
FIG. 10.
Surgical removal of a lacrimal sac infected with Rhinosporidium seeberi. Small, spherical structures (arrows) are seen on the surface of the sac; these represent the sporangia (cysts).
Since all attempts to cultivate R. seeberi have failed, histopathology forms the cornerstone of the diagnosis of rhinosporidiosis. The typical histological picture is that of a granuloma with marked inflammatory cell infiltrates (325); however, chronic nongranulomatous inflammation may also be seen (295). All stages of the life cycle can be seen in excised tissue, from small trophocytes to large sporoblasts; the latter contain spherical bodies (spherules or sporangia) varying in size from 6 to 30 μm (Fig. 7). The presence of well-defined spherical bodies of different sizes in a rather dense stroma covered by hyperplastic epithelium is a distinctive feature (325). Hematoxylin-eosin staining of infected tissue generally suffices to demonstrate the characteristic structures. The sporoblasts need to be differentiated from the spherules of C. immitis. In immunocompromised patients, unstained material could first be examined, since R. seeberi is readily identified by its brown color; special stains (Giemsa, Gridley, and toluidene blue) can then be used to differentiate R. seeberi from C. immitis (122). Serological tests have not been found useful for the diagnosis of the condition.
Two distinct phases of the tissue life cycle, namely, trophic and endosporulating, have been discerned by light and electron microscopic studies on conjunctival rhinosporidiosis (343). Electron microscopic studies suggested that the formation of the wall is a continuous morphological and biochemical spectrum throughout the cytological maturation of the organism (371). A different pattern of wall formation was observed in the conjunctiva of a patient who had concurrent rhinosporidiosis and papillomavirus infection; this modification was possibly a protective mechanism by R. seeberi against the virus (371). An additional feature noted in this patient was the absence of the marked inflammatory reaction that characterizes the histological picture of rhinosporidiosis.
No drug treatment has proven effective for ocular rhinosporidiosis. This condition is treated by surgical excision of the lesions.
Mycotic Keratitis (Keratomycosis)
Mycotic keratitis presents as a suppurative, usually ulcerative, corneal infection. This entity may account for more than 50% of all cases of culture-proven microbial keratitis and of ophthalmic mycoses (137), especially in tropical and subtropical areas.
The fungi most frequently implicated appear to vary depending on the geographical location and the period for which the infection is observed. In the first half of a 9-year study of microbial keratitis in south Florida, nine strains of C. albicans were isolated, but only one strain was isolated in the second half of the study (216). Although F. solani has been reported as the most common cause of mycotic keratitis in many parts of the world (137, 334, 364, 429), species of Aspergillus have predominated in some authentic, carefully documented recent studies from the Indian subcontinent (85, 398) and other countries (186). C. albicans was reported to be the most common cause (377, 394), or one of the most common causes (334, 398), of mycotic keratitis in the United States and Nepal, but it has been infrequently reported in several other major studies (Table 15).
TABLE 15.
Major studies of mycotic keratitisa
| Place, study population, duration (reference) | Demographic details | Risk factors | Criteria for diagnosis of fungal infection | Direct microscopy positive for fungib | Principal isolates in culture (% of total isolates) |
|---|---|---|---|---|---|
| Paraguay, 45 patients with microbial keratitis, 1 yr, (248) | Details not provided | Details not provided | Not clear; based on culture, 26 (58%) of 45 considered to have mycotic keratitis | 22 (85%) of 26 culture-proven cases | Total fungal culture positives, 26; Fusarium spp., 11 (42%); Aspergillus spp., 5; Cladosporium, 3 |
| Sri Lanka, 66 patients with corneal ulcers, 2 yr (117) | Details not provided | Trauma in 36 (55%) | Microscopy positive for fungi considered indicative of mycotic keratitis | Microscopy and culture positive in 17 | Total fungal culture-positives, 17; Aspergillus spp., 4; Fusarium spp., L. theobromae, and P. farinosus, 1 each |
| Florida, 125 pts. with mycotic keratitis, 10 yr from 1982 to 1992, retrospective analysis (334) | Male/female ratio, 4:1; avg age, 49 yr | Trauma in 58 (44%), chronic topical medications in 16 (13%), diabetes mellitus in 15 (12%), topical steroids in 9 (7%), contact lenses in 7 (6%) | Growth of fungus on ≥2 media; growth of fungus on 1 medium with positive microscopy or growth on ≥1 medium later | Gram smear positive in 27 (33%) of 80 culture-positive cases, Giemsa smear positive in 20 (27%) of 74 culture-positive cases | Total fungal culture positives, 125; Fusarium spp., 79 (68%); Candida spp., 16 (14%); Curvularia spp., 11 (9%); Aspergillus spp., 5; Paecilomyces spp., 4; Acremonium, 3; L. theobromae and Cylindrocarpon spp., 2 each |
| Bangladesh, 142 patients with suppurative keratitis, 11 mo (85) | For mycotic keratitis: avg age, 41.4 yr; male/female ratio, 2:1; agriculturalists, 29%; domestics, 46% | For mycotic keratitis: trauma in 35%, dacryocystitis in 4% | Not clearly stated; based on culture, 51 (36%) of 142 considered to have mycotic keratitis; criteria for significant growth in culture not clearly provided | Gram smear positive in 50 (98%) of 51 culture-positive cases | Total fungal culture positives, 51; Aspergillus spp., 19 (37%); Fusarium spp., 10 (20%); Curvularia spp., 9 (18%); L. theobromae, 2; S. apiospermum, 1 |
| For bacterial keratitis: avg age, 39.9 yr; male/female ratio, 2.6:1 | For bacterial keratitis: trauma in 52%, dacryocystitis in 12% | ||||
| Ghana, 199 patients with suppurative keratitis, study duration unclear (137) | Male/female ratio, 7:3; avg age, 36.3 yr; students and teachers, 20%; traders, 20%; agriculturalists, 16% | Trauma in 39.2% | Inclusion criteria and criteria for diagnosis not clearly stated; fungus culture positive in 65 patients | Details not provided | Total fungal culture positives, 65; Fusarium spp., 34 (52%); Aspergillus spp., 10 (15%); L. theobromae, 6 (9%); Cladosporium spp., 4 |
| New Delhi, India, 211 patients with mycotic keratitis, 5-yr retrospective (288) | Study done in children (≤16 yr old); male/female ratio, 6:4 | Trauma in 119 (55%), systemic illness in 24 (11%), previous eye surgery or local ocular disease in 39 (18.5%), contact lenses in 6 (3%) | Growth of ≥1 colony in 2 solid culture media; growth on 1 medium with positive microscopy | KOH mounts positive in 190 (90.2%); Gram smears positive in 68 (31.6%) | Total fungal culture positives, 211; Aspergillus spp., 85 (39.5%); Fusarium spp., 30 (14%); Alternaria spp., 22 (10.2%); Curvularia spp., 16 (7.4%); Penicillium spp., 15 (7%) |
| Madurai, India, 434 patients with central corneal ulceration, 3 mo (364) | 245 patients (56.4%) were agricultural workers | Trauma in 284 (65.4%) (mainly paddy, tree branch, thorn, dust, soil, vegetable matter); corticosteroids in 35(8%); traditional eye remedies in 162(37%) | Growth of same organism on ≥2 solid media, semiconfluent growth at inoculated site on 1 solid medium plus positive microscopy | Details not provided | Total fungal culture positives, 154; Fusarium spp., 66 (43%); Aspergillus spp., 23 (15%); Curvularia spp., 4; Exserohilum spp. and Bipolaris spp., 4; S. apiospermum, 2 |
| Singapore, retrospective review of 29 mycotic keratitis patients seen over 5 yr and 51 bacterial keratitis patients seen over 21 mo. (429) | For fungal keratitis: male/female ratio, 4:1; avg age, 40.6 yr | For fungal keratitis: trauma in 16 (55%), contact lenses in 2 (7%), ocular disease in 4 (14%), systemic disease in 5 (21%) | Corneal epithelial defect and underlying stromal infiltrate culture positive for fungus and clinical features suggestive of fungal keratitis (criteria for significant growth in culture not given) | Details not provided | Total fungal culture positives, 29; Fusarium spp., 15 (51.7%); A. flavus, 5 (17.2%); Candida spp., 3; Curvularia, Penicillium spp., S. apiospermum, 1 each |
| For bacterial keratitis: male/female ratio, 2:1; avg age, 44.5 yr | For bacterial keratitis: trauma in 16 (31%), contact lenses in 16 (31%), ocular disease in 21 (41%), systemic disease in 8 (16%) | ||||
| Philadelphia, 24 patients with mycotic keratitis, 9-yr retrospective (377) | Male/female ratio, 1.4:1; avg age, 59 yr; risk factors present in 20 patients (83%); 11 had ≥2 risk factors | Chronic ocular surface disorder, 10 (42%); contact lenses, 7 (29%); trauma, 2; topical or oral corticosteroids, 5; systemic diseases, 5 | Culture-proven fungal keratitis (no details about criteria to determine significant growth in culture) | Positive in 18 (75%) | Total fungal culture positives, 24; C. albicans, 11 (46%); Fusarium spp., 6 (25%); Scedosporium spp., 2; Cryptococcus spp., Aspergillus spp., Penicillium spp., Alternaria spp., Chrysonilia spp., 1 each |
| Hyderabad, India, 1,352 patients with mycotic keratitis, 10-yr retrospective review (120) | Male/female ratio, 2.5:1; Avg age, 40.4 yr; agricultural workers, 27.6%; manual laborers, 20%; unemployed, 22% | Recent trauma in 736 (54.4%), plant material in 188 (14%), animal origin in 28 (2%), dust in 155 (11.4%), ocular disease in 158 (12%), systemic disease (especially diabetes mellitus) in 109 (8%) | Growth in ≥1 medium if microscopy negative, growth in 1 medium with positive microscopy, confluent growth of fungi at inoculated sites | Positive in 1,277 patients (95%); positive by KOH in 1,219 (91%), by CFW in 1,224 (91.4%), by Gram stain in 1,181 (88.2%), and by Giemsa stain in 1,139 (85%) | Total fungal culture positives, 1,352; Fusarium spp., 506 (37.2%); Aspergillus spp., 417 (30.7%); Curvularia spp., 39 (2.8%); Bipolaris spp. and Exserohilum spp., 26 (1.9%); Acremonium spp., 12 (0.9%); L. theobromae, 7 (0.5%) |
| Houston, 32 patients with Curvularia keratitis, 30-yr retrospective (418) | Male/female ratio, 15:1; avg age, 43 ± 21 yr | Trauma in 16 (50%), topical corticosteroids in 11 (34%), topical antibacterials in 24 (75%) | Growth of Curvularia on ≥2 different primary media, growth on ≥1 medium with positive stained smear | Positive in corneal scrapes of 25 patients; histopathology of 2 of 3 corneal buttons revealed fungus | Total fungal culture positives, 32; C. senegalensis, 11; C. lunata, 8; Curvularia spp., 5; C. pallescens, 4; C. prasadii and C. lunata var. aeria, 2 each |
| Qingdao, China, 108 patients with suspected mycotic keratitis, 4-yr retrospective (431) | Male/female ratio, 1.75:1; age, 21-60 yr; farm workers, 86% | Recent trauma in 51 (49%), topical corticosteroids in 19 (18%); all patients had undergone keratoplasty | Confocal microscopy and light microscopy fungus positive before surgery, histopathology and culture (corneal buttons) fungus positive | KOH (corneal scrapes) positive in 78 (72.2%); PAS (sections of corneal buttons) positive in 98 (90.7%) | 97 (90%) of 108 corneal buttons grew fungi; Fusarium spp., 63 (65%); Aspergillus spp., 14 (14%); Candida spp., 9 (9%); Penicillium spp., 4; unidentified, 7 |
| Nepal, 405 patients with suppurative keratitis, 2 yr from 1985 to 1987 (398) | Male/female ratio, 1:1; age, 10-31 yr; farmers, 49.6%; homeworkers, 22.5%; children, 4.9% | Trauma in 214 (52.8%), conjunctivitis in 58 (14.3%), other ocular diseases in 24 (6%), nonocular diseases in 41 (10.1%) | Growth on ≥1 medium, abundant growth on 1 medium with positive microscopy | Positive in 38 (56%) culture-positive cases; GMS and Gram stain positive in 32 of 38 microscopy positives | Total fungal culture positives, 68; Aspergillus spp., 31 (47%); Candida spp., 9 (13.2%); Fusarium spp., 8 (11.7%) |
| Ghana and India, 1,090 patients with suppurative keratitis, 3 yr from 1999 to 2001 (208) | Details not provided | Details not provided | Growth on ≥1 medium, abundant growth in 1 medium with positive microscopy, positive microscopy (if culture negative) | Microscopy and culture positive in 462 of 1,090; microscopy only positive in 84 of 1,090 | Total fungal culture positives, 462; Fusarium spp., 187 (41%); Aspergillus spp., 95 (19.45%); Curvularia spp., 35 (5.25%); |
Reported from 1991 to 2002.
KOH, 10% potassium hydroxide wet mount; CFW, Calcofluor white; PAS, periodic acid-Schiff.
Table 15 lists the salient observations of 14 major studies of mycotic keratitis reported in the literature since 1991; 6 of these are studies done in the Indian subcontinent, that is, India, Nepal, Bangladesh and Sri Lanka (85, 117, 120, 288, 364, 398); 3 are studies done in the United States (334, 377, 418); 4 are studies done in Paraguay (248), Ghana (137), Singapore (429), and the People's Republic of China (431); and 1 recently published study was performed simultaneously in Ghana and southern India (208). Mycotic keratitis apparently occurs much more frequently in developing countries such as India than in developed countries such as the United States. Srinivasan et al. (364) reported on 139 patients with culture-proven mycotic keratitis seen over a 3-month period in Madurai, India; in contrast, 125 patients with culture-proven mycotic keratitis were seen over a 10-year period in south Florida (334), while 24 patients with mycotic keratitis were treated over a 9-year period in Philadelphia (377).
Risk factors.
Most of the studies done exclusively on mycotic keratitis (120, 288, 334, 431) have listed trauma as being the most common risk factor (occurring in 44 to 55% of patients); less frequently reported risk factors include prolonged use of topical corticosteroids or antibacterials, systemic diseases such as diabetes mellitus, preexisting ocular diseases, and contact lens wear. In all these studies, filamentous fungi, mainly Fusarium spp. or Aspergillus spp., were the most frequent isolates. Similarly, in a review of 32 patients with keratitis due to Curvularia spp. (418), trauma and prior use of corticosteroids were the most frequent risk factors (Table 15). In contrast, in a study in Philadelphia (377), the three most common risk factors were found to be chronic ocular surface disease, contact lens wear, and use of topical corticosteroids; interestingly, C. albicans was the most common isolate in this study (46%). Only two studies (85, 429) have sought to compare the most frequent risk factors in mycotic and bacterial keratitis (Table 15). In one of these studies (in Bangladesh), antecedent ocular trauma was reported by 35% of patients with mycotic keratitis and 52% of patients with bacterial keratitis; dacryocystitis was noted in 12% of those with bacterial keratitis and 4% of those with mycotic keratitis (85). Data derived from the other study, a retrospective case-control study in Singapore (429), suggested that mycotic keratitis (principally due to Fusarium spp. and Aspergillus spp.) was more likely to be related to mechanical ocular trauma and bacterial keratitis (principally due to Pseudomonas aeruginosa) was more likely to be related to contact lens wear and preexisting ocular diseases. Preexisting inflammatory ocular diseases were less frequently seen in mycotic keratitis than in bacterial keratitis, but systemic immunosuppressive conditions appeared to be of equal significance in both mycotic and bacterial keratitis. Interestingly, antecedent topical corticosteroid therapy, which is frequently perceived to be a specific risk factor for mycotic keratitis, did not appear to predispose more frequently to mycotic keratitis (25%) than to bacterial keratitis (38%) in this study (429).
One study attempted to compare the risk factors for keratitis due to filamentous fungi and that due to yeasts and yeast-like fungi (334). Ocular trauma appeared to predispose most frequently to infections due to Fusarium spp. (70%), Curvularia spp. (11%), and Aspergillus spp. (5%). Similarly, diabetes mellitus may have been a specific risk factor for keratitis due to Fusarium spp. (67% of diabetic patients had such infections) and to Candida spp. (13%). In patients who had used prolonged topical medications, Candida spp. (44%) and Fusarium spp. (38%) were the most frequent isolates. In patients who used topical corticosteroids, Candida spp., Aspergillus spp., Acremonium spp., and Curvularia spp. were the most frequent isolates (22% each) (334). Although these data are interesting, a case-control study is needed to compare the relative contribution of different risk factors to determining whether a patient develops keratitis due to filamentous fungi or to yeast or yeast-like fungi.
Fungi causing mycotic keratitis.
Filamentous fungi are the principal causes of mycotic keratitis in most parts of the world; in 12 of the 14 studies listed in Table 15, either Fusarium spp. or Aspergillus spp. were the most common isolates. Dematiaceous fungi, such as Curvularia spp. and Bipolaris spp., are the third most important cause of keratitis in a number of studies (111, 120, 208, 364), while the coelomycete L. theobromae has been reported to cause keratitis in India (111, 383, 389, 392) and the southern United States (216, 334).
Filamentous fungal keratitis appears to occur most commonly in healthy young men engaged in agricultural work or outdoor occupations (120, 334); mycotic keratitis has been reported to occur in onion harvesters in Taiwan (219). Trauma was the most common risk factor reported in all the studies listed in Table 15 in which filamentous fungi were the principal isolates. Various traumatizing agents have been reported, including vegetable matter, mud or dust particles, paddy grain, the swish of a cow's tail, tree branches, and metallic foreign bodies (120, 334). There have been reports of mycotic keratitis associated with the use of nylon-line lawn trimmers in the United States; the fungi implicated have included Curvularia spp. and F. oxysporum (65, 334). Preexisting allergic conjunctivitis (400) or vernal keratoconjunctivitis (134) may also predispose to the occurrence of filamentous fungal keratitis.
Environmental and corneal isolates of various species of Fusarium and Aspergillus have been found to be virtually indistinguishable in certain growth characteristics (71, 383). Seasonal variations have been observed in the incidence of mycotic keratitis and in the predominant genera of fungi isolated from such cases; such variations have been linked to environmental factors, such as humidity, rainfall, and wind, and also to the harvest (133, 216, 334, 383). The fungi most frequently present in the environment are also frequently found as transient commensals in the conjunctival sac in a variable percentage of healthy eyes (363); these fungi are thought to become virulent for the cornea under certain circumstances, such as following trauma or administration of corticosteroids (17, 424). However, this mechanism of infecting the cornea may be less important than the direct implantation of environmental fungi in the cornea by trauma.
Keratitis due to yeasts and yeast-like fungi is most frequently caused by C. albicans (100, 174, 216, 377). Since C. albicans is a ubiquitous commensal of mucous membranes in humans, with no geographic dominance, keratitis due to this organism tends to occur more frequently in areas where traumatic keratitis is uncommon but where other predisposing factors are important (174, 394). C. albicans was reported to be the most common fungal species isolated from patients with culture-proven mycotic keratitis in Philadelphia (377), but species of Candida accounted for only 12.5% of isolates from patients with culture-proven mycotic keratitis in Miami (334). C. albicans and related fungi have been infrequent isolates in most recent studies performed in tropical countries (85, 117, 120, 137, 208, 364), possibly due to the predominance of livelihoods, such as agriculture, which carry a higher risk for the occurrence of trauma-related keratitis caused by filamentous fungi than for keratitis due to C. albicans. Keratitis due to yeast-like and related fungi usually develops in eyes with preexisting epithelial or stromal ulceration due, for example, to previous herpes simplex keratitis or contact lens-induced corneal abrasions (100). This type of keratitis can also occur in the presence of systemic disorders or preexisting ocular abnormalities
Diagnosis.
A rapid and accurate diagnosis of mycotic keratitis improves the chances of a complete recovery, especially in the tropics, where patients may delay presenting to an ophthalmologist. A systematic approach, comprising a detailed elicitation of the clinical history, a meticulous examination with the slit-lamp or the confocal microscope, and appropriate microbiological investigations, should be adopted.
(i) History and clinical features.
Details elicited in the clinical history should include possible risk factors (trauma or use of contact lenses); prior therapy with antibacterials, corticosteroids, or other compounds; and preexisting ocular disease (allergic conjunctivitis or lagophthalmos). The clinician should then look for ocular or systemic defects that may have predisposed the patient to the keratitis, since these require correction. Symptoms are usually as in any other type of keratitis but, perhaps, are more prolonged in duration (5 to 10 days).
Filamentous fungal keratitis may involve any area of the cornea (100). The clinical features usually noted are the firm (sometimes dry) elevated necrotic slough (Fig. 11), “hyphate” lines extending beyond the ulcer edge into the normal cornea, multifocal granular (or feathery) gray-white “satellite” stromal infiltrates, “immune ring,” minimal cellular infiltration in the adjacent stroma, and mild iritis (100, 174). An endothelial plaque and hypopyon generally do not occur within the first week, but the presence of an hypopyon in an indolent ulcer may suggest a fungal etiology. An elevated firm slough and hyphate margins are found in more than 50% of culture-proven cases (388). The most common manifestations of culture-proven mycotic keratitis were reported to be (in descending order of occurrence) a gray or dirty-white surface, anterior-chamber cellular reaction, irregular feathery margins, elevated borders, dry rough texture, satellite lesions, Descemet's folds, hypopyon, ring infiltrate, endothelial plaque, and keratitic precipitates (334). However, a comparison of the most frequently occurring manifestations of bacterial and mycotic keratitis is needed.
FIG. 11.
Clinical keratitis due to Fusarium solani. The necrotic slough is elevated above the surface of the cornea. Hyphate lines are seen extending into the surrounding cornea.
Although most cases of mycotic keratitis exhibit these basic features, there may be other unique features, depending on the etiological agent. Thus, F. solani is able to completely destroy an eye in a few weeks, since the infection is usually severe and perforation, deep extension, and malignant glaucoma may supervene (408). In keratitis due to certain dematiaceous hyphomycetes (Curvularia spp., Bipolaris spp., or Exserohilum spp.), a persistent, low-grade, smoldering keratitis, with minimal structural alteration, may occur; the necrotic slough may be pigmented (Fig. 12), and simple debridement may suffice for resolution of all lesions (111, 173). However, if such dematiaceous fungal ulcers are not treated properly or if topical corticosteroids are used, the ulcers may spread to involve the deeper corneal layers, with intraocular extension or descemetocele formation supervening (366); Lecytophora verrucosa and L. mutabilis may cause a severe keratitis that is unresponsive to medical therapy (111; R. H. T. Ho, P. J. Bernard, and K. A. McClellan, Letter, Am. J. Ophthalmol. 112:728-729, 1991). L. theobromae causes a very severe type of keratitis clinically; in a study in India, severe keratitis was observed in 82% of cases of L. theobromae keratitis, 59% of cases of Aspergillus keratitis, and 53% of cases of Fusarium keratitis (389). Keratitis due to S. apiospermum (P. boydii) resembles other types of keratitis in predisposing factors and clinical features (211, 437) but may not respond satisfactorily to medical therapy alone. Most cases of keratitis due to Acremonium spp. have occurred following trauma, surgery, or application of topical steroids (93, 129, 317). P. insidiosum causes a very severe form of keratitis in Thailand, which is unresponsive to medical therapy (155, 156). Chronic, severe, filamentous fungal keratitis may resemble bacterial suppuration and may involve the entire cornea (194).
FIG. 12.
Clinical keratitis due to a Curvularia species. The necrotic slough exhibits a brownish pigmentation. Such pigmentation may be seen in corneal lesions due to dematiaceous filamentous fungi and should not be mistaken for an incarcerated iris.
The stromal keratitis caused by C. albicans and related fungi resembles bacterial keratitis, with an overlying epithelial defect, a more discrete infiltrate, and slow progression; such ulcers frequently occur in eyes with preexisting corneal disease and in areas of exposure, such as inferocentrally, at the junction of the superior two-thirds and inferior one-third of the cornea (271).
Keratitis due to a zygomycete such as Rhizopus sp. (334) or A. corymbifera (231) occurs very rarely; when it does occur, it is very fulminant and unresponsive to medical therapy. The progression of lesions was so rapid in one patient with keratitis due to A. corymbifera that penetrating keratoplasty was required within 9 days of the initial presentation; antecedent ocular trauma was the sole risk factor for the keratitis in this patient (231). Rodrigues and Laibson (332) described two patients with apparently primary exogenous keratitis due to B. dermatitidis.
(ii) Noninvasive techniques.
Confocal microscopy is an imaging technique that allows optical sectioning of almost any material, with increased axial and lateral spatial resolution and better image contrast, which may be useful for the identification of corneal pathogens in the early stages of infection. In clinical keratitis due to Aspergillus spp., fungal hyphae were imaged as high-contrast filaments, 60 to 400 μm long, and 6 μm wide (426). In one patient with keratitis due to F. solani (97), in vivo scanning slit confocal microscopy helped in first establishing the diagnosis, then demonstrating nonresponsiveness to medical therapy by showing an increased load of fungal filaments, and finally confirming that the entire fungal load was eradicated following penetrating keratoplasty, aiding the decision to administer corticosteroids and to quickly discontinue antifungals. Subsequently, there have been reports of the use of this technique, in conjunction with culture, in establishing a diagnosis of mycotic keratitis (408, 431). Thus, confocal microscopy is a potentially useful, noninvasive technique to determine the presence of fungal hyphae in vivo within the human cornea. Limitations in the use of this technique for routine diagnosis relate to instrument configuration, movement of either the tissue or the microscope, difficulty in reproducibly returning to the area of interest for serial examination, lack of a distinctive morphology of some pathogens, and limited resolution of the microscope.
(iii) Microbiological investigations.
Although there is some controversy regarding the need to perform microbiological investigations on all patients presenting with suspected microbial keratitis, it appears that such investigations are essential in the diagnosis of suspected mycotic keratitis (242). The specimens to be collected from a patient with suspected mycotic keratitis have been briefly described in Table 6. Prior to performing a corneal scraping, specimens for lid and conjunctival cultures are usually taken to ensure that the organisms isolated on the corneal media have not come from the transient commensal fungal flora of the conjunctival sac.
(a) Samples. Corneal scrapings are obtained by using an instrument (platinum spatula, Beaver blade, Bard Parker knife no. 15, or blunt cataract knife) to debride material from the base and edges of the ulcerated part of the cornea (3); this should be done several times to obtain as much material as possible. The blade or spatula may be reused if a sterile medium has been streaked but must be changed (the spatula can be flamed) if the instrument has made contact with an unsterile slide (3). Cotton swabs do not seem to be a useful means of debriding the necrotic corneal slough. However, if calcium alginate swabs, premoistened with tryptone soy broth, are used for the debridement, recovery of fungi in culture may be facilitated (163).
Corneal scrapings do not yield positive results in a small percentage of patients. In this case, corneal biopsy may aid the diagnosis since a larger amount of tissue can be obtained from a greater depth of the cornea (158, 196, 210). A corneal biopsy can be performed by using a corneal trephine which defines the precise diameter and depth (0.2 to 0.3 mm) of corneal tissue that is to be removed (196, 210). A second method involves the free dissection of the corneal lamellae by a sharp surgical knife; corneal perforation needs to be carefully guarded against in this procedure. Another method involves removal of the epithelium and necrotic debris overlying the suppurated area and then incising the corneal stroma with a Bard-Parker no. 15 blade and corneal forceps to about one-half the corneal thickness (160).
Several experimental (160) and clinical (42, 158, 196, 334) studies have highlighted the potential value of corneal biopsy samples in diagnosis of mycotic keratitis when the conventional corneal scrapes do not yield positive results. The biopsies may be relatively superficial (the procedure of keratectomy) or deep (42), and the tissue obtained may be stained with ink-KOH (158, 160) or lactophenol cotton blue (196). However, some workers (210) have reported inferior results in samples from patients with clinically evident infectious ulcerative keratitis.
(b) Direct microscopic examination. Direct microscopic examination of corneal scrapes or corneal biopsy samples permits a rapid presumptive diagnosis of mycotic keratitis to be established. Examination of a wet preparation (using KOH, ink-KOH, or lactophenol cotton blue), a smear stained by the Gram or Giemsa method, and a smear stained with special fungal stains (GMS silver, PAS, or calcofluor white) may yield valuable results. The corneal material should be spread out as thinly as possible on the microscope slides to facilitate easy visualization of the fungal structures (Fig. 9). The advantages and disadvantages of different staining techniques have already been described (Table 7). In major studies of mycotic keratitis (Table 15), the sensitivities of different staining techniques for culture-proven mycotic keratitis were 72.2% (431) to 91% (120) for KOH, 31.6% (288) to 98% (85) for the Gram-stained smears, 27% (334) to 85% (120) for Giemsa-stained smears, 91.4% for calcofluor white (120), 91% for PAS (431), and 56% for GMS (398).
In other studies, direct microscopic examination of corneal scrapes stained with lactophenol cotton blue yielded positive results in 78% of culture-proven cases of mycotic keratitis (387); Acanthamoeba cysts can also be detected in corneal scrapes stained with lactophenol cotton blue (P. A. Thomas and T. Kuriakose, Letter, Arch. Ophthalmol. 108:168, 1990). Examination of corneal scrapings from clinically suspected cases of mycotic keratitis yielded positive results in 76% of acridine orange-stained smears and in 65% of KOH wet mounts (179). Thus, microscopic examination of corneal material is an important means of arriving at a rapid presumptive diagnosis of mycotic keratitis and correlates well with culture positivity.
Excellent results were reported when the nonspecific fluorescent stain calcofluor white was used to stain corneal scrapes or biopsy specimens prior to direct microscopic examination (120, 55, 351, 372). However, not all fungi are adequately stained (314). The use of blankophor or Uvitex 2B may yield better results (314). Fungal autofluorescence and fluorescein-conjugated lectins have yielded promising results in some studies (229, 330), but these techniques need to be applied on a larger scale before conclusions can be drawn.
It is generally reported that the identity of the infecting fungus cannot be deduced from the direct microscopic examination, particularly where the infecting fungi closely resemble each other morphologically, as in Fusarium, Paecilomyces, and Acremonium; however, Liu et al. (220) studied adventitious sporulation in tissue samples, including some from corneal ulcers and found that this might serve as an aid to identify the possible genus involved. This requires further study.
(c) Culture. Culture of corneal scrapes or biopsy specimens is essential to confirm a diagnosis of mycotic keratitis and to initiate appropriate antifungal therapy. Corneal material is inoculated on the surface of solid media by making rows of “C” streaks (two rows from each scraping); only growth on the C streaks (Fig. 8) is deemed significant (175). Liquid media are inoculated by twirling the tip of the spatula, loop, or swab in the broth several times.
The media commonly used for recovery of corneal fungi are as described above (see “Etiological agents and laboratory diagnosis of ophthalmic mycoses”). Blood agar plates should be incubated at 25 and 37°C, while Sabouraud glucose-neopeptone agar is kept at 25°C. Liquid media should be included; brain heart infusion broth is perhaps the best single medium to use, especially when corneal material is scanty. An incubation temperature of 30°C and the use of liquid-shake cultures may also aid the recovery of corneal fungi.
Fungal growth on the culture media (Fig. 4 and 8) usually occurs within 3 to 4 days (334), but culture media may need to be kept for up to 4 to 6 weeks. “Sham cultures” should also be maintained to ensure that there is no contamination from the environment or media during sample collection. The criteria used to consider a fungal strain isolated in culture as significant are described in the Introduction.
(d) Histopathology. Histopathological studies offer certain advantages over culture in the diagnosis of mycotic keratitis since contamination is avoided, tissue penetration can be gauged, and the outcome of surgical procedures can be anticipated (406). In some studies (160, 334), direct examination of corneal biopsy specimens or corneal buttons was found to yield positive results when cultures of the same samples were negative, both in experimental animals and in patients; however, other investigators (8) are of the opinion that microbiological evaluation of the corneal biopsy specimen is more sensitive than histopathological examination as a diagnostic aid in microbial keratitis. Material for histopathological testing is obtained as a corneal biopsy (8, 196, 210) or button following penetrating keratoplasty(334). Fungal structures in corneal tissue can be stained by the PAS and GMS techniques, but fluorochromes such as calcofluor white and fluorescein-conjugated lectins can also be used (3, 57). The purulent inflammatory cellular reaction is usually less marked in fungal than in bacterial keratitis; filamentous fungi are usually found deep in, and arranged parallel to, the corneal stromal lamellae while being absent on the surface (Fig. 13). The inflammatory cells seen are usually lymphocytes and plasma cells, but polymorphonuclear leukocytes are also involved to various extents (3). There is coagulative necrosis of the stroma, resulting in stromal abscesses; “satellite” microabscesses may also be seen, with focal necrosis of the corneal stroma and clusters of acute inflammatory cells (3). At this stage, healing of the epithelium is seen with a coexisting active proliferation of the fungus in the deeper stroma; therefore, corneal scrapings may fail to demonstrate fungal structures. This may explain the superior results obtained by some workers when culturing corneal biopsy specimens compared to those obtained from culturing corneal scrapings (8, 42, 158, 196, 334). Invasion and penetration of an apparently intact Descement's membrane may occur rarely, perhaps when F. solani or Fusarium spp. are the infecting organisms (173, 204).
FIG. 13.
Photomicrograph of a tissue section from a corneal button removed at the time of keratoplasty. Fungal hyphae are seen below the epithelium (E), in the superficial and middle layers of the corneal stroma. The hyphae are oriented mostly in a direction parallel to that of the corneal collagen bundles, although the hyphae in the superficial stroma are oriented vertically (arrows). GMS stain; magnification, ×100.
Patients are usually reluctant to undergo even minor surgical procedures. Moreover, conventional techniques of debriding corneal ulcers require great dexterity and magnification to stay within the confines of the lesion and to avoid perforation if imminent; the material obtained is sometimes difficult to spread on slides, and the cells seen may be distorted or crushed with loss of spatial relationships (18). An impression debridement technique, using filter paper, has been described that overcomes some of these drawbacks (18). In this technique, a cellulose acetate filter paper (of the type used for conjunctival impression cytology) is applied to the ulcerated part of the cornea and gentle pressure applied; the filter paper with debrided corneal tissue sticking to it is then removed and stained. Since there is no danger of damage to the corneal tissue, both the ophthalmologist and the patient feel comfortable. Repeat debridements can be done in grossly infected corneal ulcers to reduce the load of organisms and to provide material for repeat cultures. In the study reported (18), a diagnosis of mycotic keratitis could be made in 10 patients based on the presence of fungal hyphae in the material obtained by impression debridement; however, no information was provided about whether these observations coincided with the clinical picture, the results of other investigations, or the outcome of antifungal therapy. A drawback of this technique is that it would be suitable only for superficial corneal lesions and would not be applicable to the debridement of deep intrastromal mycotic keratitis or deeply embedded corneal foreign bodies.
Management.
Mycotic keratitis is managed by medical or surgical means. Medical therapy consists of nonspecific measures and the use of specific antifungal agents. Cycloplegics are used to relieve the iridocyclitis (anterior uveitis) that usually accompanies mycotic keratitis; broad-spectrum antibacterials may be needed to combat secondary bacterial infection (334, 429).
(i) Specific antifungal therapy.
Various specific antifungals have been tried in the therapy of experimental and clinical mycotic keratitis (see “Antifungal agents used to treat ophthalmic mycoses” above). Treatment may be protracted, since the effective concentrations achieved by most antifungals in the cornea, with the possible exception of amphotericin B, only inhibit the growth of the fungus, and host defense mechanisms must eradicate the organism (267, 366).
The antifungal ultimately selected as primary therapy necessarily depends on its easy availability and on other criteria. If direct microscopic examination of corneal scrapes or corneal biopsy specimens yields unequivocal results that are consistent with the clinical picture, treatment may be initiated; otherwise, therapy may need to be withheld until culture reports become available. Topical natamycin (5%) or amphotericin B (0.15%) is usually selected as first-line therapy for superficial keratitis, whether or not septate hyphae or yeast cells have been seen by direct microscopy; if deep lesions are present, oral ketoconazole, oral itraconazole or oral fluconazole may be added to the therapeutic regimen (334, 377, 429). If hyphae have been seen by microscopy and a filamentous fungus is isolated in culture, natamycin appears to be the treatment of choice when available (334, 377); topical 0.15% amphotericin B (170, 429) is an alternative. If yeasts or pseudohyphae are seen by microscopy and species of Candida or Cryptococcus are isolated in culture, topical 0.15% amphotericin B appears to be the treatment of choice when available (334, 377), although natamycin (287, 334) and topical 1% miconazole (101, 287) have also been used as primary therapy. It is difficult to assess the validity of these choices of therapy in the absence of controlled clinical trials, especially since the number of patients dealt with is generally small. Moreover, satisfactory responses of filamentous fungal keratitis to, for example, natamycin or of yeast keratitis to, for example, topical 0.15% amphotericin B may appear so commonplace that clinicians do not deem it necessary to report their observations and will publish reports only when something out of the ordinary is encountered. Keeping these limitations in mind, an attempt has been made in this article to review the therapy of keratitis due to frequently encountered hyaline filamentous (Fusarium spp., Aspergillus spp., and S. apiospermum), dematiaceous (Curvularia spp.), and yeast (Candida spp.) fungal pathogens based on reports published in the literature.
(a) Therapy of keratitis due to Fusarium spp. An analysis was made of 85 patients reported in the literature for whom details of outcome of therapy have been provided (Table 16). A total of 29 patients apparently had superficial keratitis; 22 (76%) of these responded to antifungals alone (topical amphotericin B alone or in combination with topical natamycin, oral and/or topical ketoconazole, and oral itraconazole). Seven patients with apparently superficial keratitis required surgery; interestingly, none of these had received natamycin at any time (Table 16). A total of 49 patients appeared to have keratitis with deep lesions; only 14 (29%) of these responded to antifungals alone. Overall, 6 of the 49 patients with apparently deep keratitis received topical natamycin at some time, and 4 of these responded to medical therapy alone; the other 43 patients did not receive natamycin at any time, and only 10 (23%) of these responded to medical therapy alone (Table 16). In seven patients with culture-proven keratitis due to Fusarium spp., the severity of the keratitis was not clearly described; three of the patients responded to antifungals alone. Thus, more than 70% of patients with superficial keratitis due to F. solani and other Fusarium spp. apparently respond to medical therapy alone; although several antifungals have been found effective, administration of natamycin may forestall surgical intervention. In striking contrast, almost 70% of patients with Fusarium keratitis with deep lesions do not respond to medical therapy alone, particularly if natamycin is not used, and some form of surgical intervention is necessary.
TABLE 16.
Treatment of keratitis due to Fusarium spp.
| No. of patients (reference) | Criteria for diagnosis of mycotic keratitisb (fungus isolated) | Treatmentb | Outcome | Comment |
|---|---|---|---|---|
| 1 (408) | Detection of fungal hyphae by confocal microscopy, HPE, and culture of corneal button (F. solani) | No response to econazole, oral fluconazole, topical amphotericin B, natamycin,d oral itraconazole, oral voriconazole; PKP done 3 times | Ultimately healed; graft rejection prevented by cyclosporin | Keratitis after LASIKc Infection eradicated apparently after repeated PKP |
| 1 (322) | Immunohistochemical staining and HPE of corneal button (F. solani) | No response to antibacterials, PKP, intravenous fluconazole, topical amphotericin B, oral itraconazole | Ultimately responded to 8 wk of voriconazole (intravenous, oral, topical, intracameral) | Response to voriconazole may have been aided by prior PKP and other antifungals |
| 1 Sponsel et al., lettera | HPE of corneal button and lens, culture of repeated corneal scrapings and aqueous aspirate (F. solani and bacteria) | No response to amphotericin B (topical, intravenous), natamycin, oral ketoconazole | Most lesions cleared after administration of posaconazole (oral, topical) and PKP | Keratitis progressed to end ophthalmitis; apparently good response to posaconazole and surgery (ocular penetration of posaconazole confirmed) |
| 1 (62) | Culture of corneal scrapes (Fusarium spp.) | Partial response to natamycin and topical antibacterials; topical amphotericin B added | Resolved with intensive double antifungal therapy | Not clear whether deep lesions were pr |
| 1 (99) | Direct microscopy and culture of corneal scrapes (F. solani) | No response to natamycin, topical amphotericin B, oral azoles; cornea perforated, necessitating PKP | Resolved after PKP | Poor response to medical therapy possibly due to contact lens wear, viral keratitis, and deep corneal lesions |
| 1 (116) | Fungal hyphae detected by confocal microscopy, culture of corneal button (F. solani) | No response to antibacterials, antivirals, corticosteroids, topical amphotericin B; PKP done; endophthalmitis ensued in spite of postsurgery antifungals (natamycin, amphotericin B, ketoconazole) | Ultimately healed with ABLC (8.8 g) given over 45 days | Contact lens wearer; reason for nonresponsiveness to many antifungals not known |
| 6 (377) | Positive microscopy confirmed by culture; criteria for significant growth in culture unclear (Fusarium spp.) | Superficial keratitis (1 patient): natamycin, topical amphotericin B Keratitis with deep lesions (5 patients) Natamycin and oral ketoconazole in 2 patients Natamycin and oral itraconazole in 1 patient Natamycin, topical and oral azoles, and PKP in 2 patients | Keratitis resolved Keratitis resolved Keratitis resolved Keratitis progressed in spite of antifungals and PKP | Criteria for grading severity of keratitis not clear; nonrandomized, noncomparative case series |
| 1 (417) | HPE of corneal button, culture of vitreous (F. solani) | No response to various antifungals; PKP done | Lesions resolved after PKP; no recurrence (3 mo) | Keratitis developed following endophthalmitis |
| 1 (148) | Culture of corneal scrapes (F. solani) | No response to fluconazole, miconazole, flucytosine, natamycin | Resolved with topical 2% amphotericin B ointment | Good response to topical ointment possibly because keratitis was superficial |
| 3 (428) | DM, culture of corneal scrapes (Fusarium spp. isolated from the scrapes of all three patients) | Partial response to topical amphotericin B and natamycin; PKP done No response to topical amphotericin B No response to subconjunctival and topical miconazole, topical amphotericin B, oral ketoconazole | Graft clear, 14 mo postoperative Evisceration Evisceration | Prior use of topical corticosteroids may have contributed to poor outcome in patients with no response |
| 1 Gussler et al., letter | HPE and culture of corneal button (Fusarium spp. and Acanthamoeba) | PKP | Graft clear, no recurrence after 16 months | Keratitis after radial keratotomy; no mention of medical therapy |
| 79 (334) | DM, culture of corneal scrapes; growth in ≥2 culture media, growth in culture on ≥2 occasions (F. oxysporum, F. solani) | For superficial keratitis, natamycin; for keratitis with deep lesions, natamycin and oral ketoconazole; 6 of 79 ulcers perforated | Response to medical therapy alone unclear; 22 (28%) of 79 ulcers needed PKP; 1 of 79 eyes enucleated | Outcome of medical therapy unclear; PKP needed in 28% due to nonresponsiveness to medical therapy |
| 19 (385) | Suggestive clinical features, DM, growth of fungi from corneal scrapes in ≥2 media (F. solani in all 19 patients) | Oral itraconazole 200 mg once daily; 8 patients responded (6 with superficial keratitis), no response in 11 (5 with superficial keratitis, 6 with deep lesions) | Lesions resolved with itraconazole in 8 patients; 11 nonresponders needed PKP | Nonrandomized, noncomparative case series; natamycin not available at time of study |
| 2 (396) | Suggestive clinical features, DM, growth of fungi from corneal scrapes in ≥2 media (F. solani in both) | Topical 2% ketoconazole | Lesions completely resolved with topical ketoconazole alone | Both ulcers relatively superficial; this probably aided the response |
| No. of patients (reference) | Criteria for diagnosis of mycotic keratitisb (fungus isolated) | Treatmentb | Outcome | Comment |
| 30 (309) | Suggestive clinical features, DM, growth of fungi from corneal scrapes in ≥2 media (Fusarium spp.) | Oral ketoconazole 600 mg/day (6 patients) Oral ketoconazole plus topical 1% ketoconazole (24 patients); response in 10 (6 with superficial lesions, 4 with deep lesions) | Resolved in 2 patients Resolved in 10 patients Overall PKP done for 18 of 30 patients | Nonrandomized, noncomparative case series; natamycin not available at time of study |
| 18 (385) | Suggestive clinical features, DM, growth in ≥2 media (Fusarium spp.) | Topical 0.15% amphotericin B hourly; response in 8 of 18 patients (4 with deep lesions), no response in 10 patients (8 with deep lesions) | Lesions resolved in 8 patients; PKP done in 10 of 18 patients | Nonrandomized, noncomparative case series done before availability of natamycin |
W. E. Sponsel, J. R. Graybill, H. L. Nevarez, and D. Darg, Letter, Br. J. Ophthalmol. 86:829-830, 2002.
HPE, fungal hyphae seen by histopathological examination; DM, fungal hyphae seen in microscopy of corneal scrapes; PKP, penetrating keratoplasty; ABLC, amphotericin B-lipid complex.
LASIK, laser-assisted in situ keratomileusis.
Topical 5% natamycin.
In the series of Rosa et al. (334) in Miami, Fl., 79 patients were reported to have had keratitis due to Fusarium spp. Patients with presumably superficial keratitis received topical natamycin alone, while those with presumably deep lesions received topical natamycin and systemic antifungals; the average duration of treatment was 38 days. Details of the response to therapy were not provided, but 22 (28%) of these patients ultimately required penetrating keratoplasty; enucleation had to be done in 1patient. Although it is tempting to speculate that all the patients who did not require surgery ultimately responded to therapy with natamycin (with or without systemic antifungals), such speculation without support from concrete follow-up data may lead to serious misinterpretations.
(b) Therapy of keratitis due to Aspergillus spp. The data pertaining to the outcome of therapy have been analyzed for 61 patients (Table 17). A total of 17 patients had apparently superficial keratitis, of whom 15 (88%) responded to antifungals alone (oral itraconazole [6 patients], combined oral and topical ketoconazole [five patients], topical 2% ketoconazole [2 patients], and topical natamycin [2 patients]); the patients who responded to azole therapy had not received natamycin.
TABLE 17.
Treatment of keratitis due to Aspergillus spp.
| No. of patients (reference) | Criteria for diagnosis of mycotic keratitis (fungus isolated)a | Treatment | Outcome | Comments |
|---|---|---|---|---|
| 2 (396) | 1. Suggestive clinical features 2. DM, growth in ≥2 culture media (A. fumigatus, A. flavus) | Topical 2% ketoconazole | Keratitis resolved in both patients after 17 days therapy | Both patients apparently had only superficial keratitis |
| 22 (309) | 1. Suggestive clinical features 2. DM, growth in ≥2 culture media (A. fumigatus in 7, A. flavus in 11, Aspergillus spp. in 4) | Oral ketoconazole (600 mg/day) in 10 patients Oral ketoconazole and topical 1% ketoconazole in 12 patients Response to therapy in 7 patients (5 with superficial keratitis, 2 with deep lesions); no response in 5 (all had deep lesions) | Lesions resolved in 5 of 10 patients (3 of 8 with A. flavus, 2 of 2 with A. fumigatus). Lesions resolved in 7 of 12 patients (1 of 3 with A. flavus, 3 of 5 with A. fumigatus, 3 of 4 with Aspergillus spp.) Overall, PKP necessary in 10 patients (7 of 11 with A. flavus, 2 of 7 with A. fumigatus, 1 of 4 with Aspergillus spp.) | Nonrandomised, noncomparative case series; ketoconazole used due to nonavailability of natamycin at the time of study |
| 11 (385) | 1. Suggestive clinical features 2. DM, growth in ≥2 culture media (A. flavus, A. fumigatus) | Topical 0.15% amphotericin B | Lesions resolved in 3 patients (all 3 had keratitis with deep lesions); no response in 8 patients (2 with superficial keratitis, 6 with deep lesions); PKP done | Nonrandomized, noncomparative case series; amphotericin B used due to nonavailability of natamycin at the time of study |
| 15 (390) | 1. Suggestive clinical features 2. DM, growth in ≥2 culture media (A. flavus in 10, A. fumigatus in 5) | Oral itraconazole (200 mg/day); response in 10 patients (9 with A. flavus keratitis, 1 with A. fumigatus keratitis) | Lesions resolved in 10 patients (6 with superficial keratitis, 4 with deep lesions); No response in 5 patients (all with deep lesions); PKP done in all 5 patients | Nonrandomized, noncomparative case series; itraconazole used due to nonavailability of natamycin at the time of study |
| 1 (68) | HPE, culture (A. fischerianus) | No response to oral ketoconazole | Evisceration | |
| 1 (142) | HPE, culture (A. fumigatus) | No response to topical amphotericin B and oral itraconazole; PKP done | Lesions resolved after PKP | Keratitis following radial keratotomy |
| 1 (148) | 1. Suggestive clinical features 2. DM, culture (A. fumigatus) | No response to fluconazole (systemic, topical), miconazole, flucytosine, natamycin | Lesions resolved after amphotericin B treatment (topical 2% ointment, intravenous) | No mention of severity of keratitis |
| 1 (134) | DM, culture (A. fumigatus) | Natamycin | Lesions resolved | No mention of severity of keratitis |
| 1 (361) | DM, culture (A. flavus) | No response to natamycin, oral ketoconazole; PKP done | Lesions resolved after PKP | Keratitis after LASIK |
| 1 (203) | HPE, culture of biopsy material (A. fumigatus) | No response to antibacterials; perforation after biopsy (sealed with glue and sutures); antifungals used | Infiltrate resolved after medical therapy; perforation required other measures | Keratitis after LASIK |
| 1 (362) | DM, culture (A. fumigatus) | Natamycin | Lesions resolved | Superficial keratitis, hence resolved |
| 1 (327) | Culture (A. fumigatus) | Natamycin, topical 0.1% amphotericin B | Lesions resolved | Polymicrobial keratitis after LASIK |
| 3 (183) | DM and culture of corneal scrapes and aqueous aspirates (A. flavus from all 3 patients) | Partial response to initial natamycin, topical amphotericin B, and oral itraconazole in all 3 patients | Lesions completely resolved in all 3 patients after intracameral administration of amphotericin B (7.5 μg/0.1 ml and 10 μg/0.1 ml) | Partial response of corneal infiltrate to topical and oral antifungals; complete resolution of hypopyon probably aided by removal of aqueous prior to intracameral injection of amphotericin B |
DM, fungal hyphae seen in microscopy of corneal scrapes; culture, fungi grown in culture from corneal scrapes; HPE, fungal hyphae seen by histopathological examination of corneal biopsy specimen or button; PKP, penetrating keratoplasty; LASIK, laser-assisted in situ keratomileusis.
Twenty-nine patients apparently had keratitis with deep lesions; 12 (41%) of these responded to antifungals alone, while surgery was required for the other 17, who did not respond to medical therapy. Of the 29 patients, 4 had received topical natamycin at some time, and 3 of these responded to medical therapy; 25 patients did not receive natamycin at any time, and 16 (64%) of these ultimately required surgery. Of 20 patients who received oral azoles, 12 (60%) ultimately required surgery, as did 6 (50%) of 12 patients who received topical amphotericin B.
In 15 patients, it was not clear whether deep lesions were present; 8 (53%) of these patients responded to medical therapy alone, including 2 of 3 patients who received natamycin. Of the 15 patients, 12 did not receive natamycin, and 7 (58%) of these ultimately required surgical intervention.
These data suggest that more than 80% of patients with superficial keratitis due to A. flavus, A. fumigatus, and other Aspergillus spp. respond to medical therapy with a variety of topical or systemic antifungals, with surgery not being required. However, in the presence of deep corneal lesions, almost 60% of patients do not respond to medical therapy alone, particularly if natamycin is not used, and surgery is required to control the infection.
(c) Therapy of keratitis due to Candida spp. Details of the response to therapy of Candida spp. have been analyzed for 38 patients (Table 18). Four patients appeared to have had superficial keratitis, which resolved after administration of topical amphotericin B alone (three patients) and combined topical amphotericin B and natamycin therapy (one patient). For an additional 12 patients, it was not clear whether deep lesions were present; the corneal lesions resolved in all 12, with 7 responding to topical amphotericin B alone, 4 responding to topical natamycin alone, and 1 (who had chronic granulomatous disease) apparently responding to the intravenous amphotericin B administered for coexisting systemic candidiasis. Keratitis with deep lesions appears to have been present in 22 patients, and the corneal lesions resolved in 18 (82%) by using medical therapy; 5 responded to topical amphotericin B alone, 7 responded to combined topical amphotericin B and systemic azoles, and 6 (who had not responded to natamycin or topical miconazole) responded to topical 2% fluconazole (the source of the drug was not mentioned in this study).
TABLE 18.
Treatment of keratitis due to Candida spp.
| No. of patients (reference) | Criteria for diagnosis of mycotic keratitis (fungus isolated)a | Treatment | Outcome | Comments |
|---|---|---|---|---|
| 11 (377) | Culture-proven fungal keratitis; positive microscopy confirmed by culture of corneal scrapes (C. albicans isolated from the scrapes of all 11 patients) | Apparently nonsevere keratitis in 4 patients Topical amphotericin Bb only in 3 patients Topical amphotericin B and natamycinc in 1 patient Apparently severe keratitis in 7 patients Topical amphotericin B and oral itraconazole, fluconazole, or ketoconazole in 4 patients Topical amphotericin B and oral fluconazole in 2 patients Topical amphotericin B, oral and topical fluconazole in 1 patient | Keratitis resolved in all 3 patients Keratitis resolved Keratitis resolved in all 4 patients Keratitis resolved in both patients Poor response in spite of antifungals and penetrating keratoplasty | Severity of keratitis not clearly defined |
| 1 (219) | DM, culture of corneal scrapes (Candida spp.) | Topical amphotericin B, fluconazole (topical, intravenous), penetrating keratoplasty, anterior vitrectomy | Keratitis resolved | Severe keratitis |
| 6 (287) | DM, culture of corneal scrapes (C. albicans isolated from the scrapes of all 6 patients) | Topical 2% fluconazole (there had been no response to natamycin in 3 patients or to topical miconazole in 3 patients) | Keratitis resolved in all 6 patients (mean 22.6 ± 2.3 days of treatment with fluconazole), all patients had keratitis with deep lesions | No details of source of 2% solution or why this was used instead of commercially available 0.2% solution |
| 16 (334) | Corneal scrapes: fungal elements in smear and growth in culture or growth on ≥2 media (C. parapsilosis in 11, C. albicans in 4, C. tropicalis in 1) | Natamycin in 4 patients Topical amphotericin B in 7 patients | All 4 healed All 7 healed Outcome data only for 11 patients | No details on severity of keratitis |
| 2 (419) | DM, culture of corneal scrapes (C. albicans) | Topical amphotericin B and oral ketoconazole | Responded, but regraft needed | Both infectious crystalline keratopathy |
| Topical amphotericin B and oral ketoconazole | Resolved | |||
| 1 (80) | Culture of corneal scrapes (C. glabrata) | Intravenous amphotericin B for candidiasis of liver associated with chronic granulomatous disease | Ocular lesions resolved with the intravenous therapy alone | Severity of ocular lesion not clear |
| 1 (4) | HPE and culture of lamellar biopsy specimen (C. guilliermondii) | Partial response to topical amphotericin B, oral and topical fluconazole | Resolved after penetrating keratoplasty | |
| 5 (144) | Culture of corneal scrapes (C. albicans recovered from the scrapes of all 5 patients) | Topical amphotericin B | Lesions resolved in all 5 patients | All patients had AIDS; keratitis reported to be severe, but no details given |
DM, fungal structures in microscopy of corneal scrapes; HPE, fungal structures in histopathological examination of biopsy tissue.
Topical 0.1 to 0.5% solution of amphotericin B.
Topical 5% natamycin suspension.
To summarize, overall, 34 of 38 patients with keratitis due to C. albicans and other Candida spp. responded to antifungals alone; 15 patients responded to topical amphotericin B alone, 1 responded to intravenous amphotericin B alone, 8 responded to topical amphotericin B in combination with natamycin or systemic azoles, 6 responded to topical fluconazole alone (although the medication used appears to have been 10-fold more concentrated than the commercially available topical fluconazole formulation), and 4 responded to topical natamycin alone. It therefore appears that the medical therapy of keratitis due to Candida spp. generally has a favorable prognosis, particularly when topical amphotericin B is used alone or in combination with systemic azoles, and the presence of deep lesions is not a major hurdle.
(d) Therapy of keratitis due to Curvularia spp. Data pertaining to 42 patients reported in the literature have been analyzed (Table 19). In 35 (83%) of the 42 individuals, the corneal lesions responded to antifungals alone; 19 patients responded to topical natamycin alone, another 8 responded to natamycin and other antifungals, 6 responded to oral ketoconazole, and 1 each responded to topical miconazole and topical amphotericin B. In an additional three patients, the keratitis resolved with keratectomy and antifungal therapy. Penetrating keratoplasty was required in four patients who did not respond to medical therapy alone. These data suggest that most patients with keratitis due to species of Curvularia can be treated by antifungals alone, particularly when natamycin is used. However, most of the papers analyzed did not provide details about the severity of the corneal lesions in the patients. This is an important aspect that needs to be studied. In one study of dematiaceous fungal keratitis (111), antifungal therapy alone (principally natamycin, alone or in combination with topical clotrimazole or topical miconazole) sufficed for resolution of lesions in 88% of patients with superficial lesions; however, only 46% of patients with deep keratitis responded to antifungal therapy alone (topical antifungals combined with oral ketoconazole), and surgery was required for the other patients (111) (Table 19).
TABLE 19.
Treatment of keratitis due to Curvularia spp.
| No. of patients (reference) | Criteria for diagnosis of mycotic infection (species isolated)a | Treatment | Outcome | Comments |
|---|---|---|---|---|
| 32 (418) | DM, growth on ≥1 medium (C. senegalensis in 11, C. lunata in 8, C. pallescens in 4, C. lunata var. aeria in 2, C. prasadii in 2, Curvularia spp. in 5) | Natamycinb only (21-60 days) in 17 patients Natamycin and other antifungals (topical miconazole,c oral ketoconazole, oral fluconazole, topical amphotericin B) in 7 patients Topical miconazole only in 1 patient Natamycin, other antifungals, and keratectomy in 1 patient Natamycin and keratectomy in 2 patients N atamycin and PKP, with or without other antifungals, in 3 patients | 16 healed, 1 unknown outcome All 7 healed Healed Healed Both healed All 3 healed | Presence of hypopyon at initial presentation influenced outcome; where the delay in diagnosis was >7 days, duration of antifungal therapy was almost twice that where the delay was ≤7 days; No correlation provided between severity of keratitis at presentation and outcome; retrospective review of case records from 1970 to 1999 |
| 86 (111) | DM, growth on ≥1 medium (Curvularia spp. in 20, Bipolaris spp. and Exserohilum spp. in 13, L. theobromae in 3) | Natamycin only in 32 patients, natamycin and other topical antifungals (clotrimazole, miconazole) in 16 patients, topical antifungals, oral ketoconazole in 38 patients | Superficial keratitis (53 patients): Healed in 37 (88%), PKP in 3 (7.1%), evisceration in 2 (4.8%), unknown in 11 Deep keratitis (33 patients): healed in 12 (46.1%), PKP in 10 (38.4), evisceration in 4 (15.4%), Unknown in 7 | Outcome data not categorized according to etiological agents (fungal genera) or antifungals used; retrospective, nonrandomized, noncomparative case series |
| 1 (63) | DM, culture (Curvularia spp.) | Natamycin, topical amphotericin Bd alternately | Healed | Only known case of post-LASIK keratitis cured by medical therapy only |
| 1 (130) | DM, repeated culture (C. senegalensis) | No response to natamycin, PKP done; infection recurred; no response to oral itraconazole. | Unknown (patient awaiting repeat PKP at time of publication) | Apparently severe keratitis |
| 1 (65) | DM, culture (Curvularia sp.) | Natamycin | Healed | Follow-up inadequate |
| 1 (65) | Culture only (Curvularia spp.) | Natamycin | Healed | Doubtful because hyphae not seen in scrapes or biopsy specimens |
| 1 (228) | DM, growth in multiple media (C. brachyspora) | Topical amphotericin B | Healed | Superficial keratitis |
| 6 (309) | Suggestive clinical features, DM, growth in ≥1 medium (Curvularia spp.) | Oral ketoconazole (600 mg/day) in 3 patients Topical 1% ketoconazole suspension hourly in 2 patients Oral and topical ketoconazole in 1 patient | All 3 healed Both healed Healed | All patients had superficial keratitis; nonrandomized, noncomparative case series; ketoconazole used due to nonavailability of natamycin at the time of study |
| 5 (310) | Suggestive clinical features, DM, growth in ≥1 medium (C. geniculata in 1, C. ovoides in 1, Curvularia spp. in 3) | Oral itraconazole (200 mg/day) in 1 patient Oral plus hourly topical 1% itraconazole suspension in 4 patients | Healed 3 healed (2 with superficial keratitis, 1 with deep keratitis), no response in 1 (with deep keratitis) | Good response in patients with superficial keratitis. Nonrandomized, noncomparative case series; itraconazole used due to nonavailability of natamycin at the time of study |
DM, fungal hyphae seen in microscopy of corneal scrapes.
Topical 5% natamycin.
Topical 1% miconazole.
Topical 0.15% amphotericin B.
Keratitis due to dematiaceous fungi other than Curvularia spp. appears to respond to primary therapy with topical natamycin, oral and/or topical ketoconazole, oral ketoconazole with topical miconazole, topical amphotericin B, or oral itraconazole (111, 334, 390, 391, 429). However, therapy of keratitis due to L. theobromae is often difficult. A successful outcome of this type of dematiaceous fungal keratitis was reported for patients receiving natamycin ointment and topical or subconjunctival amphotericin B (318). Intravenous miconazole was reported to be useful, but this was based on the study of a single patient (Y. Ishifashi and Y. Matsumoto, letter). Poor results have been reported for patients receiving azoles (37, 389, 392).
(e) Therapy of keratitis due to S. apiospermum. The outcome of keratitis due to S. apiospermum is varied. A review of 13 cases reported up to 1979 revealed a generally poor outcome, with 6 of 13 patients eventually requiring enucleation or evisceration (437). Moreover, miconazole is thought to be an important drug in treatment of keratitis due to S. apiospermum in humans; a recent review of 15 patients with this condition (430) appears to endorse this view (only reports in which details of treatment regimen and visual outcome were provided were included in the review, and patients with initial scleral involvement were excluded). Four (67%) of six individuals who had received miconazole retained form vision (counting fingers or better), whereas three (33%) of nine persons who had not received miconazole retained form vision (430). However, it is difficult to draw conclusions based on the small number of patients studied; moreover, the severity of the keratitis at presentation could be an important determinant of outcome of medical therapy.
At least 14 patients with keratitis due to S. apiospermum have been reported in the literature since 1991 (Table 20). Medical therapy alone sufficed for resolution of lesions in 8 (57%) of these 14 patients (3 of the “responders” had keratitis with deep lesions); penetrating keratoplasty was needed in 3 patients (all of whom had deep keratitis), and evisceration or enucleation was needed for 3 patients (2 of whom had deep corneal lesions). Eight patients (five with deep keratitis and three with keratitis of undetermined severity) received natamycin at some time; the corneal lesions resolved with medical therapy alone in three of these, while penetrating keratoplasty was required in the eyes of three patients and enucleation had to be done for two patients. Six individuals (two with superficial keratitis, three with deep keratitis, and one with keratitis of unknown severity) received miconazole; the lesions of four of these patients resolved with medical therapy alone (two had superficial keratitis), while evisceration or enucleation was needed for two eyes (both with deep keratitis).
TABLE 20.
Treatment of keratitis due to Scedosporium apiospermuma
| Keratitis with deep lesions | Criteria for diagnosis of mycotic infectionb | Treatmentb | Outcome | Comments |
|---|---|---|---|---|
| No | CF, DM, culture of corneal scrapes | Topical miconazolec | Lesions resolved | Absence of deep lesions probably contributed to good outcome |
| No | CF, DM, culture of corneal scrapes | Topical miconazole (ointment) | Lesions resolved | Absence of deep lesions probably contributed to good outcome |
| Yes | HPE and culture of lamellar biopsy material; HPE and culture of corneal button and cornea of enucleated eye | No response to topical, subconjunctival, or intravenous miconazole, oral itraconazole, debridement, PKP | Evisceration | No growth of fungi from lens or vitreous |
| Not known | CF, DM, culture of corneal scrapes | Natamycind topical and subconjunctival miconazole, oral ketoconazole | Lesions resolved | Medical cure |
| Yes | CF, DM, culture of corneal scrapes, HPE of cornea and iris of enucleated eye | No response to natamycin, corneoscleral graft, subconjunctival miconazole | Enucleation | Panophthalmitis supervened |
| Yes | CF, DM, culture of aqueous aspirate | Oral ketoconazole, topical AB, intravenous miconazole | Lesions resolved | Medical cure of severe keratitis |
| Yes | CF, DM, culture of corneal scrapes | No response to natamycin, oral ketoconazole | PKP done | Eye saved by PKP |
| Not known | CF, DM, culture of corneal scrapes | Natamycin | Lesions resolved | No details about presence of deep lesions |
| Not known | CF, DM, culture of corneal scrapes | Natamycin, oral fluconazole | Lesions resolved | No details about presence of deep lesions |
| Yes | CF, DM, culture of corneal scrapes | No response to topical clotrimazole, oral ketoconazole, natamycin; PKP done | Lesions resolved after PKP | Coinfected with Acanthamoeba |
| Yes | DM, culture of corneal scrapes | Topical clotrimazole | Lesions resolved | Medical cure; poor vision |
| Yes | CF, DM, culture of corneal scrapes | Topical AB, oral fluconazole | Lesions resolved | Medical cure of severe keratitis |
| No | CF, DM, culture of corneal scrapes | No response to topical AB, oral fluconazole, lamellar KP, PKP | Enucleation | Poor outcome of keratitis without deep lesions |
| Yes | Culture of corneal scrapes | No response to topical AB, natamycin, oral itraconazole; PKP due to perforation | Lesions resolved after PKP | Eye saved |
Culture-proven cases reported since 1991 (modified from reference 430; also incorporates observations from references 34, 77, 79, 336, 360, and 377).
CF, clinical features suggestive of mycotic keratitis; DM, fungal hyphae seen on microscopy of scrapes; HPE, fungal hyphae seen on histopathological examination; CB, corneal button or biopsy specimen; Topical AB, topical 0.1 to 0.5% amphotericin B; PKP, penetrating keratoplasty; KP, keratoplasty.
Topical 1% miconazole.
Topical 5% natamycin.
It would appear that keratitis due to S. apiospermum more frequently has a favorable outcome currently than in the past, with evisceration or enucleation being the final result in 21% of patients recently (compared to 54% in patients reported upto 1979). However, the severity of keratitis at presentation is an important determinant of the ultimate outcome, with penetrating keratoplasy being required in addition to medical therapy. It is difficult to assess the relative efficacy of miconazole versus natamycin in view of the small numbers of patients involved.
(f) Therapy of keratitis due to other fungi. A combination of topical antifungal therapy and keratoplasy appears to provide the most adequate treatment for keratitis due to Acremonium spp. (93). A prospective evaluation of the comparative safety and efficacy of topical natamycin and 0.2% fluconazole was made for eight patients with filamentous fungal keratitis, including five cases due to Acremonium spp. and two due to Curvularia spp. (315). Corneal lesions resolved in three of four patients receiving primary natamycin treatment for a mean duration of 20 days (the keratitis worsened in the fourth patient), whereas the lesions failed to resolve in all four patients who received topical fluconazole as the primary treatment (two subsequently responded to natamycin therapy). Although the identification of Acremonium spp. in some of these patients appears to have been erroneous, this study is important in providing evidence of the efficacy of topical natamycin and the relative inefficacy of topical fluconazole in therapy of keratitis due to filamentous fungi.
A recurring corneal infection due to Fonsecaea pedrosoi was treated by a large penetrating keratoplasty and removal of the involved part of the iris and the entire lens, followed by a 5-month course of oral itraconazole; this resulted in no recurrence of the infection (27). Oral fluconazole therapy, in association with topical natamycin and intracameral amphotericin B and various surgical measures, resulted in eradication of corneal infection due to Colletotrichum graminicola (326). A combination of ketoconazole and amphotericin B therapy and keratoplasty resulted in a favorable outcome of posttraumatic keratitis due to Scopulariopsis brevicaulis in one patient (307). A common problem reported by all those who have had to treat Pythium insidiosum keratitis is that it is not sensitive to any of the currently available antifungals; wide surgical excision including penetrating keratoplasty has been advised for such patients, with enucleation or evisceration being required in patients who fail to respond to these measures (22, 156, 381, 411).
(g) Other considerations. The collagen shield, which is shaped like a contact lens and is packaged in a dehydrated form and rehydrated before use, may protect the corneal epithelium from the action of the eyelids, and the collagen in the lens may promote healing; shields lasting up to 72 h may more conveniently protect the cornea than does repeated patching (107).
Triturated (crushed and suspended) ketoconazole has been recommended for the treatment of mycotic keratitis when commercial antifungal eye drops are not obtainable (136). Ketoconazole and itraconazole tablets were triturated to 20 mg/ml in polyvinyl alcohol, boric acid, olive oil, or balanced salt solution and applied topically to deepithelialized rabbit corneas (one drop/15 min for 2 h). The concentrations of ketoconazole in corneal tissue treated with the triturated drug in balanced salt solution, olive oil, polyvinyl alcohol, and boric acid were calculated to be 512, 773, 1,221, and 1,492 μg/g, respectively; the concentrations of itraconazole were about half those of ketoconazole (136). Therefore, since the vehicle used to triturate the antifungals may affect the tissue concentration, the development of effective vehicles may have an impact on the therapy of mycotic keratitis.
Mycotic keratitis usually responds slowly (over a period of weeks) to antifungal therapy. Clinical signs of improvement include diminution of pain, decrease in the size of the infiltrate, disappearance of satellite lesions, rounding out of the feathery margins of the ulcer, and appearance of hyperplastic masses or fibrous sheets in the region of healing fungal lesions (170, 173). Conjunctival chemosis and injection and punctate epithelial keratopathy may indicate toxicity of the antifungal agent being used. Although repeat scrapings taken during treatment may not yield growth in culture, this does not necessarily indicate that the fungus has been eradicated, since it may have become deep seated; therefore, therapy should be continued for at least 6 weeks (170).
There is a danger of antagonistic effects developing when certain antifungal agents are combined, for example, amphotericin B and miconazole (170). Therefore, methods to enhance the efficacy of existing antifungal agents require careful study.
(ii) Measures to suppress corneal damage due to microbe- or host tissue-derived factors.
Corticosteroids are sometimes used in ocular infections in an attempt to reduce tissue damage wrought by the inflammatory reaction directed against an infecting microorganism. This approach seems to work well in disciform keratitis and central stromal keratitis due to herpes simplex virus (D. M. O'Day, Editorial Ophthalmology 98:845-846, 1991); corneal inflammation and ultimately scarring is reduced. This seems to have been the rationale, in a study in Miami, for the administration of topical corticosteroids to 19 of 125 patients after the diagnosis of mycotic keratitis had been made and after a period of antifungal therapy averaging 14 days (the average duration of corticosteroid therapy was 24 days); unfortunately, corneal lesions progressed in 2 patients in spite of the concurrent antifungal therapy (334). It is already well known that corticosteroid administration is frequently necessary to create experimental models of mycotic keratitis (158, 160, 276). Moreover, corticosteroids have been found to worsen the course of existing but unrecognized mycotic keratitis (366, 394). In one patient from whom a Fusarium sp. was ultimately isolated, the initial corticosteroid therapy appeared to contribute to a fulminant course (the eye was eventually enucleated), while in another patient from whom a Curvularia sp. was isolated, the infection progressed rapidly (a therapeutic penetrating keratoplasty had to be done) (366).
These data support the contention that corticosteroid use is definitely contraindicated when a fungal pathogen is present (366), perhaps even when specific antifungal therapy is given. Possible “inflammatory rebound,” a potentially devastating complication that occurs when corticosteroid therapy is abruptly terminated, also needs to be guarded against, since this could be confused with a worsening of infection (O'Day, editorial).
In an animal model of C. albicans keratitis, ketorolac (a nonsteroidal anti-inflammatory compound) satisfactorily reduced the tissue necrosis occurring as a result of inflammatory mechanisms, without permitting progression of the infection (105). That paper reflected an important aspect of current research on corneal ulceration, i.e., the attempt to develop molecules other than corticosteroids which would inhibit the deleterious effects of inflammatory mechanisms in keratitis. Administration of a synthetic thiol peptide, which appeared to suppress corneal ulceration by inhibiting the action of corneal collagenase and by reducing infiltration by polymorphonuclear leukocytes (47), and application of inhibitors of oxidative metabolism, which reduced the release of free radicals (11), were found to exert beneficial effects on experimental keratitis caused by alkali burns. Symptoms of keratitis have been elicited in rabbit eyes by application of lipid mediators (395); antagonists of these mediators and inhibitors of lipid mediator synthesis may thus serve as alternatives to topical corticosteroid therapy. Platelet-activating factor was found to induce expression of MMP-1 and MMP-9 in the corneal epithelium, leading to corneal ulceration; it was suggested that specific antagonists of platelet-activating factor might deter corneal ulcer formation, thus facilitating corneal wound healing (378). Specific studies are necessary to determine whether such factors influence the progression or outcome of mycotic keratitis. Caution must be exercised in extrapolating the results obtained with sterile corneal ulceration to the different situation in infectious corneal ulcers, since the results obtained may vary dramatically (12).
(iii) Therapeutic surgery.
Surgery may be necessary when mycotic keratitis responds poorly, or not at all, to medical therapy or when perforation or descemetocele formation is imminent. Every attempt should be made, however, to prolong medical therapy for as long as possible, since this will render the infecting fungus nonviable, thereby improving the outcome of surgery. In mycotic keratitis, surgery may aid medical management by increasing drug penetration, by bringing in blood vessels in the form of conjunctival flaps, by stabilizing the corneal epithelial surface, by removing infected corneal tissue (therein reducing or eliminating the microbial load), or by providing tectonic support to the globe when integrity is threatened, as in thinning or perforation of the cornea (3).
(a) Surgical management of small superficial corneal fungal infections. The methods advocated include debridement and pedicle (racquet) conjunctival flaps (for peripheral ulcers), in association with antifungal therapy; tissue adhesives and a bandage contact lens have also been advocated (10, 174, 334).
In mycotic keratitis, regular debridement of the base of the ulcer helps the elimination of fungi and necrotic material (3) and also facilitates the penetration of antifungal drugs into the corneal stroma (273). In a model of deep stromal C. albicans infection in rabbits, a significant reduction of the number of fungi occurred when daily debridement of the corneal epithelium and topical administration of amphotericin B or natamycin was performed; when the epithelium was left intact, this antifungal effect was much reduced. Debridement can be performed under topical anesthesia, with a Bard-Parker blade no. 15, ensuring that a margin of 1 to 2 mm is left at the limbus (3).
Superficial lamellar keratectomy helps to remove the thick mat of fungal filaments on the cornea and facilitates increased drug penetration in patients with dematiaceous fungal keratitis (111, 418).
It may be possible to ablate superficial stromal corneal infiltrates by using the excimer laser. The 193-nm excimer laser was used to ablate experimental keratitis due to Fusarium spp. (123). The infections were allowed to proceed for 24 and 72 h, and then ablation with the 193-nm excimer laser with 5.0-mm treatment zones was performed until all suppurative areas were treated; all cultures of excised corneas were negative in the 24-h group but positive in the 71-h group. Although excimer laser photoablation might be useful to eradicate early, localized microbial infections, it appears that advanced infections, with deep stromal involvement and suppuration, would not be eradicated by this technique. Moreover, caution is required when using the excimer laser for infectious keratitis (123).
Conjunctival flaps help in achieving a stable conjunctival surface in cases of persistent or recurrent epithelial defects and progressive ulceration (3, 10); such flaps are especially helpful in chronic peripheral disease, where the flap does not encroach onto the visual axis (300). Blood vessels present in the flap brought in to cover the ulcerated area help in healing of peripheral fungal corneal ulcers; a superficial lamellar keratectomy should first be done to remove the necrotic stroma, and then a thin conjunctival flap should be anchored over the ulcerated site (3, 10).
Recently, Kim et al. (189) reported that permanent or temporary amniotic membrane transplantation resulted in successful healing of the corneal surface in 21 eyes of 21 consecutive patients with microbial keratitis (including 2 with mycotic keratitis) who had already been treated with sufficient quantities of antimicrobial drugs to eradicate the infecting microorganisms; there was no recurrence of microbial infection in any patient. Prior to transplantation, the amniotic membrane was soaked in antimicrobials; after transplantation, follow-up times ranged from 4 to 28 months (mean, 18 months). Although amniotic membrane transplantation may be a potentially useful adjunctive surgical procedure for the management of microbial keratitis since it promotes wound healing and reduced inflammation, the extremely small number of patients with mycotic keratitis enrolled in this study does not allow firm conclusions to be drawn. This technique may not be successful in patients who have extensive corneal epithelial ulceration and stromal infiltration. Moreover, it is unclear whether the infecting fungus is eradicated in this procedure or whether foci of viable fungi continue to persist in the corneal tissue; these fungi may become reactivated under undefined circumstances to cause corneal damage.
Tissue adhesives (cyanoacrylate “glue”) provide support to a thinned-out cornea and can seal a corneal perforation that is 2 mm or less in size (100). In addition, cyanoacrylate adhesive has been found to be bacteriostatic for gram-positive bacteria (3). Prior to application of the adhesive, necrotic stroma or epithelium and other debris must be removed from the base of the ulcer; a bandage contact lens is usually fitted after the application (3). The adhesive is left in place until it loosens spontaneously, the bed becomes vascularized, or keratoplasty is performed.
(b) Surgical management of keratitis with deep lesions. A Gunderson conjunctival flap has been advocated for deep keratitis; however, there are several limitations to this technique. The procedure is technically difficult to perform since the tissue bleeds profusely and the view of the ulcer is obscured, rendering follow-up examination difficult. Moreover, perforation of the flap and ulcer may occur, the infected material is not removed, and penetration of antifungals may be hindered (10). This procedure is now advocated only in desperate situations, where penetrating keratoplasty is not possible.
Full-thickness corneal grafting (penetrating keratoplasty) is indicated if there is impending perforation, if a perforation exceeding 2 mm has occurred, or if there is no response to medical therapy. The donor button is usually cut so as to be about 0.5 mm bigger than the recipient corneal bed. As far as possible, the lens should be left undisturbed to prevent spread of the infection to the posterior segment; however, where the lens is already exposed preoperatively due to a large perforation, lens extraction should be performed through the trephination wound (300, 384).
Due to the availability of specific antibacterial drugs, penetrating keratoplasty is rarely required for the treatment of active bacterial keratitis. However, it is required in 15 to 28% of patients with mycotic keratitis since medical treatment may be ineffective (111, 334). One study in Singapore indicated that fungal keratitis was associated with a five- to sixfold higher risk of subsequent perforation and need for penetrating keratoplasty than was bacterial keratitis (429). In Miami, penetrating keratoplasty was required in 22 (28%) of 79 patients with Fusarium keratitis, 4 (25%) of 16 patients with keratitis due to different Candida spp., 2 (18%) of 11 patients with Curvularia keratitis, 3 (60%) of 5 patients with Aspergillus keratitis, 2 (67%) of 3 patients with keratitis due to Acremonium spp., and 1 (50%) of 2 patients with keratitis due to Cylindrocarpon spp. (334). In Philadelphia, penetrating keratoplasty was performed for 2 of 11 patients with C. albicans keratitis, 2 of 6 patients with keratitis due to Fusarium spp., and the only patient who had Aspergillus keratitis (377). Other fungi that may cause a severe keratitis that does not respond to medical therapy and necessitates penetrating keratoplasty include L. theobromae (37, 389), P. insidiosum (155), and P. lilacinus (121, 197, 280).
When penetrating keratoplasty is performed for mycotic keratitis, the grafts may opacify in about 4.0 weeks, in contrast to grafts done for bacterial keratitis, where opacification may occur in about 12.9 weeks (70). Similarly, the reported success rate for grafts in mycotic keratitis (20 to 60%) appears to be much lower than the 70 to 75% success rate reported for bacterial keratitis (188, 289). In one study, 25% of grafts performed for mycotic keratitis showed reinfection (334). To decrease the incidence of recurrence, at least 0.5 mm of clear tissue all around the infected area should be excised. Postoperative antifungal therapy should be continued. When donor grafts 8 mm or less in diameter were used for penetrating keratoplasty in fungal corneal ulcers, the outcome was better than when larger grafts were used (188).
To prevent graft rejection in penetrating keratoplasty for mycotic keratitis, topical corticosteroids are given postoperatively, but these need to be used cautiously (366). Topical cyclosporin A has been suggested as an alternative to the use of topical corticosteroids (297). In a prospective, nonrandomized interventional case series, three patients with culture-proven mycotic keratitis who had undergone therapeutic keratoplasties were treated with topical 0.5% cyclosporin A as a primary or adjunctive therapy for prevention of allograft rejection (follow-up was performed for 15 to 42 months); two of the three patients maintained clear grafts, while the remaining patient developed an opacified graft secondary to preexisting ocular surface disease (297). These promising results require verification in studies with a larger number of patients and studies by other workers.
Mycotic Scleritis
Although uncommon, mycotic lesions of the sclera are important. Scleritis arising due to spread of infection from keratitis due to A. corymbifera (231), Acremonium spp., and L. theobromae (37) has been reported. Similarly, scleritis due to Aspergillus spp. or S. schenckii has been reported to occur following ocular trauma (333; Brunette and Stulting, Letter). Endogenous infections have also been reported. A unique subset of microbial scleritis following ocular surgical procedures is being increasingly reported (see below).
One patient with S. prolificans corneoscleritis responded to intensive antifungal therapy and aggressive scleral debridement (202), whereas another patient responded poorly to medical therapy (topical natamycin and amphotericin B, oral itraconazole and ketoconazole) and eventually required enucleation (370). The outcome of scleritis due to S. apiospermum is also reported to be varied, with good results being obtained in some patients (254) and poor results being obtained in others (379). S. schenckii infection has been successfully treated with oral potassium iodide (50 mg/drop), 10 drops three times daily slowly increasing to 24 drops three times daily (Brunette and Stulting, Letter). Scleritis due to A. fumigatus following an injury to the eye by a tree branch worsened in spite of oral fluconazole and topical amphotericin B therapy; cryotherapy and duramater grafting were then performed, which appeared to control the infection (333). Oral itraconazole therapy resulted in resolution of inflammation in A. flavus scleritis; the patient's condition had worsened during therapy with oral ketoconazole and topical amphotericin B (51).
Intraocular Mycoses (Excluding Endophthalmitis)
The uveal tract is the heavily pigmented, highly vascularized middle layer of the eye situated between the sclera externally and the retina internally. It is composed of three distinct regions, namely, the iris, the ciliary body, and the choroid. Infection of the anterior uveal tract is termed “iritis” when inflammation manifests chiefly anterior to the lens and “iridocyclitis” when inflammatory cells are seen in front of and behind the lens. Posterior uveitis and choroiditis are synonymous. The retina usually becomes involved secondary to lesions in the choroid, which manifests as chorioretinitis (424). Intraocular lesions may be caused by many different fungi but are caused chiefly by certain dimorphic fungi (B. dermatitidis, C. immitis, H. capsulatum var. capsulatum, and S. schenckii), yeasts (Candida spp. and Cryptococcus spp.), and P. carinii.
Latent disseminated blastomycosis with choroidal involvement was described in a 36-year-old man who developed blurred vision and a cough 5 months after traveling to an area where a large outbreak of acute blastomycosis had been reported (214); the patient had skin and pulmonary lesions, in addition to the choroidal lesions. Histopathology of the skin lesions confirmed the diagnosis of blastomycosis, and intravenous amphotericin B produced a rapid resolution of both his choroidal and pulmonary lesions (214). Safneck et al. (338) reported the occurrence of endophthalmitis due to B. dermatitidis. Their critical review of the world literature yielded nine cases of intraocular infection due to B. dermatitidis, of which six were verified by histological examination of the enucleated globe. In their case, and in the six cases reviewed, the organisms seen in infected tissue were in the highly characteristic yeast stage, which is found at temperatures of 37°C or greater. These workers contended that the microscopic appearance is sufficiently distinctive to permit presumptive identification without culture. However, culture should be done wherever possible. Pars plana vitrectomy and intravitreal amphotericin B may have a role to play, in addition to intravenous amphotericin B, in therapy of intraocular blastomycosis.
Intraocular coccidioidomycosis may occur in otherwise healthy individuals. Multiple, yellow-white, juxtapapillary chorioretinal lesions with pigmented borders are usually seen; retinal exudates or serous retinal detachment (331), unilateral granulomatous iridocyclitis with multiple iris nodules (72), or papilledema and multifocal choroiditis (72, 331) may also occur. In a report (72) on two patients with intraocular coccidioidomycosis in association with the disseminated form, the diagnosis was established by detection of C. immitis spherules in skin biopsy samples. However, the diagnosis of intraocular coccidioidomycosis is usually made if the suspicious chorioretinal lesions are present in association with anticoccidioidal antibodies in the serum and a positive coccidioidin skin test. Amphotericin B (local and systemic) and oral fluconazole have been used with success in treatment of C. immitis chorioretinitis (72); vitrectomy is necessary if these lesions are associated with endophthalmitis.
A. flavus retinitis was reported in two patients who had undergone bone marrow transplantation 120 days before; fungi were recovered in culture from the vitreous (69). The eyes responded poorly to antifungals. Another four patients developed endophthalmitis due to Candida spp. (69).
Intraocular cryptococcosis usually results from cryptococcal septicemia with severe meningeal infection (67); such sequelae may be seen in patients with AIDS (see “Ophthalmic mycoses associated with AIDS” below). However, isolated ocular cryptococcosis in an apparently immunocompetent individual has been reported (146). Hence, ocular cryptococcal infection must be suspected, even in the absence of predisposing factors or systemic findings. Cryptococcosis may produce visual loss by damaging multiple areas of the anterior visual pathway (67). The diagnosis of cryptococcal chorioretinitis is a presumptive one in a patient with characteristic fundus lesions, with or without vitritis, and documented cryptococcal meningitis or disseminated cryptococcosis. In one patient, transscleral needle biopsy of a subretinal mass was used to establish the diagnosis of subretinal cryptococcosis (146). A vitreous tap or biopsy may be done if vitritis is present. Cryptococcal chorioretinitis can be treated with intravenous amphotericin B (146) or oral fluconazole (2); vitrectomy may be needed if chorioretinitis progresses to endophthalmitis.
Patients with AIDS are at risk of developing pulmonary disease due to P. carinii; aerosolized pentamidine may be given as prophylaxis in such patients. However, patients receiving aerosolized pentamidine therapy are not protected against extrapulmonary disease. Dugel et al. (83) and Foster et al. (104) described the occurrence of choroidal lesions which appeared to be typical of P. carinii in two and three patients, respectively, who were receiving prophylactic aerosolized pentamidine therapy. The lesions resolved after administration of intravenous pentamidine therapy in four of the patients, while the lesions in the remaining patient resolved after administration of intravenous trimethoprim and sulfamethoxazole. None of these patients had clinical or laboratory evidence of P. carinii infection other than in the eye. The choroidal lesions of P. carinii manifest as yellow-white to orange spots without vitreous inflammation (255). Early ophthalmologic examination may detect these lesions before they are threatening to sight and allow systemic therapy to be instituted before widely disseminated infection due to P. carinii results in a fatal outcome.
Ocular involvement by species of Candida is a well-documented sequel to fungemia (81). Candida may spread hematogenously to the choroid and retina without extending into the vitreous to cause endophthalmitis. In a prospective multicenter study with observational design, 118 patients with candidemia were evaluated for the presence of intraocular candidiasis (81). None of the patients were shown to have endophthalmitis, and Candida chorioretinal lesions were observed in only 9% of the patients. Risk factors for Candida chorioretinitis included fungemia with C. albicans (in contrast to non-albicans species), multiple positive blood cultures, visual symptoms, and immunosuppression. It was suggested that when systemic antifungal agents are given early in the course of Candida fungemia, chorioretinal lesions do not progress to endophthalmitis. Choroidal neovascularization is a potential cause of late visual loss in patients who have had sepsis and endogenous chorioretinitis due to C. albicans (166); this complication may occur in spite of adequate antifungal therapy and apparently complete resolution of the chorioretinal lesions. Laser photocoagulation or surgical excision of the neovascular complex may be of benefit in selected cases.
The “presumed ocular histoplasmosis syndrome” is characterized by the presence of multifocal choroiditis scattered throughout the fundus, the peripapillary area, and sometimes the macular area; some lesions show healing with variable chorioretinal scarring (180). This syndrome is not associated with intraocular inflammation and is well tolerated by the eye, unless complications of subretinal neovascularization arise (118). H. capsulatum var. capsulatum has been isolated from the eyes of patients suffering from this syndrome, suggesting that this fungus is the etiologic agent (180). Thomas and Kaplan (382) treated two patients with presumed ocular histoplasmosis, subfoveal neovascular membranes, and progressive loss of visual acuity. Vitreoretinal surgical techniques were used to remove the subfoveal membranes, and good visual recovery was obtained. Therefore, vitreoretinal surgical techniques may be successful in mechanically removing subfoveal neovascular membranes with preservation of the overlying neurosensory retina, and hence preservation of central visual acuity, in the presumed ocular histoplasmosis syndrome.
Until 1990, 17 episodes of endophthalmitis due to S. schenckii had been reported (427). Since then, there have been additional reports of S. schenckii causing endophthalmitis (52, 427) and uveitis (410). Vieira-Dias et al. (410) reported the occurrence of concomitant ocular and cutaneous sporotrichosis, in which the fungus was isolated from skin lesions and the aqueous humor. Risk factors for endophthalmitis due to S. schenckii include AIDS (205) and trauma (427); however, this ocular infection may occur even in the absence trauma or systemic infection (52). Endophthalmitis due to S. schenckii usually presents initially as a granulomatous uveitis (52, 205) which may be treated with corticosteroids, leading to progression of the lesion. Improperly treated uveitic lesions may result in frank endophthalmitis (205) or scleral perforation (52). The only patient with successfully treated endophthalmitis due to S. schenkii responded to amphotericin B (topical and intravitreal) and vitrectomy (427); enucleation had to be peformed for all other patients with S. schenkii endophthalmitis reported in the literature.
FUNGAL OCULAR INFECTIONS AFTER OPHTHALMIC SURGICAL PROCEDURES
Certain surgical procedures are unique to ophthalmology. The most important ophthalmic surgical procedure is cataract extraction, and the most important fungal infection following cataract extraction is fungal endophthalmitis. Fungal endophthalmitis is beyond the scope of this review. Fungal infections such as keratitis and scleritis have been reported following corneal refractive surgical procedures (radial keratotomy, photorefractive keratectomy, laser-assisted in situ keratomileusis, keratoplasty [corneal transplantation], pterygium excision, and cataract extraction) (Table 21).
TABLE 21.
Ophthalmic mycoses following ocular surgerya
| Ocular surgery | Ophthalmic lesion | Criteria for diagnosis of mycosisb | Fungus isolated | Response to antifungals (antifungals used)c | Surgery needed (type of surgery) | Reference |
|---|---|---|---|---|---|---|
| Radial keratotomy | Keratitis | CF, HPE, CC | Fusarium spp. | Details not provided | Yes (keratoplasty) | Gussler et al., letter |
| Keratitis | CF, HPE, CC | A. fumigatus | No (AB, IC) | Yes (keratoplasty) | 142 | |
| Keratitis | C | C. parapsilosis | Yes (AB, KC) | No | 232 | |
| Keratitis | CF, DM, C | Fusarium spp. | Yes (NT) | No | 285 | |
| Keratitis | CF, DM, C | Aspergillus spp. | Yes (NT) | No | 285 | |
| LASIKd | Keratitis | CF, DM, C | Curvularia spp. | Yes (NT, AB) | No | 63 |
| Keratitis | CF, DM, CC | S. apiospermum | No (NT, KC) | Yes (keratoplasty) | 360 | |
| Keratitis | CF, DM, C | A. flavus | No (NT, KC) | Yes (keratoplasty) | 361 | |
| Keratitis | CF, HPE, CC | Acremonium atrogriseum | No | Yes (keratoplasty) | 317 | |
| Keratitis | HPE, CC | A. fumigatus | Yes | No | 203 | |
| Keratitis | HPE, CC | F. solani | No (econazole, FC, AB, NT, IC, voriconazole) | Yes (keratoplasty 3 times) | 408 | |
| Lamellar keratoplasty | Corneal interface infection | HPE, CC | Rhodotorula spp. | No (NT, AB) | Yes (keratoplasty) | 286 |
| Penetrating keratoplasty | Keratitis, intraocular infection | DM, HPE, CC, pathogenic in animal cornea | Exophiala dermatitidis | No (NT, KC, miconazole) | Yes (keratoplasty twice) | 212 |
| Keratitis, intraocular infection | HPE, CC | Exophiala dermatitidis | No (IC, AB) | Yes (keratoplasty) | 29 | |
| Crystalline keratopathy | HPE, CC | C. guilliermondii | Partial (AB, FC) | Yes (keratoplasty) | 4 | |
| Crystalline keratopathy | DM, C | C. albicans | Partial (AB, KC) | Yes (keratoplasty) | 419 | |
| Keratoplasty dehiscence repair | Keratitis | DM, C | Beauveria bassiana | Partial (NT, FC) | Yes (keratoplasty) | 191 |
| Pterygium excision (no radiation) | Anterior and posterior scleritis | C | S. apiospermum | No (AB, IC, miconazole) | Yes (enucleation) | 379 |
| Pterygium excision (radiation) | Corneoscleritis complicating radionecrosis | HPE, CC HPE, culture of scleral tissue | Scedosporium prolificans Fusarium spp. | Yes (NT, FC, AB) Yes (NT,KC) | Yes (scleral resection) Yes (scleral debridement, plaque removal) | 254 254 |
| HPE, culture of scleral tissue | S. apiospermum | Yes (NT,KC,AB) | Yes (plaque removal) | 254 | ||
| HPE, CC | S. apiospermum | No (NT, AB) | Yes (enucleation) | 254 | ||
| HPE, CC | S. prolificans | Yes | Yes (scleral) | 202 | ||
| debridement) | ||||||
| Scleritis and panophthalmitis | Aspergillus spp. | No (AB, flucytosine, miconazole) | Yes (enucleation) | 230 | ||
| Sclerokeratitis | S. prolificans | No (AB, NT) | Yes (enucleation) | 370 | ||
| Cataract extraction | Scleritis | CF, C | Rhizopus spp.e | No (AB)f | Yes (enucleation) | 221 |
| Scleritis (2 patients) | HPE, culture of scleral tissue | Aspergillus spp. | Yes (IC, econazole, AB) | Yes (scleral excision, patch graft) | 31 | |
| Scleritis | CF, HPE, CC | A. flavus | Yes (AB, KC, IC) | Yes (scleral debridement) | 51 | |
| Trabeculectomy | Scleritis | HPE, culture of scleral tissue | Aspergillus spp. | Yes (AB, IC, econazole) | No | 31 |
| Phacoemulsification and intraocular lens implantation | Keratitis secondary to endophthalmitis | HPE, culture of corneal scrapes and vitreous HPE, culture of vitreous | A. kiliense F. solani | No No | Yes (keratoplasty) Yes (keratoplasty) | 417 417 |
| CF, DM, C (repeated) | C. parapsilosis | Partial (AB, FC) | Yes (debridement) | 88 |
Excluding endophthalmitis following cataract surgery.
CF, clinical features suggestive of fungal infection; DM, fungal elements in microscopy of scrapes or necrotic material; HPE, fungal elements seen by histopathological examination of biopsy specimens or tissue bits; C, fungi grown in culture from scrapes or necrotic material; CC, fungi grown in culture from corneal button or biopsy specimen.
AB, amphotericin B; IC, itraconazole; KC, ketoconazole; NT, natamycin; FC, fluconazole.
LASIK, laser-assisted in situ keratomileus.
Significance of this isolate is doubtful.
Also received hyperbaric oxygen therapy.
Postoperative infectious keratitis is an uncommon but serious complication of radial keratotomy; the use of topical corticosteroids and the presence of corneal incisions are probably risk factors. There have been at least five reported cases of mycotic keratitis following radial keratotomy, two each due to Fusarium spp and Aspergillus spp. and one due to C. parapsilosis (142, 232, 285; J. R. Gussler, D. Miller, M. Jaffe, and E. C. Alfonso, Letter, Am. J. Ophthalmol. 119:798-799, 1995). Three of these cases responded to antifungals alone, while the other two required penetrating keratoplasty, wherein the lesions resolved (Table 21).
Laser-assisted in situ keratomileusis combines the precision of excimer laser photoablation with the advantages of an intrastromal procedure that maintains the integrity of Bowman's layer and the overlying corneal epithelium. Therefore, theoretically speaking, the risk of infectious keratitis after this procedure should be minimal. However, microbial contamination of the stromal bed may occur during surgery due to the proximity of the eyelids, eyelashes, conjunctiva, and microkeratome. The use of topical corticosteroids, unstable epithelium at the edge of the lamellar flap, reduced corneal sensitivity, and use of contact lenses all render these eyes more susceptible to infection. While risk of infection is high after photorefractive keratectomy because of the presence of a large epithelial defect, laser-assisted in situ keratomileusis can also be associated with severe, vision-threatening infectious keratitis.
Since the first reported case of mycotic keratitis following laser-assisted in situ keratomileusis in 2000 (317), there have been at least five other reports of this condition (Table 21). Six different species of fungi from five genera have been implicated. Only two of these patients responded to medical therapy alone; penetrating keratoplasty was ultimately required for the other four patients. Some workers (203, 327, 360, 361) feel that although mycotic keratitis following this surgical procedure is rare, it may pose an important therapeutic challenge due to poor intracorneal penetration of antifungals, especially through an intact epithelium. Sampling at the site of infection provides the best chance of obtaining a positive culture. A favorable outcome of such infections may be ensured by prompt and proper management, collection of corneal scrapings from underneath the flap, quick microbial identification, irrigation of the stromal bed with antimicrobials, and intensive treatment with specific antimicrobials.
Mycotic keratitis has also been reported to occur following lamellar (286) or penetrating (4, 29, 212, 419) keratoplasty, following keratoplasty dehiscence repair (191), and secondary to the endophthalmitis that occurred after phacoemulsification and intraocular lens implantation surgery (88, 417); all nine patients involved required surgery (therapeutic penetrating keratoplasty in seven, optical keratoplasty in one, and debridement in one) (Table 21). Four different yeast species (C. albicans, C. guilliermondii, C. parapsilosis, and Rhodotorula sp.) were isolated from the lesions of four patients, and four different species of filamentous fungi (Exophiala dermatitidis in two patients, and A. kiliense, Beauveria bassiana, and a presumed Fusarium sp. in one patient each) were isolated from the other patients.
Fungal scleritis has been reported to occur in at least 13 patients (Table 21) following various ophthalmic surgical procedures, including excision of pterygium (a fleshy conjunctival growth) without (379) or with beta irradiation (202, 230, 254,370) or cataract extraction (31, 51, 221), and after trabeculectomy (filtering surgery for glaucoma). For management of fungal scleritis, early debridement and culture, close microbiologic assistance, systemic antimicrobials for a prolonged period, and penetrating keratoplasty for perforation or incipient perforation are the measures that have been advocated (254). In the actual clinical setting, however, various modalities of antifungal therapy, as well as surgical debridement, were not found useful in 5 of 13 patients, with enucleation eventually having to be performed (Table 21). S. apiospermum was incriminated in two of the patients, S. prolificans was found in one, and Aspergillus sp. was found in one (230, 254, 370, 379); the identification of Rhizopus spp. in the remaining patient is contentious, since fungal hyphae were not visualized in the samples collected and since just one colony of a Rhizopus spp. was recovered in culture (221). Of the 13 patients, 8 required some form of surgical intervention, such as scleral debridement or resection or removal of plaque, to ensure resolution of the infection; the fungi involved were A. flavus and Aspergillus sp. in five patients, S. prolificans in two patients (one of the isolates had been described by the older name, Scedosporium inflatum), and S. apiospermum and Fusarium sp. (in one patient each) (31, 51, 202, 254). In view of the difficulty of managing mycotic scleritis following excision of pterygium, the following preventive measures have been advocated (254): limited use of low-dose radiotherapy after pterygium excision; adequate sterilization before covering of ulcer beds and calcific plaques at sites of radionecrosis; and careful removal of plaques, since ulcer beds and plaques might harbor infective agents.
OPHTHALMIC MYCOSES ASSOCIATED WITH AIDS
Infections by opportunistic microorganisms constitute an important ocular manifestation of AIDS, although ocular findings are infrequent in human immunodeficiency virus (HIV)-infected, asymptomatic individuals (162). Cytomegalovirus retinitis is reported to be the most common intraocular infection in AIDS patients (162, 348), while other opportunistic ocular infections are considerably less common (162, 348). Various types of ophthalmic mycoses, principally affecting the orbit and intraocular structures, have been reported to occur in association with AIDS (Table 22).
TABLE 22.
Ophthalmic mycoses associated with AIDSa
| Ophthalmic lesion and no. of patients (reference) | Criteria for diagnosis of fungal infection (fungus isolated) | Treatment | Outcome | Comment |
|---|---|---|---|---|
| Rhino-orbital zygomycosis; 1 patient (Blat et al., letter) | CL, CT, HPE, culture (Rhizopus arrhizus) | Debridement, intravenous amphotericin B | Orbital lesions improved | Died of cytomegalovirus infection |
| P. carinii orbital infection; 1 patient (Friedberg et al., Letter) | CL, CT, HPE (open biopsy) | Trimethoprim-sulfamethoxazole | Orbital infection resolved | Died (19 mo) |
| Orbital aspergillosis; 1 patient (Vitale et al., Letter) | CL, CT, DM and culture of fine-needle aspirated material (A. fumigatus) | Sinus surgery, amphotericin B (intravenous, local) | Orbital infection resolved | Survived |
| Orbital aspergillosis; 1 patient (245) | CL, CT, HPE, culture (A. fumigatus) | Surgery (sinus, orbit), amphotericin B (intravenous, local) | Orbital infection did not respond | Died (1 wk) |
| Orbital aspergillosis; 4 patients (201) | CL, CT, HPE, culture (A. fumigatus in all 4) | Surgery (sinus, orbit), amphotericin B (intravenous, local), oral itraconazole | (i) Orbital infection did not respond in 3 patients (ii) Response to surgery and amphotericin B in 1 patient | All 3 died (intracranial disease) Survived (no intracranial disease) |
| Rhino-orbital zygomycosis; 1 patient (143) | CL, MRI, HPE (no culture) | Surgery (sinus), debridement, amphotericin B for 3 mo | Orbital infection resolved; no recurrence | Survived (only focal disease) |
| Orbital aspergillosis; 5 patients (172) | CL, CT, HPE, culture (A. fumigatus in all 5) | (i) Surgery, local and intravenous amphotericin B in 2 patients | Both died | Died (16 mo) |
| (ii) Only local and intravenous amphotericin B in 1 patient | Died | Died (7 mo) | ||
| (iii) Local and intravenous amphotericin B, orbital exenteration, oral itraconazole in 1 patient | Died | Died (28 mo) | ||
| (iv) Debridement, ABLC, orbital exenteration in 1 patient | Survived | Factors aiding survival not elucidated | ||
| Rhino-orbito-cerebral zygomycosis and optic nerve invasion; 1 patient (209) | HPE of orbit, sinuses, optic nerve tissue obtained at autopsy (no culture) | Intravenous amphotericin B (6 days) | Died | Patient had presented with blindness of sudden onset |
| Optic neuritis; 1 patient (433) | MRI, CT, HPE, and culture of biopsy specimen (H. capsulatum) | No details provided | No details provided | No details provided |
| Keratitis; 5 patients (144) | CL, culture of scrapes (C. albicans in all 5) | Topical 0.15% amphotericin B | Resolved in all 5 (good vision in 4) | Severity of lesions unclear; microscopy details not provided. |
| Retinitis, uveitis, optic neuritis; 1 patient (357) | CL, HPE; electron microscopy of eyes obtained at autopsy; blood culture during life (H. capsulatum) | Intravenous amphotericin B | Ocular condition deteriorated | Died |
| Limbal nodule and multifocal choroiditis, 1 patient (259) | HPE (limbal nodule biopsy); presumptive C. neoformans (no culture) | No details provided | No details provided | Presumptive diagnosis of cryptococcal infection by HPE |
| Keratitis; 1 patient (291) | Culture; no mention of microscopy (C. parapsilosis) | Topical 0.15% amphotericin B | Keratitis resolved | Died of malignant lymphoma; diagnosis of AIDS made postmortem |
| Iris inflammatory mass; 1 patient (60) | DM of aqueous tap, HPE of enucleated eye; presumptive C. neoformans (no culture) | Intravenous amphotericin B, pars plana vitrectomy | Enucleation | Died (7 days) |
| Retinitis and choroiditis; 1 patient each (162) | HPE of eyes obtained at autopsy (culture not done) | No details provided | No details provided | Presumed fungal retinitis and choroiditis (autopsy) |
| Bilateral endogenous endophthalmitis; 1 patient (293) | Culture of vitreous (B. hawaiiensis) | Intravitreal and intravenous amphotericin B, pars plana vitrectomy, oral fluconazole | Resolved | HPE of vitreous biopsy did not reveal fungal structures |
| Bilateral endogenous endophthalmitis; 1 patient (115) | CL, US, CT, culture of vitreous; culture of blood and cerebrospinal fluid; HPE of enucleated eyes (Fusarium spp.) | Amphotericin B (intravenous, intravitreal), vitrectomy, intravenous fluconazole | Ocular lesions progressed, necessitating enucleation | Died (2 wk) |
| Endophthalmitis; 1 patient (205) | CL, DM, and culture of aqueous tap and vitreous; HPE of enucleated eye (S. schenckii) | Amphotericin B (intravenous, intravitreal, intracorneal), potassium iodide | Enucleation | |
| Bilateral chorioretinitis; 1 patient (224) | HPE of autopsied organs and eyes, immunofluorescence with antisera to H. capsulatum (no culture) | Intravenous amphotericin B | Ocular lesions did not respond to therapy | Died |
| Endogenous endophthalmitis; 1 patient (118) | CL and culture of vitreous (H. capsulatum var. capsulatum) | Surgery (pars plana vitrectomy, scleral buckle), amphotericin B (intravenous, intravitreal) | Improved and then deteriorated | |
| Multifocal choroiditis; 14 patients (255) | HPE and electron microscopy; presumed C. neoformans (no culture) in 7; P. carinii in 4; H. capsulatum and Candida spp. in 1 each; A. fumigatus in 1 (diagnostic criteria unclear) | Intravenous amphotericin B | No details provided | Study done on autopsied eyes of patients with AIDS dying of various complications, e.g., disseminated cryptococcosis, candidiasis, histoplasmosis; only HPE—no culture |
| Choroidopathy; 21 patients (350) | CL, immunofluorescence with antisera to P. carinii, HPE (2 patients); choroidopathy presumed to be due to P. carinii | No details provided | No details provided | These patients had pneumonia or disseminated infection due to P. carniii; choroidopathy was an incidental observation |
Abbreviations: CL, clinical features suggestive of mycosis; CT, evidence of sinusitis, bony erosion, and increased soft tissue density within the orbit on computed tomography; MRI, evidence of sinusitis, bony erosion, and increased soft tissue density within the orbit on magnetic resonance imaging; DM, fungal structures seen by microscopy of necrotic material or scrapes; HPE, fungal structures seen by histopathological examination; US, ultrasound findings suggestive of space-occupying lesion; ABLC, amphotericin B-lipid complex.
Autopsy findings in 25 patients who died of AIDS revealed opportunistic ocular infections in 8 patients; this included retinitis due to Candida spp. in 1 patient and choroiditis due to C. neoformans var. neoformans in 1 patient (162). Earlier, Schuman and Friedman (346) had reported the occurrence of retinitis due to C. albicans and C. neoformans in 2 of 34 patients with AIDS; bacterial corneal ulceration was noted in 2%, and fungal corneal ulceration was not noted at all.
Opportunistic infections of the orbit from bacterial, fungal, and parasitic organisms are a serious complication of systemic HIV infection and are associated with high ocular morbidity and mortality. Orbital mycoses associated with AIDS (Table 22) have been reported in 15 patients since 1991 (Friedberg et al., letter; 143, 172, 201, 209, 245; S. P. Blatt, D. R. Lucey, D. DeHoff, and R. B. Zellmer, Letter, J. Infect. Dis. 164:215-216, 1991; A. T. Vitale, R. F. Spaide, F. A. Warren, H. F. Moussouris, and R. A. D'Amico, Letter, Am. J. Ophthalmol. 113:725-726, 1992). The outcome was generally poor in these patients (Table 22), with resolution or improvement of the orbital mycotic infection in just 6 of 15 patients. Surprisingly, there was resolution or improvement following debridement and intravenous amphotericin B therapy in two of the three patients with rhinoorbital zygomycosis, perhaps because these had relatively focal lesions (143; Blatt et al., letter). There was complete resolution of lesions in the one patient with P. carinii infection after treatment with trimethoprim and sulfamethoxazole (Friedberg et al., letter). Eleven patients had infections due to A. fumigatus, and 10 of these were treated with surgery and intravenous amphotericin B; the mycotic infection resolved and the patient survived in only 3 of these cases. Two of the three survivors underwent surgery and received amphotericin B (intravenous and local irrigation), while the third survivor was treated with debridement, amphotericin B lipid complex, liposomal amphotericin B, and orbital exenteration (172, 201; Vitale et al., letter); none of these three patients had intracranial disease, and all appeared to have relatively focal orbital lesions, which may explain the successful outcome. Overall, it appears that the outcome of orbital aspergillosis in patients with AIDS is poor.
There are many causes of optic neutritis in AIDS patients. There have been two reports of optic neuritis due to H. capsulatum var. capsulatum (357, 433); in one of these, the optic neuritis occurred in association with retinitis and uveitis (Table 22).
Although bacterial and fungal corneal infections appear to be infrequent in HIV-infected patients, they may be severe and associated with corneal perforation when they do occur. Known risk factors for ulcerative keratitis may be absent in HIV-infected patients (144). There have been reports of six patients with mycotic keratitis associated with AIDS (in one patient, the diagnosis of AIDS was made postmortem); C. albicans was the fungus implicated in all six patients (144, 291). The keratitis resolved in all six with topical 0.15% amphotericin B therapy.
Other mycotic infections of the anterior segment reported in AIDS include limbal nodules (and multifocal choroiditis) in one patient (259) and an iris inflammatory mass in another (60); a presumptive diagnosis of infection due to C. neformans var. neoformans was made in both patients by histopathological studies (Table 22).
Presumed mycoses of the posterior segment in patients with AIDS include multifocal choroiditis (choroidopathy) due to cryptococcosis, histoplasmosis, candidiasis, and P. carinii infection (162, 224, 255, 259, 350, 407), and retinitis (162, 357). Culture-proven endogenous endophthalmitis due to Bipolaris hawaiiensis, Fusarium sp., S. schenckii and H. capsulatum var. capsulatum has also been reported (115, 118, 205, 293); complete resolution of lesions was achieved by surgery and amphotericin B and fluconazole therapy only in the patient with B. hawaiiensis infection (Table 22). Although central nervous system infection with C. neoformans var. neoformans is common in patients with AIDS, actual invasion of the intraocular structures by this fungus appears to be uncommon. In one study of 80 HIV-seropositive patients with cryptococcal infections, ophthalmic manifestations included papilledema (32.5%), visual loss and abducens nerve palsy (9%) and optic atrophy (2.5%); interestingly, visual loss caused by optic nerve involvement was less frequent among the 62 patients who had received oral ketoconazole, itraconazole, or fluconazole only than among the 18 patients who had received amphotericin B alone or in combination with the azoles, and actual invasion of the intraocular structures was an uncommon complication (185).
OPHTHALMIC MYCOSES ASSOCIATED WITH OCULAR BIOMATERIALS
The topic of ophthalmic mycoses associated with ocular biomaterials has been extensively reviewed by Wilson (422). Microbial colonization of indwelling and implanted biomedical devices, such as shunts or catheters, can lead to serious, often lethal, infection. Polymers, silicones, and metals used to fabricate various devices may be implicated (422). The organisms responsible for such biomaterial-related infections are usually part of the resident microbial flora at a particular area of the body and hence pose a constant threat.
Several factors are though to contribute to the mechanisms of infection associated with biomedical devices (422). Intraoperative contamination during surgical implantation, or extraluminal migration of organisms, permits potential pathogens to transcend normal protective barriers. Production of mucoid substances by microorganisms facilitates the adhesion of colonizing microorganisms and also protects them from various host defense mechanisms. The presence of plasma proteins (especially fibronectin) on the surface of the biopolymer may promote attachment of staphylococci and Candida species to the surface.
Contact lens plastics and their storage cases and intraocular lens implants constitute the two most important categories of biomaterials used in ophthalmology. Infection associated with contaminated intraocular lenses results in endophthalmitis, which is beyond the scope of this review.
Contact lens wear is frequently implicated in the occurrence of bacterial (especially P. aeruginosa) keratitis, and Acanthamoeba keratitis, particularly in the United States (217, 218, 348). The likely route for the normal ocular microbiota colonizing contact lenses during wear is via the lid margins, whereas colonization by gram-negative bacteria, including potential agents of microbial keratitis, is likely to be from the domestic water supply (217, 421). Contact lens-associated mycotic keratitis may be comparatively uncommon because fungi isolated from the healthy outer eye only transiently colonize this area and are not normally resident in the outer eye (217, 363). When soft lenses are worn continuously, fungal conidia adhere to the lens surface and, under favorable conditions, germinate; fungal hyphae are able to enter the matrix of the soft lens, project through the posterior surface, and then penetrate the corneal epithelium, resulting in fungal infection (354). Filamentous fungi of the genera Acremonium, Aspergillus, Alternaria, Cladosporium, Curvularia, and Fusarium were found to penetrate the matrix of soft contact lenses both during normal usage and in laboratory studies. Growth of the fungal hyphae (which were coiled within the lens matrix) increased with increasing water content of the lens. Some species penetrated completely through the lens in 96 h (354). Disinfection of lenses after exposure to potentially high concentrations of these fungi in the environment is prudent (93).
Fungal infection was reported in 4 (4%) of 90 contact lens wearers and in 4 (27%) of 15 patients who wore therapeutic bandage contact lenses (420). If fungal conidia alight on the surface of a contact lens, they are normally removed by surface cleaning of the lens. If lenses are worn for an extended duration without proper cleaning, fungi may adhere and penetrate the contact lens (422). This explains why, when such infections have occurred, soft lenses for aphakia and therapeutic extended wear have been the most frequently implicated (140, 218, 365, 420, 423). Interestingly, soft lenses for cosmetic and aphakic extended wear are frequently associated with infections due to filamentous fungi whereas soft lenses for therapeutic use are frequently associated with yeasts and yeast-like fungus (420).
Rosa et al. (334) reported that 6 of their 125 patients with mycotic keratitis in south Florida wore extended-wear contact lenses; F. oxysporum was isolated from four patients, and C. albicans and Paecilomyces sp. were isolated from one patient each. In one patient who wore a bandage contact lens, keratitis due to C. parapsilosis developed. Liesegang and Forster (216) had earlier reported the occurrence of fungal keratitis in three patients who wore soft contact lenses; the fungi isolated were A. flavus and F. dimerum. Filamentous fungi (A. flavus, F. dimerum, and Fusarium sp.) had also been isolated from the corneal scrapes of several other patients in South Florida who had contact lens-associated fungal keratitis (9, 216).
Perry et al. (296) reported the occurrence of a conjunctival mass and keratoconjunctivitis in an immunocompetent patient; detailed examination revealed that at the posterior aspect of this mass, and covered by mucoid material, was a soft contact lens. Simple removal of the lens resulted in a resolution of all signs and symptoms; the contact lens grew Aspergillus fumigatus (296). Keratouveitis due to Scedosporium prolificans was recently reported in an elderly female patient; the keratouveitis was associated with the intraocular long-term retention of a contact lens (19). Although there is no doubt that a fungal pathogen was involved in this patient, the exact identity of the fungal isolate has recently been questioned (Guarro and Gené, letter).
A relationship has been demonstrated between the occurrence of contact lens-associated bacterial and Acanthamoeba keratitis and the presence of bacteria and Acanthamoeba in contact lens cases (240, 348). Whether such a relationship occurs in contact lens-associated fungal keratitis is unknown. However, Wilson et al. (425) demonstrated the adherence of C. albicans within a biofilm to polyethylene contact lens case plastic; this species was found to be more resistant to the action of contact lens disinfectants than bacteria were. A survey of contact lens cases from 101 asymptomatic daily-wear, cosmetic contact lens wearers in a domiciliary contact lens practice revealed contamination in 82 (81%) cases; 77% grew bacteria, 24% grew fungi, and 20% grew protozoa (125). These authors provided electron microscopic evidence of the polymicrobial nature of the biofilm found in many cases. Ritterband et al. (328) reported a unique case of keratitis due to C. laurentii and F. solani in a diabetic male patient who wore a gas-permeable contact lens; both fungi (and Staphylococcus aureus) were isolated from the patient's corneal button, infected toenails, and contact lens storage case, and enucleation eventually had to be done. C. parapsilosis keratitis, associated with contact lens wear, was reported in an elderly Israeli patient who developed stromal infiltration at the donor-recipient interface 2 years after penetrating keratoplasty, while wearing a “piggyback” type of contact lens; the infection resolved after treatment with amphotericin B and flucytosine (198).
Overnight soaking of soft lenses in 3% hydrogen peroxide (longer than 4 h), with neutralization in the morning with thiosulfate solution, catalase solution, or catalase tablets, is perhaps the safest way to ensure killing of bacteria, Acanthamoeba, and fungi (58, 422). However, Gray et al. (125) reported that 81% of contact lens cases surveyed were contaminated with microbes and that 75% of the subjects used hydrogen peroxide disinfection for their contact lenses. All the contaminating microorganisms were found to possess catalase (which breaks down hydrogen peroxide to water and oxygen). Recommendations for contact lens wearers to prevent microbial contamination of the lens and case include regular scrubbing of the interior of contact lens cases to disrupt biofilms, exposure of the contact lens case to very hot water (≥70°C), air drying of the contact lens case between use, use of a two-step system for hydrogen peroxide disinfection, and regular replacement of the contact lens case (125, 348).
Punctal occluders or plugs are used to facilitate the management of dry-eye syndrome. Since these devices are left in situ for a long duration, nonspecific microbial attachment, surface colonization, and biofilm formation may occur (422). Fungi are rarely implicated. In one instance, in a patient with mycotic keratitis due to C. lunata, the same fungus was isolated from the plug on removal (422).
Several alloplastic biomaterials have been used for orbital floor repair (243) and to restore the anophthalmic socket (113). Oestreicher et al. (279) described a patient who developed an Aspergillus abscess within a hydroxyapatite orbital implant 58 months following uncomplicated implant surgery; the symptoms resolved following removal of the implant.
Patients with corneal or scleral defects have been treated with Gore-Tex grafting; although this material offers some advantages, there are disadvantages, such as poor epithelialization, poor adhesion between the graft and the surrounding tissue, and the possibility of infection. Huang et al. (153) reported the occurrence of fungal contamination in one such graft, which ultimately led to fungal endophthalmitis some months after graft removal and penetrating keratoplasty.
FUTURE RESEARCH IN OPHTHALMIC MYCOSES
Future research in ophthalmic mycoses needs to focus on improvement in diagnostic techniques, development of new antifungal compounds and a better understanding of the pathogenesis of the conditions.
Diagnostic Methods
A rapid and accurate identification of the fungal species causing an ocular infection will permit the immediate institution of specific antifungal therapy. The nonspecific fluorescent staining techniques (55), immunohistochemical methods (311), and DNA hybridization and DNA amplification techniques (165) are very promising methods, but they need to be simplified and standardized before they can be applied on a large scale. However, culture continues to provide many advantages. New culture media need to be developed.
New Antifungal Compounds
Amphotericin B continues to be the mainstay of therapy of many, especially severe, ophthalmic mycoses, since fungicidal concentrations may be achieved in ocular tissues (267). Research needs to focus on methods to increase the concentrations of other available antifungals in ocular tissues following administration of therapeutic doses. A change in the vehicle used, or the method of application, may help to do this (136). However, to achieve improved outcomes of ocular infections due to Fusarium spp., various zygomycetes, and P. insidiosum, new compounds need to be developed. Many new azole antifungals and other compounds against Aspergillus spp. have been developed (267) but must still be evaluated for their efficacy in severe ophthalmic mycoses due to Aspergillus spp. O'Day et al. (277) recently described a model of experimental keratitis due to C. albicans, where a standardized inoculum of blastoconidia was placed on the corneal surface and covered with a contact lens. These workers found that invasive corneal disease was established by this surface inoculum and that administration of corticosteroid increased corneal penetration of hyphae. This model mimics human disease since the only fungi present are those actively growing within the cornea. Such a model may prove valuable in evaluation of antifungals in the future.
Pathogenesis of Ophthalmic Mycoses
A better understanding of pathogenetic mechanisms in ophthalmic mycoses is required. In particular, the possible role of fungal extracellular proteinases (438) and fungal morphogenesis (392) in ophthalmic mycoses requires clarification. The role of nonspecific inflammatory mechanisms and specific immunological mechanisms in the pathogenesis of ophthalmic mycoses needs to be studied. A better understanding of these various pathogenetic mechanisms will permit the development of molecules and methods to neutralize these mechanisms and to augment antifungal therapy.
Acknowledgments
I am grateful to P. Geraldine, J. Kaliamurthy, C. M. Kalavathy, C. Madhavan, D. Arvind Prasanth, A. Geetha, and R. T. Rajagowthamee for their abundant help and to C. A. Nelson Jesudasan for permitting me to utilize the patient and laboratory records and other facilities available at the Institute of Ophthalmology, Joseph Eye Hospital, in the preparation of this review.
REFERENCES
- 1.Adler, D. E., T. H. Milhorat, and J. I. Miller. 1998. Treatment of rhinocerebral mucormycosis with intravenous, interstitial and cerebrospinal fluid administration of amphotericin B: case report. Neurosurgery 42:644-648. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal, A., A. Gupta, V. Sakhuja, P. Talwar, K. Joshi, and K. S. Chugh. 1991. Retinitis following disseminated cryptococcosis in a renal allograft recipient. Efficacy of oral fluconazole. Acta Ophthalmol. Copenh. 69:402-405. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal, V., J. Biswas, H. N. Madhavan, G. Mangat, M. K. Reddy, J. S. Saini, S. Sharma, and M. Srinivasan. 1994. Current perspectives in infectious keratitis. Indian J. Ophthalmol. 42:171-191. [PubMed] [Google Scholar]
- 4.Ainbinder, D. J., V. C. Parmley, T. H. Mader, and M. L. Nelson. 1998. Infectious crystalline keratopathy caused by Candida guilliermondii. Am. J. Ophthalmol. 125:723-725. [DOI] [PubMed] [Google Scholar]
- 5.Ajayi, B. G., B. Osuntokun, O. Olurin, O. O. Kale, and T. A. Junaid. 1986. Orbital histoplasmosis due to Histoplasma capsulatum var. duboisii: successful treatment with septrin. J. Trop. Med. Hyg. 89:179-187. [PubMed] [Google Scholar]
- 6.Alexandrakis, G., M. Sears, and P. Gloor. 1996. Postmortem diagnosis of Fusarium panophthalmitis by the polymerase chain reaction. Am. J. Ophthalmol. 121:221-223. [DOI] [PubMed] [Google Scholar]
- 7.Alexandrakis, G., S. Jalali, and P. Gloor. 1998. Diagnosis of Fusarium keratitis in an animal model using the polymerase chain reaction. Br. J. Ophthalmol. 82:306-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexandrakis, G., R. Haimovici, D. Miller, and E. C. Alfonso. 2000. Corneal biopsy in the management of progressive microbial keratitis. Am. J. Ophthalmol. 129:571-576. [DOI] [PubMed] [Google Scholar]
- 9.Alfonso, E., S. Mandelbaum, M. J. Fox, and R. K. Forster. 1986. Ulcerative keratitis associated with contact lens wear. Am. J. Ophthalmol. 108:64-67. [DOI] [PubMed] [Google Scholar]
- 10.Alino, A. M., H. D. Perry, A. J. Kanellopoulos, E. D. Donnenfeld, and E. K. Rahn. 1998. Conjunctival flaps. Ophthalmology 105:1120-1123. [DOI] [PubMed] [Google Scholar]
- 11.Alio, J. L., M. J. Ayala, E. Mulet, A. Ratola, J. L. Bellot, and J. M. Ruiz-Moreno. 1993. Treatment of experimental acute corneal inflammation with inhibitors of oxidative metabolism. Ophthalmic Res. 25:331-336. [DOI] [PubMed] [Google Scholar]
- 12.Alio, J. L., A. Artola, A. Serra, M. J. Ayala, and M. E. Mulet. 1995. Effect of topical antoxidant therapy on experimental infectious keratitis. Cornea 14:175-179. [PubMed] [Google Scholar]
- 13.Alleyne, C. H., Jr., A. G. Vishteh, R. F. Spetzler, and P. W. Detwiler. 1999. Long-term survival of a patient with invasive cranial base rhinocerebral mucormycosis treated with combined endovascular, surgical and medical therapies: case report. Neurosurgery 45:1461-1463. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez, H., and K. F. Tabbara. 1996. Infections of the eyelids, p. 559-570. In K. F. Tabbara and R. A. Hyndiuk (ed.), Infections of the eye, 2nd ed. Little, Brown & Co., Boston, Mass.
- 15.Anand, V. K., G. Alemar, and J. A. Griswold, Jr. 1992. Intracranial complications of mucormycosis: an experimental model and clinical review. Laryngoscope 102:656-662. [DOI] [PubMed] [Google Scholar]
- 16.Anderson, R. L., T. F. Carroll, J. T. Harvey, and M. G. Myers. 1984. Petriellidium (Allescheria) boydii orbital and brain abscess treated with intravenous miconazole. Am. J. Ophthalmol. 97:771-775. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong, R. A. 2000. The microbiology of the eye. Ophthalmic Physiol. Opt. 20:429-441. [PubMed] [Google Scholar]
- 18.Arora, I., and S. Singhvi. 1994. Impression debridement of corneal lesions. Ophthalmology 101:1935-1940. [DOI] [PubMed] [Google Scholar]
- 19.Arthur, S., L. L. Steed, D. J. Apple, Q. Peng, G. Howard, and M. Escobar-Gomez. 2001. Scedosporium prolificans keratouveitis in association with a contact lens retained intraocularly over a long term. J. Clin. Microbiol. 39:4579-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashbee, H. R., and E. G. V. Evans. 2002. Immunology of diseases associated with Malassezia species. Clin. Microbiol. Rev. 15:21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attapattu, M. C. 1995. Acute rhinocerebral mucomycosis caused by Rhizopus arrhizus from Sri Lanka. J. Trop. Med. Hyg. 98:355-358. [PubMed] [Google Scholar]
- 22.Badenoch, P. R., D. J. Coster, B. I. Wetherall, H. T. Brettig, M. A. Rozenbilds, A. Drenth, and G. Wagels. 2001. Pythium insidiosum keratitis confirmed by DNA sequence analysis. Br. J. Ophthalmol. 85:502-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balch, K., P. H. Phillips, and N. J. Newman. 1997. Painless orbital apex syndrome from mucormycosis. J. Neuroophthalmol. 17:178-182. [PubMed] [Google Scholar]
- 24.Baron, E. J., L. R. Peterson, and S. M. Finegold. 1994. Bailey and Scott's diagnostic microbiology, 9th ed. C. V. Mosby, St. Louis, Mo.
- 25.Reference deleted.
- 26.Bartley, G. B. 1995. Blastomycosis of the eyelid. Ophthalmology 102:2020-2023. [DOI] [PubMed] [Google Scholar]
- 27.Barton, K., D. Miller, and S. C. Pflugfelder. 1997. Corneal chromoblastomycosis. Cornea 16:235-239. [PubMed] [Google Scholar]
- 28.Behrens-Baumann, W. 1999. Mycoses of the eye and its adnexa. Dev. Ophthalmol. 32:27-107. [PubMed]
- 29.Benaoudia, F., M. Assouline, Y. Pouliquen, A. Bouvet, and E. Gueho. 1999. Exophiala (Wangiella) dermatitidis keratitis after keratoplasty. Med. Mycol. 37:53-56. [PubMed] [Google Scholar]
- 30.Berman, M. B. 1993. Regulation of corneal fibroblast MMP-1 collagenase secretion by plasmin. Cornea 12:420-432. [DOI] [PubMed] [Google Scholar]
- 31.Bernauer, W., B. D. S. Allan, and J. K. G. Dart. 1998. Successful management of Aspergillus scleritis by medical and surgical treatment. Eye 12:311-316. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharya, A. K., A. R. Deshpande, S. R. Nayak, M. V. Kirtane, M. V. Ingle, and I. M. Vora. 1992. Rhinocerebral mucormycosis: an unusual case presentation. J. Laryngol. Otol. 106:48-49. [DOI] [PubMed] [Google Scholar]
- 33.Reference deleted.
- 34.Bloom, P. A., D. A. Laidlaw, D. L. Easty, and D. W. Warnock. 1992. Treatment failure in a case of fungal keratitis caused by Pseudallescheria boydii. Br. J. Ophthalmol. 76:367-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boelart, J. R. 1994. Mucormycosis: is there news for the clinician? J. Infect. 28(Suppl. 1):1-6. [DOI] [PubMed] [Google Scholar]
- 36.Boralkar, A. N., P. R. Dindore, R. P. Fule, B. N. Bangde, M. V. Albel, and A. M. Saoji. 1989. Microbiological studies in conjunctivitis. Indian J. Ophthalmol. 37:94-95. [PubMed] [Google Scholar]
- 37.Borderie, V. M., T. M. Bourcier, J.-L. P. Poirot, M. Baudrimont, P. P. de Saint-Maur, and L. Laroche. 1997. Endophthalmitis after Lasiodiplodia theobromae corneal abscess. Graefe's Arch. Clin. Exp. Ophthalmol. 235:259-261. [DOI] [PubMed] [Google Scholar]
- 38.Borruat, F. X., J. Bogousslavsky, S. Uffer, G. Klainguti, and N. J. Schatz. 1993. Orbital infarction syndrome. Ophthalmology 100:562-568. [DOI] [PubMed] [Google Scholar]
- 39.Bouchara, J. P., A. Bouali, G. Tronchin, R. Robert, D. Chabasse, and J. M. Senet. 1988. Binding of fibrinogen to the pathogenic Aspergillus species. J. Med. Vet. Mycol. 26:327-334. [PubMed] [Google Scholar]
- 40.Bouchon, C. L., D. L. Greer, and C. F. Genre. 1994. Corneal ulcer due to Exserohilum longirostratum. Am. J. Clin. Pathol. 101:452-455. [DOI] [PubMed] [Google Scholar]
- 41.Brook, I., and E. H. Frazier. 1998. Aerobic and anaerobic microbiology of dacryocystitis. Am. J. Ophthalmol. 125:552-554. [DOI] [PubMed] [Google Scholar]
- 42.Brooks, J. G., and D. J. Coster. 1993. Non-ulcerative fungal keratitis diagnosed by posterior lamellar biopsy. Aust. N. Z. J. Ophthalmol. 21:115-119. [DOI] [PubMed] [Google Scholar]
- 43.Brown, S. R., I. A. Shah, and M. Grinstead. 1998. Rhinocerebral mucormycosis caused by Apophysomyces elegans. Am. J. Rhinol. 12:289-292. [DOI] [PubMed] [Google Scholar]
- 44.Brummund, W., V. P. Kurup, G. J. Harris, J. A. Duncavage, and J. A. Arkins. 1986. Allergic sino-orbital mycosis. A clinical and immunologic study. JAMA 256:3249-3253 [PubMed] [Google Scholar]
- 45.Reference deleted.
- 46.Burnier, S. V., and A. E. Sant'Anna. 1997. Palpebral paracoccidioidomycosis. Mycopathologia 140:29-33. [DOI] [PubMed] [Google Scholar]
- 47.Burns, F. R., R. D. Gray, and C. A. Paterson. 1990. Inhibition of alkali-induced corneal ulceration and perforation by a thiol peptide. Investig. Ophthalmol. Visual Sci. 31:107-114. [PubMed] [Google Scholar]
- 48.Cailliez, J. C., N. Séguy, C. M. Denis, E. M. Aliouat, E. Mazars, L. Polonelli, D. Camus, and E. Dei-Cas. 1996. Pneumocystis carinii: an atypical fungal micro-organism. J. Med. Vet. Mycol. 34:227-239. [DOI] [PubMed] [Google Scholar]
- 49.Calli, C., R. Savas, M. Parildar, G. Pekindil, H. Alper, and N. Yunten. 1999. Isolated pontine infarction due to rhinocerebral mucormycosis. Neuroradiology 41:179-181. [DOI] [PubMed] [Google Scholar]
- 50.Campbell, C. K., E. M. Johnson, C. M. Philpot, and D. W. Warnock. 1996. Identification of pathogenic fungi. Public Health Laboratory Service, London, England.
- 51.Carlson, A. N., G. N. Foulks, J. R. Perfect, and J. H. Kim. 1992. Fungal scleritis after cataract surgery. Successful outcome using itraconazole. Cornea 11:151-154. [DOI] [PubMed] [Google Scholar]
- 52.Cartwright, M. J., M. Promersberger, and G. A. Stevens. 1993. Sporothrix schenckii endophthalmitis presenting as granulomatous uveitis. Br. J. Ophthalmol. 77:61-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carubell, R., R. E. Nordquist, and J. J. Rowsey. 1990. Role of active oxygen species in corneal ulceration: effect of hydrogen peroxide generated in situ. Cornea 9:161-169. [PubMed] [Google Scholar]
- 54.Castelino, A. M., S. K. Rao, J. Biswas, L. Gopal, H. N. Madhavan, and S. K. Kumar. 2000. Conjunctival rhinosporidiosis associated with scleral melting and staphyloma formation: diagnosis and management. Cornea 19:30-33. [DOI] [PubMed] [Google Scholar]
- 55.Chander, J., A. Chakrabarti, A. Sharma, J. S. Saini, and D. Panigrahi. 1993. Examination of calcofluor staining in the diagnosis of fungal corneal ulcer. Mycoses 36:243-245. [DOI] [PubMed] [Google Scholar]
- 56.Chander, J., and A. Sharma. 1994. Prevalence of fungal corneal ulcers in northern India. Infection 22:207-209. [DOI] [PubMed] [Google Scholar]
- 57.Chandler, F. W. 1991. Histologic diagnosis of mycotic diseases, p. 235-242. In F. Gatti, C. de Vroey, and A. Persi (ed.), Human mycoses in tropical countries. Health Cooperation Papers no. 13. Organizzazione per la Cooperazione Sanitaria Internazionale, Bologna, Italy.
- 58.Chandler, J. W. 1990. Biocompatibility of hydrogen peroxide in soft contact lens disinfection: antimicrobial activity vs. biocompatibility — the balance. CLAO J. 16(1 Suppl.):S43-S45. [PubMed] [Google Scholar]
- 59.Reference deleted.
- 60.Charles, N. C., C. A. Boxrud, and E. A. Small. 1992. Cryptococcosis of the anterior segment in acquired immune deficiency syndrome. Ophthalmology 99:813-816. [DOI] [PubMed] [Google Scholar]
- 61.Chitravel, V., S. Subramanian, B. M. Sundaram, and K. Thiruneelakantan. 1980. Rhinosporidiosis in a south Indian village. Sabouraudia 18:241-247. [DOI] [PubMed] [Google Scholar]
- 62.Choi, D. M., M. H. Goldstein, A. Salierno, and W. T. Driebe. 2001. Fungal keratitis in a daily disposable soft contact lens wearer. CLAO J. 27:111-112. [PubMed] [Google Scholar]
- 63.Chung, M. S., M. H. Goldstein, W. T. Driebe, Jr., and B. Schwartz. 2000. Fungal keratitis after laser in situ keratomileusis: a case report. Cornea 19:236-237. [DOI] [PubMed] [Google Scholar]
- 64.Clancy, C. J., and M. H. Nguyen. 1998. Invasive sinus aspergillosis in apparently immunocompetent hosts. J. Infect. 37:229-240. [DOI] [PubMed] [Google Scholar]
- 65.Clinch, T. E., M. J. Robinson, B. A. Barron, M. S. Insler, K. Liang, and H. E. Kaufman. 1992. Fungal keratitis from nylon line lawn trimmers. Am. J. Ophthalmol. 114:437-440. [DOI] [PubMed] [Google Scholar]
- 66.Coccia, L., D. Calista, and A. Boschini. 1999. Eyelid nodule: a sentinel lesion of disseminated cryptococcosis in a patient with acquired immunodeficiency syndrome. Arch. Ophthalmol. 117:271-272. [PubMed] [Google Scholar]
- 67.Cohen, D. B., and B. J. Glasgow. 1993. Bilateral optic nerve cryptococcosis in sudden blindness in patients with acquired immunodeficiency syndrome. Ophthalmology 100:1689-1694. [DOI] [PubMed] [Google Scholar]
- 68.Coriglione, G., G. Stella, L. Gafa, G. Spata, S. Oliveri, A. A. Padhye, and L. Ajello. 1990. Neosartorya fischeri var. fischeri (Wehmer) Malloch and Cain 1972 (anamorph: Aspergillus fischerianus Samson and Gams, 1985) as a cause of mycotic keratitis. Eur. J. Epidemiol. 6:382-385. [DOI] [PubMed] [Google Scholar]
- 69.Coskuncan, N. M., D. A. Jabs, J. P. Dunn, J. A. Haller, W. R. Green, G. B. Vogelsang, and G. W. Santos. 1994. The eye in bone marrow transplantation. VI. Retinal complications. Arch. Ophthalmol. 112:372-379. [DOI] [PubMed] [Google Scholar]
- 70.Cristol, S. M., E. C. Alfonso, J. H. Guildford, T. J. Roussel, and W. W. Culbertson. 1996. Results of large penetrating keratoplasty in microbial keratitis. Cornea 15:571-576. [PubMed] [Google Scholar]
- 71.Cuero, R. G. 1980. Ecological distribution of Fusarium solani and its opportunistic action related to mycotic keratitis in Cali, Colombia. J. Clin. Microbiol. 12:455-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cunningham, E. T., Jr., S. R. Seiff, T. G. Berger, P. E. Lizotte, E. L. Howes, Jr., and J. C. Horton. 1998. Intraocular coccidioidomycosis diagnosed by skin biopsy. Arch. Ophthalmol. 116:674-677. [DOI] [PubMed] [Google Scholar]
- 73.Reference deleted.
- 74.Daly, A. L., L. A. Velazquez, S. F. Bradley, and C. A. Kauffman. 1989. Mucormycosis association with deferoxamine therapy. Am. J. Med. 87:468-471. [DOI] [PubMed] [Google Scholar]
- 75.Dantas, A. M., R. Yamane, and A. G. Camara. 1990. South American blastomycosis: ophthalmic and oculomotor nerve lesions. Am. J. Trop. Med. Hyg. 43:386-388. [DOI] [PubMed] [Google Scholar]
- 76.de Garcia, M. C., M. L. Arboleda, F. Barraquer, and E. Grose. 1997. Fungal keratitis caused by Metarhizium anisopliae var. anisopliae. J. Med. Vet. Mycol. 35:361-363. [PubMed] [Google Scholar]
- 77.del Palacio, A., E. Perez-Blazquez, M. S. Cuetara, M. Garcia-Bravo, D. Criado, C. Gimeno, and A. R. Noriega. 1991. Keratomycosis due to Scedosporium apiospermum. Mycoses 34:483-487. [DOI] [PubMed] [Google Scholar]
- 78.de Shazo, R. D., M. O'Brien, K. Chapin, M. Soto-Aguilar, L. Gardner, and R. Swain. 1997. A new classification and diagnostic criteria for invasive fungal sinusitis. Arch. Otolaryngol. Head Neck Surg. 123:1181-1188. [DOI] [PubMed] [Google Scholar]
- 79.Diaz-Valle, D., J. M. Benitez del Castillo, E. Amor, N. Toledano, M. M. Carretero, and T. Diaz-Valle. 2002. Severe keratomycosis secondary to Scedosporium apiosperum. Cornea 21:516-518. [DOI] [PubMed] [Google Scholar]
- 80.Djalilian, A. R., J. A. Smith, T. J. Walsh, H. L. Malech, and M. R. Robinson. 2001. Keratitis caused by Candida glabrata in a patient with chronic granulomatous disease. Am. J. Opthalmol. 132:782-783. [DOI] [PubMed] [Google Scholar]
- 80a.D'Mellow, G., L. W. Hirst, M. Whitby, G. Nimmo, and K. Stallard. 1991. Intralenticular infections. Ophthalmology 98:1376-1378. [DOI] [PubMed] [Google Scholar]
- 81.Donahue, S. P., C. M. Greven, J. J. Zuravleff, A. W. Eller, M. H. Nguyen, J. E. Peacock, Jr., M. W. Wagener, and V. L. Yu. 1994. Intraocular candidiasis in patients with candidemia. Clinical implications derived from a prospective multicenter study. Ophthalmology 101:1302-1309. [DOI] [PubMed] [Google Scholar]
- 82.Doorenbos-Bot, A. C., J. M. Hooymans, and L. J. Blanksma. 1990. Periorbital necrotising fasciitis due to Cryptococcus neoformans in a healthy young man. Doc. Ophthalmol. 75:315-320. [DOI] [PubMed] [Google Scholar]
- 83.Dugel, P. U., N. A. Rao, D. J. Foster, L. P. Chong, G. T. Frangieh, and F. Sattler. 1990. Pneumocystis carinii choroiditis in patients receiving inhaled pentamidine. Am. J. Ophthalmol. 110:113-117. [DOI] [PubMed] [Google Scholar]
- 84.Duguid, I. G., J. K. Dart, N. Morlet, B. D. Allan, M. Matheson, L. Ficker, and S. Tuft. 1997. Outcome of Acanthamoeba keratitis treated with polyhexamethylene biguanide and propamidine. Ophthalmology 104:1587-1592. [DOI] [PubMed] [Google Scholar]
- 85.Dunlop, A. A., E. D. Wright, S. A. Howlader, I. Nasrul, R. Husain, K. McClellan, and F. A. Billson. 1994. Suppurative corneal ulceration in Bangladesh: a study of 142 cases, examining the microbiological diagnosis, clinical and epidemiological features of bacterial and fungal keratitis. Aust. N. Z. J. Ophthalmol. 22:105-110. [DOI] [PubMed] [Google Scholar]
- 86.Reference deleted.
- 87.Fairley, C., T. J. Sullivan, P. Bartley, T. Allworth, and R. Lewandowski. 2000. Survival after rhino-orbital-cerebral mucormycosis in an immunocompetent patient. Ophthalmology 107:555-558. [DOI] [PubMed] [Google Scholar]
- 88.Fekrat, S., J. A. Haller, W. R. Green, and J. D. Gottsch. 1995. Pseudophakic Candida parapsilosis endophthalmitis with a consecutive keratitis. Cornea 14:212-216. [PubMed] [Google Scholar]
- 89.Feman, S. S., and R. H. Tilford. 1985. Ocular findings in patients with histoplasmosis. JAMA 253:2534-2537. [PubMed] [Google Scholar]
- 90.Ferguson, B. J., T. G. Mitchell, R. Moon, E. M. Camporesi, and J. Farmer. 1988. Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev. Infect. Dis. 10:551-559. [DOI] [PubMed] [Google Scholar]
- 91.Ferrer, C., F. Colom, S. Frasés, E. Mulet, J. L. Abad, and J. L. Alió. 2001. Detection and identification of fungal pathogens by PCR and by ITS2 and 5.8S ribosomal DNA typing in ocular infections. J. Clin. Microbiol. 39:2873-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferrer, C., G. Munoz, J. L. Alio, J. L. Abad, and F. Colom. 2002. Polymerase chain reaction diagnosis in fungal keratitis caused by Alternaria alternata. Am. J. Ophthalmol. 133:398-399. [DOI] [PubMed] [Google Scholar]
- 93.Fincher, R.-M. E., J. F. Fisher, R. D. Lovell, C. L. Newman, A. Espinel-Ingroff, and H. J. Shadomy. 1991. Infection due to the fungus Acremonium (Cephalosporium). Medicine (Baltimore) 70:398-409. [DOI] [PubMed] [Google Scholar]
- 94.Fine, M. E., M. T. Girard, and M. Matsubara. 1992. Collagenolytic/gelatinolytic enzymes in corneal wound healing. Acta Ophthalmol. (Suppl.) 202:26-33. [DOI] [PubMed] [Google Scholar]
- 95.Fiscella, R. G., M. Moshifar, C. R. Messick, S. L. Pendland, J. W. Chandler, and M. Viana. 1997. Polyhexamethylene biguanide (PHMB) in the treatment of experimental Fusarium keratomycosis. Cornea 16:447-449. [PubMed] [Google Scholar]
- 96.Fitzsimons, R., and A. L. Peters. 1986. Miconazole and ketoconazole as a satisfactory first-line treatment for keratomycosis. Am. J. Ophthalmol. 101:605-608. [DOI] [PubMed] [Google Scholar]
- 97.Florakis, G. J., G. Moazami, H. Schubert, C. J. Koester, and J. D. Auran. 1997. Scanning slit confocal microscopy of fungal keratitis. Arch. Ophthalmol. 115:1461-1463. [DOI] [PubMed] [Google Scholar]
- 98.Font, R. L., and F. A. Jakobiec. 1976. Granulomatous necrotising retinochoroiditis caused by Sporotrichum schenckii. Report of a case, including immunofluorescence and electron microscopical studies. Arch. Ophthalmol. 94:1513-1519. [DOI] [PubMed] [Google Scholar]
- 99.Foroozan, R., R. C. Eagle, Jr., and E. J. Cohen. 2000. Fungal keratitis in a soft contact lens wearer. CLAO J. 26:166-168. [PubMed] [Google Scholar]
- 100.Forster, R. K. 1994. Fungal keratitis and conjunctivitis: clinical aspects, p. 239-252. In G. Smolin and R. A. Thoft (ed). The cornea: scientific foundations and clinical practice, 3rd ed. Little, Brown & Co., Boston, Mass.
- 101.Foster, C. S. 1981. Miconazole therapy for keratomycosis. Am. J. Ophthalmol. 91:622-629. [DOI] [PubMed] [Google Scholar]
- 102.Foster, C. S., J. H. Lass, K. Moran-Wallace, and R. Giovanoni. 1981. Ocular toxicity of topical antifungal agents. Arch. Ophthalmol. 99:1081-1084. [DOI] [PubMed] [Google Scholar]
- 103.Foster, C. S., and M. Stefanyszyn. 1979. Intraocular penetration of miconazole in rabbits. Arch. Ophthalmol. 97:1703-1706. [DOI] [PubMed] [Google Scholar]
- 104.Foster, R. E., C. Y. Lowder, D. M. Meisler, S. S. Huang, and D. L. Longworth. 1991. Presumed Pneumocystis carinii choroiditis. Unifocal presentation, regression with intravenous pentamidine and choroiditis recurrence. Ophthalmology 98:1360-1365. [PubMed] [Google Scholar]
- 105.Fraser-Smith, E. B., and T. R. Matthews. 1987. Effect of ketorolac on Candida albicans ocular infection in rabbits. Arch. Ophthalmol. 105:264-267. [DOI] [PubMed] [Google Scholar]
- 106.Reference deleted.
- 107.Friedberg, M. L., U. Pleyer, and B. J. Mondino. 1991. Device drug delivery to the eye. Collagen shields, iontophoresis and pumps. Ophthalmology 98:725-732. [DOI] [PubMed] [Google Scholar]
- 108.Gaines, J. J., Jr., J. R. Clay, F. W. Chandler, M. E. Powell, P. A. Sheffield, and A. P. Keller III. 1996. Rhinosporidiosis: three domestic cases. South. Med. J. 89:65-67. [DOI] [PubMed] [Google Scholar]
- 109.Gamba, J. L., W. Woodruff, W. T. Djang, and A. E. Yeates. 1986. Craniofacial mucormycosis: assessment with CT. Radiology 160:207-212. [DOI] [PubMed] [Google Scholar]
- 110.Garcia, M. L., J. M. Herreras, E. Dios, P. Argueso, and A. Almaraz. 2002. Evaluation of lectin staining in the diagnosis of fungal keratitis in an experimental rabbit model. Mol. Vis. 8:10-16. [PubMed] [Google Scholar]
- 111.Garg, P., U. Gopinathan, K. Choudhary, and G. N. Rao. 2000. Keratomycosis— clinical and microbiologic experience with dematiaceous fungi. Ophthalmology 107:574-580. [DOI] [PubMed] [Google Scholar]
- 112.Gaudio, P. A., U. Gopinathan, V. Sangwan, and T. E. Hughes. 2002. Polymerase chain reaction based detection of fungi in infected corneas. Br. J. Ophthalmol. 86:755-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Georgiadis, N. S., C. D. Terzidou, and A. S. Dimitriadis. 1998. Restoration of the anophthalmic socket with secondary implantation of a corraline hydroxyapatite sphere. Ophthalmic Surg. Lasers 29:808-814. [PubMed] [Google Scholar]
- 114.Ghose, S., and V. M. Mahajan. 1990. Fungal flora in congenital dacryocystitis. Indian J. Ophthalmol. 38:189-190. [PubMed] [Google Scholar]
- 115.Glasgow, B. J., R. E. Engstrom, Jr., G. N. Holland, A. E. Kreiger, and M. G. Wool. 1996. Bilateral endogenous Fusarium endophthalmitis associated with the acquired immunodeficiency syndrome. Arch. Ophthalmol. 114:873-877. [DOI] [PubMed] [Google Scholar]
- 116.Goldblum, D., B. E. Frueh, S. Zimmerli, and M. Böhnke. 2000. Treatment of post-keratitis Fusarium endophthalmitis with amphotericin B. Lipid complex. Cornea 19:853-856. [DOI] [PubMed] [Google Scholar]
- 117.Gonawerdena, S. A., K. P. Ranasinghe, S. N. Arseculeratne, C. R. Seimon, and L. Ajello. 1994. Survey of mycotic and bacterial keratitis in Sri Lanka. Mycopathologia 127:77-81. [DOI] [PubMed] [Google Scholar]
- 118.Gonzales, C. A., I. U. Scott, N. A. Chaudhry, K. M. Luu, D. Miller, T. G. Murray, and J. L. Davis. 2000. Endogenous endophthalmitis caused by Histoplasma capsulatum var. capsulatum. A case report and literature review. Ophthalmology 107:725-729. [DOI] [PubMed] [Google Scholar]
- 119.Gopinathan, U., T. Ramakrishna, M. Willcox, C. M. Rao, D. Balasubramanian, A. Kulkarni, G. K. Vemuganti, and G. N. Rao. 2001. Enzymatic, clinical and histologic evaluation of corneal tissues in experimental fungal keratitis in rabbits. Exp. Eye Res. 72:433-442. [DOI] [PubMed] [Google Scholar]
- 120.Gopinathan, U., P. Garg, M. Fernandes, S. Sharma, S. Athmanathan, and G. N. Rao. 2002. The epidemiological features and laboratory results of fungal keratitis. A 10-year review at a referral eye care center in South India. Cornea 21:555-559. [DOI] [PubMed] [Google Scholar]
- 121.Gordon, M. A., and S. W. Norton. 1985. Corneal transplant infection by Paecilomyces lilacinus. Sabouraudia 23:295-301. [PubMed] [Google Scholar]
- 122.Gori, S., and A. Scasso. 1994. Cytologic and differential diagnosis of rhinosporidiosis. Acta Cytol. 38:361-366. [PubMed] [Google Scholar]
- 123.Gottsch, J. D., M. L. Gilbert, D. F. Goodman, M. E. Sulewski, J. D. Dick, and W. J. Stark. 1991. Excimer laser ablation of microbial keratitis. Ophthalmology 98:146-149. [DOI] [PubMed] [Google Scholar]
- 124.Grant, S. M., and S. P. Clissold. 1989. Itraconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in superficial and systemic mycoses. Drugs 37:310-344. [DOI] [PubMed] [Google Scholar]
- 125.Gray, T. B., R. T. Cursons, J. F. Sherwan, and P. R. Rose. 1995. Acanthamoeba bacterial and fungal contamination of contact lens storage cases. Br. J. Ophthalmol. 79:601-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Groden, L. R., J. Rodnite, J. H. Brinser, and G. I. Genvert. 1990. Acridine orange and Gram stains in infectious keratitis. Cornea 9:122-124. [PubMed] [Google Scholar]
- 127.Reference deleted.
- 128.Guarro, J., C. de Vroey, and J. Gené. 1991. Concerning the implication of Arthrobotrys oligospora in a case of keratitis. J. Med. Vet. Mycol. 29:349-352. [Google Scholar]
- 129.Guarro, J., W. Gams, I. Pujol, and J. Gené. 1997. Acremonium species: new emerging fungal opportunists—in vitro antifungal susceptibilities and review. Clin. Infect. Dis. 25:1222-1229. [DOI] [PubMed] [Google Scholar]
- 130.Guarro, J., T. Akiti, R. Almada-Horta, L. A. M. Leite-Filho, J. Gené, S. Ferreira-Gomes, C. Aguilar, and M. Ortoneda. 1999. Mycotic keratitis due to Curvularia senegalensis and in vitro antifungal susceptibilities of Curvularia spp. J. Clin. Microbiol. 37:4170-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guarro, J., L. A. Vieira, D. De Freitas, J. Gené, I. Zaror, A. L. Hofling-Lima, O. Fischman, C. Zorat-Yu, and M. J. Figueras. 2000. Phaeoisaria clematidis as a cause of keratomycosis. J. Clin. Microbiol. 38:2434-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guarro, J., A. L. Hofling-Lima, J. Gené, D. De Freitas, P. Godoy, M. L. Zorat-Yu, L. Zaror, and O. Fischman. 2002. Corneal ulcer caused by the new fungal species Sarcopodium oculorum. J. Clin. Microbiol. 40:3071-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gugnani, H. C., S. Gupta, and R. S. Talwar. 1978. Role of opportunistic fungi in ocular infections in Nigeria. Mycopathologia 65:155-166. [DOI] [PubMed] [Google Scholar]
- 134.Gupta, A., A. Sharma, K. Mohan, and A. Gupta. 1999. Mycotic keratitis in non-steroid exposed vernal keratoconjunctivitis. Acta Ophthalmol. Scand. 77:229-231. [DOI] [PubMed] [Google Scholar]
- 135.Reference deleted.
- 136.Guzek, J. P., J. M. Roosenberg, D. L. Gano, and I. F. Wessels. 1998. The effect of vehicle on corneal penetration of triturated ketoconazole and itraconazole. Ophthalmic Surg. Lasers 29:926-929. [PubMed] [Google Scholar]
- 137.Hagan, M., E. Wright, M. Newman, P. Dolin, and G. Johnson. 1995. Causes of suppurative keratitis in Ghana. Br. J. Ophthalmol. 79:1024-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Harril W. C., M. G. Stewart, A. G. Lee, and P. Cernoch. 1996. Chronic rhinocerebral mucormycosis. Laryngoscope 106:1292-1297. [DOI] [PubMed] [Google Scholar]
- 139.Harrousseau, J. L., A. W. Dekker, A. Stamatoullas-Bastard, A. Fassas, W. Linkesch, J. Gouveia, R. De Bock, M. Rovira, W. F. Seifert, H. Joosen, M. Peeters, and K. De Beule. 2000. Itraconazole oral solution for primary prophylaxis of fungal infections in patients with haematological malignancy and profound neutropaenia: a randomised, double-blind, double-placebo, multicenter trial comparing itraconazole and amphotericin B. Antimicrob. Agents Chemother. 44:1887-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hay, J., S. Patel, and D. V. Seal. 1995. Exophiala jeanselmei: a potential ocular pathogen. J. Br. Contact Lens Assoc. 18:99-101. [Google Scholar]
- 141.Hazen, P. G., A. E. Walker, J. J. Stewart, J. F. Carney, C. W. Engstrom, and K. L. Turgeon. 1992. Keratitis, ichthyosis and deafness (KID) syndrome: management with chronic oral ketoconazole therapy. Int. J. Dermatol. 31:58-59. [DOI] [PubMed] [Google Scholar]
- 142.Heidemann, D. G., S. P. Dunn, and J. C. Watts. 1995. Aspergillus keratitis after radial keratotomy Am. J. Ophthalmol. 120:254-256. [DOI] [PubMed] [Google Scholar]
- 143.Hejny, C., J. B. Kerrison, N. J. Newman, and C. M. Stone. 2001. Rhinoorbital mucormycosis in a patient with acquired immunodeficiency syndrome (AIDS) and neutropenia. Am. J. Ophthalmol. 132:111-112 [DOI] [PubMed] [Google Scholar]
- 144.Hemady, R. K. 1995. Microbial keratitis in patients infected with the human immunodeficiency virus. Ophthalmology 102:1026-1030. [DOI] [PubMed] [Google Scholar]
- 145.Hemady, R. K., W. Chu, and C. S. Foster. 1992. Intraocular penetration of ketoconazole in rabbits. Cornea 11:329-333. [DOI] [PubMed] [Google Scholar]
- 146.Hester, D. E., J. A. Kylstra, and D. E. Eifrig. 1992. Isolated ocular cryptococcosis in an immunocompetent patient. Ophthalmic Surg. 23:129-131. [PubMed] [Google Scholar]
- 147.Hidalgo, J. A., G. J. Alangaden, D. Eliott, R. A. Akins, J. Puklin, G. Abrams, and J. A. Vasquez. 2000. Fungal endophthalmitis diagnosis by detection of Candida albicans DNA in intraocular fluid by use of a species-specific polymerase chain reaction assay. J. Infect. Dis. 181:1198-1201. [DOI] [PubMed] [Google Scholar]
- 148.Hirose, H., H. Terasaki, S. Awaya, and T. Yasuma. 1997. Treatment of fungal corneal ulcers with amphotericin B ointment. Am. J. Ophthalmol. 124:836-838. [DOI] [PubMed] [Google Scholar]
- 149.Hiruma, M., A. Kawada, T. Shimizu, and A. Kukita. 1991. Tinea of the eyebrow showing kerion celsi: report of one case. Cutis 48:149-150. [PubMed] [Google Scholar]
- 150.Reference deleted.
- 151.Hostetter, M. K. 1994. Adhesins and ligands involved in the interaction of Candida spp. with epithelial and endothelial surfaces. Clin. Microbiol. Rev. 7:29-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Howard, D. H. 1999. Acquisition, transport and storage of iron by pathogenic fungi. Clin. Microbiol. Rev. 12:394-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huang, W. J., F. R. Hu, and S. W. Chang. 1994. Clinicopathologic study of Gore-Tex patch graft in corneoscleral surgery. Cornea 13:82-86. [DOI] [PubMed] [Google Scholar]
- 154.Huber-Spitzy, V., I. Baumgartner, K. Bohler-Sommeregger, and G. Grabner. 1991. Blepharitis— a diagnostic and therapeutic challenge. Graefe's Arch. Clin. Exp. Ophthalmol. 229:224-227. [DOI] [PubMed] [Google Scholar]
- 155.Imwidthaya, P. 1994. Human pythiosis in Thailand. Postgrad. Med. J. 70:558-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Imwidthaya, P. 1995. Mycotic keratitis in Thailand. J. Med. Vet. Mycol. 33:81-82. [DOI] [PubMed] [Google Scholar]
- 157.Ishibashi, Y. 1983. Oral ketoconazole therapy for keratomycosis. Am. J. Ophthalmol. 95:342-345. [DOI] [PubMed] [Google Scholar]
- 158.Ishibashi, Y., and H. E. Kaufman. 1986. Corneal biopsy in the diagnosis of keratomycosis. Am. J. Ophthalmol. 101:288-293. [DOI] [PubMed] [Google Scholar]
- 159.Reference deleted.
- 160.Ishibashi, Y., S. Hommura, and Y. Matsumoto. 1987. Direct examination vs. culture of biopsy specimens for the diagnosis of keratomycosis. Am. J. Ophthalmol. 103:636-640. [DOI] [PubMed] [Google Scholar]
- 161.Ishibashi, Y., Y. Matsumoto, and K. Takei. 1984. The effects of intravenous miconazole on fungal keratitis. Am. J. Ophthalmol. 98:433-437. [DOI] [PubMed] [Google Scholar]
- 162.Jabs, D. A., W. R. Green, R. Fox, B. F. Polk, and J. G. Bartlett 1989. Ocular manifestations of acquired immune deficiency syndrome. Ophthalmology 96:1092-1099. [DOI] [PubMed] [Google Scholar]
- 163.Jacob, P., U. Gopinathan, S. Sharma, and G. N. Rao. 1995. Calcium alginate swabs versus Bard-Parker blade in the diagnosis of microbial keratitis. Cornea 14:360-364. [DOI] [PubMed] [Google Scholar]
- 164.Jacobson, M., S. L. Galetta, S. W. Atlas, M. T. Curtis, and A. W. Wulc. 1992. Bipolaris-induced orbital cellulitis. J. Clin. Neuroophthalmol. 12:250-256. [PubMed] [Google Scholar]
- 165.Jaeger, E. E., N. M. Carroll, S. Choudhury, A. A. Dunlop, H. M. Towler, M. M. Matheson, P. Adamson, N. Okhravi, and S. Lightman. 2000. Rapid detection and identification of Candida, Aspergillus and Fusarium species in ocular samples using nested PCR. J. Clin. Microbiol. 38:2902-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jampol, L. M., J. Sung, J. D. Walker, J. C. Folk, W. A. Townsend-Pico, C. Y. Lowder, E. M. Dodds, D. Westrich, and J. Terry. 1996. Choroidal neovascularization secondary to Candida albicans chorioretinitis. Am. J. Ophthalmol. 121:643-649. [DOI] [PubMed] [Google Scholar]
- 167.Jay, W. M., R. W. Bradsher, B. LeMay, N. Snyderman, and E. J. Angtuaco. 1988. Ocular involvement in mycotic sinusitis caused by Bipolaris. Am. J. Ophthalmol. 105:366-370. [DOI] [PubMed] [Google Scholar]
- 168.Jiang, R. S., and C. Y. Hsu. 1999. Endoscopic sinus surgery for rhinocerebral mucormycosis. Am. J. Rhinol. 13:105-109. [DOI] [PubMed] [Google Scholar]
- 169.Jimenez, J. F., D. E. Young, and A. J. Hough, Jr. 1984. Rhinosporidiosis: a report of two cases from Arkansas. Am. J. Clin. Pathol. 82:611-615. [DOI] [PubMed] [Google Scholar]
- 170.Johns, K. J., and D. M. O'Day. 1988. Pharmacologic management of keratomycoses. Surv. Ophthalmol. 33:178-188. [DOI] [PubMed] [Google Scholar]
- 171.Johnson, G. J. 1998. External eye infections. J. Community Eye Health 12:1-2. [PMC free article] [PubMed] [Google Scholar]
- 172.Johnson, T. E., R. R. Casiano, J. W. Kronish, D. T. Tse, M. Meldrum, and W. Chang. 1999. Sino-orbital aspergillosis in acquired immunodeficiency syndrome. Arch. Ophthalmol. 117:57-64. [DOI] [PubMed] [Google Scholar]
- 173.Jones, B. R. 1975. Principles in the management of oculomycosis. Am. J. Ophthalmol. 79:719-751. [DOI] [PubMed] [Google Scholar]
- 174.Jones, D. B. 1980. Strategy for the initial management of suspected microbial keratitis, p. 86-119. In J. I. Barraquer, P. S. Binder, J. N. Buxton, M. Fine, D. B. Jones, P. R. Laibson, A. B. Nesburn, D. Paton, and C. Troutman (ed.), Symposium on medical and surgical diseases of the cornea. Transactions of the New Orleans Academy of Ophthalmology. C. V. Mosby, St. Louis, Mo.
- 175.Jones, D. B., T. J. Liesegang, and N. M. Robinson. 1981. Cumitech 13, Laboratory diagnosis of ocular infections. Coordinating ed., J. A. Washington III. American Society for Microbiology, Washington, D.C.
- 176.Jones, J., S. E. Katz, and M. Lubow. 1999. Scedosporium apiospermum of the orbit. Arch. Ophthalmol. 117:272-273. [PubMed] [Google Scholar]
- 177.Kalavathy, C. M., P. A. Thomas, and J. Rajasekaran. 1986. Spectrum of microbial infection in dacryocystitis. J. Madras State Ophthalmol. Assoc. 24:24-27. [Google Scholar]
- 178.Kalavathy, C. M., P. A. Thomas, S. Thangaraj, and C. A. N. Jesudasan. 1998. Rhinosporidiosis of the lacrimal sac: a report of two cases. J. Tamilnadu Ophthalmol.Assoc. 37:11-12. [Google Scholar]
- 179.Kanungo, R., R. Srinivasan, and R. S. Rao. 1991. Acridine orange staining in early diagnosis of mycotic keratitis. Acta Ophthalmol. Copenh. 69:750-753. [DOI] [PubMed] [Google Scholar]
- 180.Katz, B. J., W. E. Scott, and J. C. Folk. 1997. Acute histoplasmosis choroiditis in 2 immunocompetent brothers. Arch. Ophthalmol. 115:1470-1471. [DOI] [PubMed] [Google Scholar]
- 181.Kaufman, L., A. A. Padhye, and S. Parker. 1988. Rhinocerebral zygomycosis caused by Saksenaea vasiformis. J. Med. Vet. Mycol. 26:237-241. [PubMed] [Google Scholar]
- 182.Kaushik, S., J. Ram, A. Chakarabarty, M. R. Dogra, G. S. Brar, and A. Gupta. 2001. Curvularia lunata endophthalmitis with secondary keratitis. Am. J. Ophthalmol. 131:140-142. [DOI] [PubMed] [Google Scholar]
- 183.Kaushik, S., J. Ram, G. S. Brar, A. K. Jain, A. Chakraborti, and A. Gupta. 2001. Intracameral amphotericin B: Initial experience in severe keratomycosis. Cornea 20:715-719. [DOI] [PubMed] [Google Scholar]
- 184.Kenyon, K. R. 1985. Inflammatory mechanisms in corneal ulceration. Trans. Am. Ophthalmol. Soc. 83:610-663. [PMC free article] [PubMed] [Google Scholar]
- 185.Kestelyn, P., H. Taelman, J. Bogaerts, A. Kagame, M. A. Aziz, J. Batungwanayo, A. M. Stevens, and P. van de Perre. 1993. Ophthalmic manifestations of infections with Cryptococcus neoformans in patients with the acquired immunodeficiency syndrome. Am. J. Ophthalmol. 116:721-727. [DOI] [PubMed] [Google Scholar]
- 186.Khairallah, S. H., K. A. Byrne, and K. F. Tabbara. 1992. Fungal keratitis in Saudi Arabia. Doc. Ophthalmol. 79:269-276. [DOI] [PubMed] [Google Scholar]
- 187.Khoo, S. H., J. Bond, and D. W. Denning. 1994. Administering amphotericin B—a practical approach. J. Antimicrob. Chemother. 33:203-213. [DOI] [PubMed] [Google Scholar]
- 188.Killingsworth, D. W., G. A. Stern, W. T. Driebe, A. Knapp, and D. M. Dragon. 1993. Results of therapeutic penetrating keratoplasty. Ophthalmology 100:534-541. [DOI] [PubMed] [Google Scholar]
- 189.Kim, J. S., J. C. Kim, T. W. Hahn, and W. C. Park. 2001. Amniotic membrane transplantation in infectious corneal ulcer. Cornea 20:720-726. [DOI] [PubMed] [Google Scholar]
- 190.Kiryu, H., S. Yoshida, Y. Suenaga, and M. Asahi. 1991. Invasion and survival of Fusarium solani in the dexamethasone-treated cornea of rabbits. J. Med. Vet. Mycol. 29:395-406. [PubMed] [Google Scholar]
- 191.Kisla, T. A., A. Cu-Unjieng, L. Sigler, and J. Sugar. 2000. Medical management of Beauveria bassiana keratitis. Cornea 19:405-406. [DOI] [PubMed] [Google Scholar]
- 192.Klapper, S. R., A. G. Lee, J. R. Patrinely, M. Stewart, and E. L. Alford. 1997. Orbital involvement in allergic fungal sinusitis. Ophthalmology 104:2094-2100. [DOI] [PubMed] [Google Scholar]
- 193.Klippenstein, K., D. M. O'Day, R. D. Robinson, T. E. Williams, and W. S. Head. 1993. The qualitative evaluation of the pharmacokinetics of subconjunctivally injected antifungal agents in rabbits. Cornea 12:512-516. [DOI] [PubMed] [Google Scholar]
- 194.Klotz, S. A., C. C. Penn, G. J. Negvesky, and S. I. Butrus. 2000. Fungal and parasitic infections of the eye. Clin. Microbiol. Rev. 13:662-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kohn, R., and R. Hepler. 1985. Management of limited rhino-orbital mucormycosis without exenteration. Ophthalmology 92:1440-1443. [DOI] [PubMed] [Google Scholar]
- 196.Kompa, S., S. Langefeld, B. Kirchkof, and N. Schrage. 1999. Corneal biopsy in keratitis performed with the microtrephine. Graefe's Arch. Clin. Exp. Ophthalmol. 237:915-919. [DOI] [PubMed] [Google Scholar]
- 197.Kozarsky, A. M., R. D. Stulting, G. O. Waring III, F. M. Cornell, L. A. Wilson, and H. D. Cavanaugh. 1984. Penetrating keratoplasty for exogenous Paecilomyces lilacinus keratitis followed by post-operative endophthalmitis. Am. J. Ophthalmol. 98:552-557. [DOI] [PubMed] [Google Scholar]
- 198.Kremer, I., M. Goldenfeld, and D. Shmueli. 1991. Fungal keratitis associated with contact lens wear after penetrating keratoplasty. Ann. Ophthalmol. 23:342-345. [PubMed] [Google Scholar]
- 199.Krishnan, M. M., V. K. Kawatra, V. A. Rao, and C. Ratnakar. 1986. Diverticulum of the lacrimal sac associated with rhinosporidiosis. Br. J. Ophthalmol. 70:867-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kristinsson, J. H., and H. Sigurdsson. 1998. Lacrimal sac plugging caused by Aspergillus fumigatus. Acta Ophthalmol. Scand. 76:241-242. [DOI] [PubMed] [Google Scholar]
- 201.Kronish, J. W., T. E. Johnson, S. M. Gilberg, G. F. Corrent, W. M. McLeish, and K. R. Scott. 1996. Orbital infections in patients with human immunodeficiency virus infection. Ophthalmology 103:1483-1492. [DOI] [PubMed] [Google Scholar]
- 202.Kumar, B., G. J. Crawford, and G. C. Morlet. 1997. Scedosporium prolificans corneoscleritis: a successful outcome. Aust. N. Z. J. Ophthalmol. 25:169-171. [DOI] [PubMed] [Google Scholar]
- 203.Kuo, I. C., T. P. Margolis, V. Cevallos, and D. G. Hwang. 2001. Aspergillus fumigatus keratitis after laser in situ keratomileusis. Cornea 20:342-344. [DOI] [PubMed] [Google Scholar]
- 204.Kuriakose, T., and P. A. Thomas. 1991. Keratomycotic malignant glaucoma. Indian J. Ophthalmol. 39:118-121. [PubMed] [Google Scholar]
- 205.Kurosawa, A., S. C. Pollock, M. P. Collins, C. R. Kraff, and M. O. Tso. 1988. Sporothrix schenckii endophthalmitis in a patient with human immunodeficiency virus infection. Arch. Ophthalmol. 106:376-380. [DOI] [PubMed] [Google Scholar]
- 206.Langford, J. D., D. L. MacCartney, and R. C. Wang. 1997. Frozen section-guided surgical debridement for management of rhino-orbital mucormycosis. Am. J. Ophthalmol. 124:265-267. [DOI] [PubMed] [Google Scholar]
- 207.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Leck, A. K., P. A. Thomas, M. Hagan, J. Kaliamurthy, E. Ackuaku, M. John, M. J. Newman, F. S. Codjoe, J. A. Opintan, C. M. Kalavathy, V. Essuman, C. A. N. Jesudasan, and G. J. Johnson. 2002. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br. J. Ophthalmol. 86:1211-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lee, B. L., G. N. Holland, and B. J. Glasgow. 1996. Chiasmal infarction and sudden blindness caused by mucormycosis in AIDS and diabetes mellitus. Am. J. Ophthalmol. 125:895-896. [DOI] [PubMed] [Google Scholar]
- 210.Lee, P., and W. R. Green. 1990. Corneal biopsy. Indications, techniques and a report of a series of 87 cases. Ophthalmology 97:718-721. [DOI] [PubMed] [Google Scholar]
- 211.Legeais, J. M., V. Blanc, D. Basset, F. D'Hermies, S. Harrabi, E. Frau, L. Goichot, C. Renard, and Y. Pouliquen. 1994. Keratomycoses severes: diagnostic et traitment. J. Fr. Ophthalmol. 17:568-573 [PubMed] [Google Scholar]
- 212.Levenson, J. E., R. M. Duffin, S. K. Gardner, and T. H. Pettit. 1984. Dematiaceous fungal keratitis following penetrating keratoplasty. Ophthalmic Surg. 15:578-582. [PubMed] [Google Scholar]
- 213.Levin, L. A., R. Avery, J. W. Shore, J. J. Woog, and A. S. Baker 1996. The spectrum of orbital aspergillosis: a clinicopathological review. Surv. Ophthalmol. 41:142-154. [DOI] [PubMed] [Google Scholar]
- 214.Lewis, H., T. M. Aaberg, D. R. Fary, and T. S. Stevens. 1988. Latent disseminated blastomycosis with choroidal involvement. Arch. Ophthalmol. 106:527-530. [DOI] [PubMed] [Google Scholar]
- 215.Li, S., J. I. Perlman, D. P. Edward, and R. Weiss. 1998. Unilateral Blastomyces dermatitidis endophthalmitis and orbital cellulitis: a case report and literature review. Ophthalmology 105:1466-1470. [DOI] [PubMed] [Google Scholar]
- 216.Liesegang, T. J., and R. K. Forster. 1980. Spectrum of microbial keratitis in South Florida. Am. J. Ophthalmol. 90:38-47. [DOI] [PubMed] [Google Scholar]
- 217.Liesegang, T. J. 1997. Contact lens-related microbial keratitis. I. Epidemiology. Cornea 16:125-131. [PubMed] [Google Scholar]
- 218.Liesegang, T. J. 1997. Contact lens-related microbial keratitis. II. Pathophysiology. Cornea 16:265-273. [PubMed] [Google Scholar]
- 219.Lin, S. H., C. P. Lin, H. Z. Wang, R. K. Tsai, and C. K. Ho. 1999. Fungal corneal ulcers of onion harvesters in southern Taiwan. Occup. Environ. Med. 56:423-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu, K., D. N. Howell, J. R. Perfect, and W. A. Schell. 1998. Morphologic criteria for the preliminary identification of Fusarium, Paecilomyces and Acremonium species by histopathology. Am. J. Clin. Pathol. 109:45-54. [DOI] [PubMed] [Google Scholar]
- 221.Locher, D. H., A. Adesina, T. C. Wolf, C. B. Imes, and J. Chodosh. 1998. Post-operative Rhizopus scleritis in a diabetic man. J. Cataract Refract. Surg. 24:562-565. [DOI] [PubMed] [Google Scholar]
- 222.Luttrull, J. K., W. L. Wan, B. M. Kubak, M. D. Smith and H. A. Oster. 1995. Treatment of ocular fungal infections with oral fluconazole. Am. J. Ophthalmol. 119:477-481. [DOI] [PubMed] [Google Scholar]
- 223.Lyman, C. A., and T. J. Walsh. 1992. Systemically administered antifungal agents. A review of their clinical pharmacology and therapeutic applications. Drugs 44:9-35. [DOI] [PubMed] [Google Scholar]
- 224.Macher, A., M. M. Rodrigues, W. Kaplan, M. C. Pistole, A. McKittrick, W. E. Lawrinson, and C. M. Reichert. 1985. Disseminated bilateral chorioretinitis due to Histoplasma capsulatum in a patient with the acquired immunodeficiency syndrome. Ophthalmology 92:1159-1164. [DOI] [PubMed] [Google Scholar]
- 225.Maguire, L. J., R. J. Campbell, and R. S. Edson. 1994. Coccidioidomycosis with necrotising granulomatous conjunctivitis. Cornea 13:539-542. [PubMed] [Google Scholar]
- 226.Mahajan, V. M. 1992. Rhinosporidiosis, p. 175-184. In E. S. Maghoub (ed.), Tropical mycoses. Janssen Research Council, Beerse, Belgium.
- 227.Manzouri, B., G. C. Vafidis, and R. K. Wyse. 2001. Pharmacotherapy of fungal eye infections. Expert Opin. Pharmacother. 2:1849-1857. [DOI] [PubMed] [Google Scholar]
- 228.Marcus, L., H. F. Vismer, H. J. van der Hoven, E. Gove, and P. Meewes. 1992. Mycotic keratitis caused by Curvularia brachyspora (Boedjin). A report of the first case. Mycopathologia 119:29-33. [DOI] [PubMed] [Google Scholar]
- 229.Margo, C. E., and T. Bombardier. 1985. The diagnostic value of fungal autofluorescence. Surv. Ophthalmol. 29:374-376. [DOI] [PubMed] [Google Scholar]
- 230.Margo, C. E., F. M. Polack, and C. I. Hood. 1988. Aspergillus panophthalmitis complicating treatment of pterygium. Cornea 7:285-289. [PubMed] [Google Scholar]
- 231.Marshall, D. H., S. Brownstein, W. B. Jackson, G. Mintsioulis, S. M. Gilberg, and B. F. Al-Zeerah. 1997. Post-traumatic corneal mucormycosis caused by Absidia corymbifera. Ophthalmology 104:1107-1111. [DOI] [PubMed] [Google Scholar]
- 232.Maskin, S. L., and E. Alfonso. 1992. Fungal keratitis after radial keratotomy. Am. J. Ophthalmol. 114:369-370. [DOI] [PubMed] [Google Scholar]
- 233.Maskin, S. L., R. J. Fetchick, C. R. Leone, Jr., P. K. Sharkey, and M. G. Rinaldi. 1989. Bipolaris hawaiiensis—caused phaeohyphomycotic orbitopathy. A devastating fungal sinusitis in an apparently immunocompetent host. Ophthalmology 96:175-179. [DOI] [PubMed] [Google Scholar]
- 234.Massry, G. G., A. Hornblass, and W. Harrison. 1996. Itraconazole in the treatment of orbital aspergillosis. Ophthalmology 103:1467-1470. [DOI] [PubMed] [Google Scholar]
- 235.Mathews, M. S., and T. Kuriakose. 1995. Keratitis due to Cephaliophora irregularis Thaxter. J. Med. Vet. Mycol. 33:359-360. [PubMed] [Google Scholar]
- 236.Mauger, T. F., and E. L. Craig. 1994. Havener's ocular pharmacology, 9th ed. C. V. Mosby, St. Louis, Mo.
- 237.McGinnis, M. R. 1980. Laboratory handbook of medical mycology. Academic Press, Inc., New York, N.Y.
- 238.McGinnis, M. R., M. G. Rinaldi, and R. E. Winn. 1986. Emerging agents of phaeohyphomycosis: pathogenic species of Bipolaris and Exserohilum. J. Clin. Microbiol. 24:250-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.McGuire, T. W., J. D. Bullock, J. D. Bullock, Jr., B. L. Elder, and J. W. Funkhouser. 1991. Fungal endophthalmitis. An experimental study with a review of 17 human ocular cases. Arch. Ophthalmol. 109:1289-1296. [DOI] [PubMed] [Google Scholar]
- 240.McLaughlin-Borlace, L., F. Stapleton, M. Matheson, and J. K. G. Dart. 1998. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J. Appl. Microbiol. 84:827-838. [DOI] [PubMed] [Google Scholar]
- 241.McLean, F. M., L. E. Ginsberg, and C. A. Stanton. 1996. Perineural spread of rhinocerebral mucormycosis. Am. J. Neuroradiol. 17:114-116. [PMC free article] [PubMed] [Google Scholar]
- 242.McLeod, S. D. 1997. The role of cultures in the management of ulcerative keratitis. Cornea 16:381-382. [PubMed] [Google Scholar]
- 243.McVicar, I., P. V. Hatton, and I. M. Brook. 1995. Self-reinforced polyglycollic acid membrane: a bioresorbable material for orbital floor repair. Br. J. Oral Maxillofac. Surg. 33:220-223. [DOI] [PubMed] [Google Scholar]
- 244.Mendonza L., L. Ajello, and M. R. McGinnis. 1996. Infections caused by the oomycetous pathogen Pythium insidiosum. J. Mycol. Med. 6:151-164. [Google Scholar]
- 245.Meyer, R. D., C. R. Gaultier, J. T. Yamashita, R. Babapour, H. E. Pitchon, and P. R. Wolfe. 1994. Fungal sinusitis in patients with AIDS: report of 4 cases and review of the literature. Medicine (Baltimore) 73:69-78. [DOI] [PubMed] [Google Scholar]
- 246.Mian, U. K., M. Mayers, Y. Garg, Q. F. Liu, G. Newcomer, C. Madu, W. Liu, A. Louie, and M. H. Miller. 1998. Comparison of fluconazole pharmacokinetics in serum, aqueous humour, vitreous humour, and cerebrospinal fluid following a single dose and a steady state. J. Ocul. Pharmacol. Ther. 14:459-471. [DOI] [PubMed] [Google Scholar]
- 246a.Miller, J. M., and H. T. Holmes. 1999. Specimen collection, transport and storage, p. 33-63. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 247.Mills, R., and G. Garrett. 1992. Pseudallescheria boydii keratitis. Aust. N. Z. J. Ophthalmol. 20:253-256. [DOI] [PubMed] [Google Scholar]
- 248.Mino de Kaspar, H., G. Zoulek, M. E. Paredes, R. Alborno, D. Medina, M. Centurion de Morinigo, M. Ortiz de Fresco, and F. Aguero. 1991. Mycotic keratitis in Paraguay. Mycoses 34:251-254. [DOI] [PubMed] [Google Scholar]
- 249.Miyamoto, H., Y. Ogura, M. Hashizoe, N. Kunou, Y. Honda, and Y. Ikada. 1997. Biodegradable scleral implant for intravitreal controlled release of fluconazole. Curr. Eye Res. 16:930-935. [DOI] [PubMed] [Google Scholar]
- 250.Mochizuki, K., Y. Yamashita, T. Torisaki, K. Komatsu, T. Tanahashi, and H. Sakai. 1992. Intraocular penetration and effect on the retina of fluconazole. Lens Eye Toxic Res. 9:537-546. [PubMed] [Google Scholar]
- 251.Mohan, M., S. K. Gupta, V. K. Kalra, R. B. Vajpayee, and M. S. Sachdev. 1988. Topical silver sulphadiazine: a new drug for ocular keratomycosis. Br. J. Ophthalmol. 72:192-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Moll, G. W., Jr. F. A. Raila, G. C. Liu, and A. W. Conerly, Sr. 1994. Rhinocerebral mucormycosis in IDDM. Sequential magnetic resonance imaging of long-term survival with intensive therapy. Diabetes Care 17:1348-1353. [DOI] [PubMed] [Google Scholar]
- 253.Moorthy, R. S., N. A. Rao, Y. Sidikaro, and R. Y. Foos. 1994. Coccidioidomycosis iridocyclitis. Ophthalmology 101:1923-1928. [DOI] [PubMed] [Google Scholar]
- 254.Moriarty, A. P., G. J. Crawford, I. L. McAllister, and I. J. Constable. 1993. Severe corneoscleral infection. A complication of beta irradiation scleral necrosis following pterygium excision. Arch. Ophthalmol. 111:947-951. [DOI] [PubMed] [Google Scholar]
- 255.Morinelli, E. N., P. U. Dugel, R. Riffenburgh, and N. A. Rao. 1993. Infectious multifocal choroiditis in patients with acquired immunodeficiency syndrome. Ophthalmology 100:1014-1021. [DOI] [PubMed] [Google Scholar]
- 256.Morrison, V. A., and P. B. McGlave. 1993. Mucormycosis in the BMT population. Bone Marrow Transplant. 11:383-388. [PubMed] [Google Scholar]
- 257.Moses, A. E., G. Rahav, Y. Barenholz, J. Elidan, B. Azaz, S. Gillis, M. Brickman, I. Polacheck, and M. Shapiro. 1998. Rhinocerebral mucormycosis treated with amphotericin B colloidal dispersion in three patients. Clin. Infect. Dis. 26:1430-1433. [DOI] [PubMed] [Google Scholar]
- 258.Moses, J. S., C. Balachandran, S. Sandhanam, N. Ratnasamy, S. Thanappan, J. Rajaswar, and D. Moses. 1990. Ocular rhinosporidiosis in Tamilnadu, India. Mycopathologia 111:5-8. [DOI] [PubMed] [Google Scholar]
- 259.Muccioli, C., R. Belfort, Jr., R. Neves, and N. Rao. 1995. Limbal and choroidal Cryptococcus infection in the acquired immunodeficiency syndrome. Am. J. Ophthalmol. 120:539-540. [DOI] [PubMed] [Google Scholar]
- 260.Murdoch, D., and D. Parr. 1997. Pythium insidiosum keratitis. Aust. N. Z. J. Ophthalmol. 25:177-179. [DOI] [PubMed] [Google Scholar]
- 261.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27A. NCCLS, Wayne, Pa.
- 262.Nelson, M. E., G. Midgley, and N. R. Blatchford. 1990. Ketoconazole in the treatment of blepharitis. Eye 4:151-159. [DOI] [PubMed] [Google Scholar]
- 263.Nelson, P. E., M. C. Dignani, and E. J. Anaissie. 1994. Taxonomy, biology and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 7:479-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nunery, W. R., M. G. Welsh, and R. L. Saylor. 1985. Pseudallescheria boydii (Petriellidium boydii) infection of the orbit. Ophthalmic Surg. 16:296-300. [PubMed] [Google Scholar]
- 265.Nussbaum, E. S., and W. A. Hall. 1994. Rhinocerebral mucormycosis: changing patterns of disease. Surg. Neurol. 41:152-156. [DOI] [PubMed] [Google Scholar]
- 266.Nwufo, M. I., and A. O. Fajola. 1988. Production of amylolytic enzymes in culture by Botryodiplodia theobromae and Sclerotium rolfsii associated with the corn rots of Colocasia esculenta. Acta Microbiol. Hung. 35:371-377. [PubMed] [Google Scholar]
- 267.O'Brien, T. P. 1999. Therapy of ocular fungal infections. Ophthalmol. Clin. North Am. 12:33-50. [Google Scholar]
- 268.O'Brien, T. P., and L. D. Hazlett. 1996. Pathogenesis of ocular infection, p. 200-214. In J. S. Pepose, G. N. Holland, and K. R. Wilhelmus (ed.), Ocular infection and immunity. Mosby Year Book, St. Louis, Mo.
- 269.O'Day, D. M. 1996. Fungal keratitis, p. 1048-1061. In J. S. Pepose, G. N. Holland, and K. R. Wilhelmus (ed.), Ocular infection and immunity. Mosby Year Book, St. Louis, Mo.
- 270.Reference deleted.
- 271.O'Day, D. M., and E. M. Burd. 1994. Fungal keratitis and conjunctivitis. Mycology, p. 229-239. In G. Smolin and R. A. Thoft (ed.), The cornea: scientific foundations and clinical practice, 3rd ed. Little, Brown & Co., Boston, Mass.
- 272.O'Day, D. M., G. Foulds, T. E. Williams, R. D. Robinson, R. H. Allen, and W. S. Head. 1990. Ocular uptake of fluconazole after oral administration. Arch. Ophthalmol. 108:1006-1008. [DOI] [PubMed] [Google Scholar]
- 273.O'Day, D. M., W. A. Ray, W. S. Head, and R. D. Robinson. 1984. Influence of the corneal epithelium on the efficacy of topical antifungal agents. Investig. Ophthalmol. Visual Sci. 25:855-859. [PubMed] [Google Scholar]
- 274.O'Day, D. M., W. S. Head, R. D. Robinson, and J. A. Clanton. 1986. Corneal penetration of topical amphotericin B and natamycin. Curr. Eye Res. 5:877-882. [DOI] [PubMed] [Google Scholar]
- 275.O'Day, D. M., W. S. Head, R. D. Robinson, T. E. Williams, and S. J. Gedde. 1991. Anomalous effect of subconjunctival miconazole on Candida albicans keratitis in rabbits. Am. J. Ophthalmol. 112:562-566. [DOI] [PubMed] [Google Scholar]
- 276.O'Day, D. M., W. A. Ray, W. S. Head, R. D. Robinson, and T. E. Williams. 1991. Influence of corticosteroid on experimentally-induced keratomycosis. Arch. Ophthalmol. 109:1601-1604. [DOI] [PubMed] [Google Scholar]
- 277.O'Day, D. M., W. S. Head, R. D. Robinson, R. Yang, D. Shetlar, and M. X. Wang. 1999. Contact lens-induced infection—a new model of Candida albicans keratitis. Investig. Ophthalmol. Visual Sci. 40:1607-1611. [PubMed] [Google Scholar]
- 278.Odds, F. C., F. van Gerven, A. Espinel-Ingroff, M. S. Bartlett, M. A. Ghannoum, M. V. Lancaster, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, and T. J. Walsh. 1998. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob. Agents Chemother. 42:282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Oestreicher, J. H., M. Bashour, R. Jung, and B. Chiu. 1999. Aspergillus mycetoma in a secondary hydroxyapatite orbital implant: a case report and literature review. Ophthalmology 106:987-991. [DOI] [PubMed] [Google Scholar]
- 280.Okhravi, N., J. K. G. Dart, H. M. Towler, and S. Lightman. 1997. Paecilomyces lilacinus endophthalmitis with secondary keratitis: a case report and literature review. Arch. Ophthalmol. 115:1320-1324. [DOI] [PubMed] [Google Scholar]
- 281.Okhravi, N., P. Adamson, R. Mant, M. M. Matheson, G. Midgley, H. M. Towler, and S. Lightman. 1998. Polymerase chain reaction and restriction fragment length polymorphism mediated detection and speciation of Candida spp. causing intraocular infection. Investig. Ophthalmol. Visual Sci. 39:859-866. [PubMed] [Google Scholar]
- 282.Or, M., H. Akbatur, B. Hasanerisoglu, K. Bilgihan, and N. Cakir. 1993. Ketoconazole-induced papilledema. Acta Ophthalmol. Copenh. 71:270-272. [DOI] [PubMed] [Google Scholar]
- 283.Orgel, I. K., and K. L. Cohen. 1989. Postoperative zygomycete endophthalmitis. Ophthalmic Surg. 20:584-587. [PubMed] [Google Scholar]
- 284.Owor, R., and W. M. Wamukota. 1978. Rhinosporidiosis in Uganda: a review of 51 cases. East Afr. Med. J. 55:582-586. [PubMed] [Google Scholar]
- 285.Panda, A., G. K. Das, M. Vanathi, and A. Kumar. 1998. Corneal infection after radial keratotomy. J. Cataract Refractive Surg. 24:331-334. [DOI] [PubMed] [Google Scholar]
- 286.Panda, A., N. Pushker, S. Nainiwal, G. Satpathy, and N. Nayak. 1999. Rhodotorula sp. infection in corneal interface following lamellar keratoplasty—a case report. Acta Ophthalmol. Scand. 77:227-228. [DOI] [PubMed] [Google Scholar]
- 287.Panda, A., N. Sharma, and S. K. Angra. 1996. Topical fluconazole therapy of Candida keratitis. Cornea 15:373-375. [DOI] [PubMed] [Google Scholar]
- 288.Panda, A., N. Sharma, G. Das, N. Kumar, and G. Satpathy. 1997. Mycotic keratitis in children: epidemiologic and microbiologic evaluation. Cornea 16:295-299. [PubMed] [Google Scholar]
- 289.Panda, A., R. B. Vajpayee, and T. S. Kumar. 1991. Critical evaluation of therapeutic keratoplasty in cases of keratomycosis. Ann. Ophthalmol. 23:373-376. [PubMed] [Google Scholar]
- 290.Parfrey, N. A. 1986. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine (Baltimore) 65:113-123. [DOI] [PubMed] [Google Scholar]
- 291.Parrish, C. M., D. M. O'Day, and T. C. Hoyle. 1987. Spontaneous fungal corneal ulcer as an ocular manifestation of AIDS. Am. J. Ophthalmol. 104:302-303. [DOI] [PubMed] [Google Scholar]
- 292.Parthiban K., S. Gnanaguruvelan, C. Janaki, G. Sentamilselvi, and J. M. Boopalraj. 1998. Rhinocerebral zygomycosis. Mycoses 41:51-53. [DOI] [PubMed] [Google Scholar]
- 293.Pavan, P. R., and C. E. Margo. 1993. Endogeneous endophthalmitis caused by Bipolaris hawaiiensis in a patient with acquired immunodeficiency syndrome. Am. J. Ophthalmol. 110:200-202. [DOI] [PubMed] [Google Scholar]
- 294.Pavilack, M. A., and B. R. Frueh. 1992. Thorough curettage in the treatment of chronic canaliculitis. Arch. Ophthalmol. 110:200-202. [DOI] [PubMed] [Google Scholar]
- 295.Pe'er, J., H. Gnessin, S. Levinger, E. Averbukh, Y. Levy, and I. Polacheck. 1996. Conjunctival rhinosporidiosis caused by Rhinosporidium seeberi. Arch. Pathol. Lab. Med. 120:854-858. [PubMed] [Google Scholar]
- 296.Perry, H. D., E. D. Donnenfeld, G. A. Grossman, M. Stein, and A. B. Epstein. 1998. Retained Aspergillus contaminated contact lens inducing conjunctival mass and keratoconjunctivitis in an immunocompetent patient. CLAO J. 24:57-58. [PubMed] [Google Scholar]
- 297.Perry, H. D., S. J. Doshi, E. D. Donnenfeld, and G. S. Bai. 2002. Topical cyclosporin A in the management of therapeutic keratoplasty for mycotic keratitis. Cornea 21:161-163. [DOI] [PubMed] [Google Scholar]
- 298.Pfeifer, J. D., M. G. Grand, M. A. Thomas, A. R. Berger, M. J. Lucarelli, and M. E. Smith. 1991. Endogenous Pseudallescheria boydii endophthalmitis. Clinicopathologic findings in two cases. Arch. Ophthalmol. 109:1714-1717. [DOI] [PubMed] [Google Scholar]
- 299.Pleyer, U., A. Legmann, B. J. Mondino, and D. A. Lee. 1992. Use of collagen shields containing amphotericin B in the treatment of experimental Candida albicans-induced keratomycosis in rabbits. Am. J. Ophthalmol. 113:303-307. [DOI] [PubMed] [Google Scholar]
- 300.Portnoy, S. L., M. S. Insler, and H. E. Kaufman. 1989. Surgical management of corneal ulceration and perforation. Surv. Ophthalmol. 34:47-58. [DOI] [PubMed] [Google Scholar]
- 301.Powers, C. N. 1998. Diagnosis of infectious diseases: a cytopathologist's perspective. Clin. Microbiol. Rev. 11:341-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Press, G. A., S. M. Weindling, J. R. Hesselink, J. W. Ochi, and J. P. Harris. 1988. Rhinocerebral mucormycosis. MR manifestations. J. Comput. Assist. Tomogr. 12:744-749. [DOI] [PubMed] [Google Scholar]
- 303.Pulido, J. S., R. Folberg, K. D. Carter, and P. Coonan. 1990. Histoplasma capsulatum endophthalmitis after cataract extraction. Ophthalmology 97:217-220. [DOI] [PubMed] [Google Scholar]
- 304.Purgason, P. A., A. Hornblass, and M. Loeffler. 1992. Atypical presentation of fungal dacryocystitis. A report of two cases. Ophthalmology 99:1430-1432. [DOI] [PubMed] [Google Scholar]
- 305.Puttana, S. T. 1967. Mycotic infections of the cornea. J. All-India Ophthalmol. Soc. 15:11-18. [PubMed] [Google Scholar]
- 306.Radner, A. B., M. D. Witt and J. E. Edwards, Jr. 1995. Acute invasive rhinocerebral zygomycosis in an otherwise healthy patient: case report and review. Clin. Infect. Dis. 20:163-166. [DOI] [PubMed] [Google Scholar]
- 307.Ragge, N., J. C. Hart, D. L. Easty, and A. G. Tyers. 1990. A case of fungal keratitis caused by Scopulariopsis brevicaulis: treatment with antifungal agents and penetrating keratoplasty. Br. J. Ophthalmol. 74:561-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Raj, P., E. J. Vella, and R. C. Bickerton. 1998. Successful treatment of rhinocerebral mucormycosis by a combination of aggressive surgical debridement and the use of systemic liposomal amphotericin B and local therapy with nebulized amphotericin—a case report. J. Laryngol. Otol. 112:367-370. [DOI] [PubMed] [Google Scholar]
- 309.Rajasekaran, J., P. A. Thomas, and R. Srinivasan. 1987. Ketoconazole in keratomycosis, p. 2462-2467. In F. Blodi, R. Brancato, G. Cristini, F. d'Ermo, I. Esente, A. Musini, B. Philipson, F. Pintucci, F. Ponte, and G. Scuderi (ed.), Acta XXV Concilium Ophthalmologicum. Kugler Ghedini, Amsterdam, The Netherlands.
- 310.Rajasekaran, J., P. A. Thomas, C. M. Kalavathy, P. C. Joseph, and D. J. Abraham. 1987. Itraconazole therapy for fungal keratitis. Indian J. Ophthalmol. 35:157-160. [PubMed] [Google Scholar]
- 311.Rajeev, B., and J. Biswas. 1998. Molecular biologic techniques in ophthalmic pathology. Indian J. Ophthalmol. 46:3-13. [PubMed] [Google Scholar]
- 312.Ramadan, A. M., R. B. Nussenblatt, and M. D. de Smet. 1997. Long-term follow-up of patients with chronic uveitis affecting the posterior pole treated with combination cyclosporine and ketoconazole. Ophthalmology 104:706-711. [DOI] [PubMed] [Google Scholar]
- 313.Rangel-Guerra, R. A., H. R. Martinez, C. Saenz, F. Bosques-Padilla, and I. Estrada-Bellmann. 1997. Rhinocerebral and systemic mucormycosis. Clinical experience with 36 cases. J. Neurol. Sci. 143:19-30. [DOI] [PubMed] [Google Scholar]
- 314.Rao, N. A. 1989. A laboratory approach to rapid diagnosis of ocular infections and prospects for the future. Am. J. Ophthalmol. 107:283-291. [DOI] [PubMed] [Google Scholar]
- 315.Rao, S.K., H. N. Madhavan, G. Rao, and P. Padmanabhan. 1997. Fluconazole in filamentous fungal keratitis. Cornea 16:700. [PubMed] [Google Scholar]
- 316.Raza, S. K., A. I. Mallett, S. A. Howell, and P. A. Thomas. 1994. An in vitro study of the sterol content and toxin production of Fusarium isolates from mycotic keratitis. J. Med. Microbiol. 41:204-208. [DOI] [PubMed] [Google Scholar]
- 317.Read, R. W., R. S. Chuck, N. A. Rao, and R. E. Smith. 2000. Traumatic Acremonium atrogriseum keratitis following laser-assisted in situ keratomileusis. Arch. Ophthalmol. 118:418-421. [DOI] [PubMed] [Google Scholar]
- 318.Rebell, G., and R. K. Forster. 1976. Lasiodiplodia theobromae as a cause of keratomycosis. Sabouraudia 14:155-170. [DOI] [PubMed] [Google Scholar]
- 319.Rehman, M. R., G. J. Johnson, R. Husain, S. A. Howlader, and D. C. Minassian. 1998. Randomised trial of 0.2% chlorhexidine gluconate and 2.5% natamycin for fungal keratitis in Bangladesh. Br. J. Ophthalmol. 82:919-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Reichard, U., S. Buttner, H. Eiffert, F. Staib, and R. Ruchel. 1990. Purification and characterisation of an extracellular serine proteinase from Aspergillus fumigatus and its detection in tissue. J. Med. Microbiol. 33:243-251. [DOI] [PubMed] [Google Scholar]
- 321.Reidy, J. J., S. Sudesh, A. B. Klafter, and C. Olivia. 1997. Infection of the conjunctiva by Rhinosporidium seeberi. Surv. Ophthalmol. 41:409-413. [DOI] [PubMed] [Google Scholar]
- 322.Reis, A., R. Sundmacher, K. Tintelnot, H. Agostini, H. E. Jenson, and C. Althaus. 2000. Successful treatment of ocular invasive mould infection (fusariosis) with the new antifungal agent voriconazole. Br. J. Opthalmol. 84:932-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ribes, J. A., C. L. Vanover-Sams, and D. J. Baker. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13:236-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Richardson, M. D., and G. S. Shankland. 1999. Rhizopus, Rhicomucor, Absidia, and other agents of systemic and subcutaneous zygomycoses, pp. 1242-1258. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 325.Rippon, J. W. 1988. Medical mycology: the pathogenic fungi and the pathogenic actinomycetes, 3rd ed. The W. B. Saunders Co., Philadelphia, Pa.
- 326.Ritterband, D. C., M. Shah, and J. A. Seedor. 1997. Colletotrichum graminicola: a new corneal pathogen. Cornea 16:362-364. [PubMed] [Google Scholar]
- 327.Ritterband, D., J. Kelly, T. McNamara, M. Kresloff, R. Koplin, and J. Seedor. 2002. Delayed onset multifocal polymicrobial keratitis after laser in situ keratomileusis. J. Cataract Refractive Surg. 28:898-899. [DOI] [PubMed] [Google Scholar]
- 328.Ritterband, D. C., J. A. Seedor, M. K. Shah, S. Waheed, and I. Schorr. 1998. A unique case of Cryptococcus laurentii keratitis spread by a rigid gas-permeable contact lens in a patient with onychomycosis. Cornea 17:115-118. [DOI] [PubMed] [Google Scholar]
- 329.Roberts, G. D. 1994. Laboratory methods in basic mycology, p. 689-775. In E. J. Baron, L. R. Peterson, and S. M. Finegold (ed.), Bailey and Scott's diagnostic microbiology, 9th ed. C. V. Mosby, St. Louis, Mo.
- 330.Robin, J. R., R. Chan, N. A. Rao, S. Sharma, and M. Srinivasan. 1989. Fluorescein-conjugated lectin visualization of fungi and acanthamoebae in infectious keratitis. Ophthalmology 96:1198-1202. [DOI] [PubMed] [Google Scholar]
- 331.Rodenbiker, H. T., and J. P. Ganley. 1980. Ocular coccidioidomycosis. Surv. Ophthalmol. 24:263-290. [DOI] [PubMed] [Google Scholar]
- 332.Rodrigues, M. M., and P. R. Laibson. 1973. Exogenous mycotic keratitis caused by Blastomyces dermatitidis. Am. J. Ophthalmol. 75:782-789. [DOI] [PubMed] [Google Scholar]
- 333.Rodriguez-Arres, M. T., M. V. de Rojas Silva, M. Pereiro, B. Fente-Sampayo, G. Gallegos-Chamas, and M. S. Salorio. 1995. Aspergillus fumigatus scleritis. Acta Ophthalmol. Scand. 73:467-469. [DOI] [PubMed] [Google Scholar]
- 334.Rosa, R. H., Jr., D. Miller, and E. C. Alfonso. 1994. The changing spectrum of fungal keratitis in South Florida. Ophthalmology 101:1005-1013. [DOI] [PubMed] [Google Scholar]
- 335.Roychoudhury, B., S. Sharma, M. K. Reddy, and T. Das. 1997. Fluorescent Gram stain in the microbiologic diagnosis of infectious keratitis and endophthalmitis. Curr. Eye Res. 16:620-623. [DOI] [PubMed] [Google Scholar]
- 336.Ruben, S. 1991. Pseudallescheria boydii keratitis. Acta Ophthalmol. Copenh. 69:684-686. [DOI] [PubMed] [Google Scholar]
- 337.Ruchel, R., and M. Schaffrinski. 1999. Versatile fluorescent staining of fungi in clinical specimens by using the optical brightener blankophor. J. Clin. Microbiol. 37:2694-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Safneck, J. R., G. R. Hogg and L. B. Napier. 1990. Endophthalmitis due to Blastomyces dermatitidis. Ophthalmology 97:212-216. [DOI] [PubMed] [Google Scholar]
- 339.Sahin, B., S. Paydas, E. Cosar, K. Bicakci, and B. Hazan. 1996. Role of granulocyte colony-stimulating factor in the treatment of mucormycosis. Eur. J. Clin. Microbiol. Infect. Dis. 15:866-869. [DOI] [PubMed] [Google Scholar]
- 340.Saltoglu, N., Y. Tasova, S. Zorludemir, and I. H. Dundar. 1998. Rhinocerebral zygomycosis treated with liposomal amphotericin B and surgery. Mycoses 41:45-49. [DOI] [PubMed] [Google Scholar]
- 341.San-Blas, G., L. R. Travassos, B. C. Fries, D. L. Goldman, A. Casadevall, A. K. Carmona, T. F. Barros, R. Puccia, M. K. Hostetter, S. G. Shanks, V. M. S. Copping, Y. Knox, and N. A. R. Gow. 2000. Fungal morphogenesis and virulence. Med. Mycol. 38(Suppl. 1):79-86. [PubMed] [Google Scholar]
- 342.Savani, D. V., J. R. Perfect, L. M. Cobo, and D. T. Durack. 1987. Penetration of new azole compounds into the eye and efficacy in experimental Candida endophthalmitis. Antimicrob. Agents Chemother 31:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Savino, D. F., and C. E. Margo. 1983. Conjunctival rhinosporidiosis: light and electron microscopic study. Ophthalmology 90:1482-1489. [PubMed] [Google Scholar]
- 344.Schulman, J. A., G. A. Peyman, J. Dietlein, and R. Fiscella. 1991. Ocular toxicity of experimental intravitreal itraconazole. Int. Ophthalmol. 15:21-24. [DOI] [PubMed] [Google Scholar]
- 345.Schulman, J. A., G. Peyman, R. Fiscella, G. Small, M. Coats, C. P. Wajszczuk, and L. Steahly. 1987. Toxicity of intravitreal injection of fluconazole in the rabbit. Can. J. Ophthalmol. 22:304-306. [PubMed] [Google Scholar]
- 346.Schuman, J. S., and A. H. Friedman. 1983. Retinal manifestations of the acquired immune deficiency syndrome (AIDS): cytomegalovirus, Candida albicans, Cryptococcus, Toxoplasmosis and Pneumocystis carini. Trans. Ophthalmol. Soc. U. K. 103:177-190. [PubMed] [Google Scholar]
- 347.Schwartz, S. D., S. A. Harrison, R. E. Engstrom, Jr., R. E. Bawdon, D. A. Lee, and B. J. Mondino. 1990. Collagen shield delivery of amphotericin B. Am. J. Ophthalmol. 109:701-704. [DOI] [PubMed] [Google Scholar]
- 348.Seal, D. V., J. Bron, and J. Hay. 1998. Ocular infection: investigation and treatment in practice. Martin Dunitz, London, England.
- 349.Seiff, S. R., P. H. Choo, and S. R. Carter. 1999. Role of local amphotericin B therapy for sino-orbital fungal infections. Ophthalmic Plast. Reconstr. Surg. 15:28-31. [DOI] [PubMed] [Google Scholar]
- 350.Shami, M. J., W. Freeman, D. Friedberg, E. Siderides, A. Listhaus, and E. Ai. 1991. A multicenter study of Pneumocystis choroidopathy. Am. J. Ophthalmol. 112:15-22. [DOI] [PubMed] [Google Scholar]
- 351.Sharma, S., M. Silverberg, P. Mehta, U. Gopinathan, V. Agrawal, and T. J. Naduvilath. 1998. Early diagnosis of mycotic keratitis: predictive value of potassium hydroxide preparation. Indian J. Ophthalmol. 46:31-35. [PubMed] [Google Scholar]
- 352.Shrestha, S. P., A. Hennig, and S. C. Parija. 1998. Prevalence of rhinosporidiosis of the eye and its adnexa in Nepal. Am. J. Trop. Med. Hyg. 59:231-234. [DOI] [PubMed] [Google Scholar]
- 353.Silva, M. R., R. P. Mendes, J. C. Lastoria, B. Barraviera, S. A. Marques, and A. Kamegasawa. 1988. Paracoccidioidomycosis: study of six cases with ocular involvement. Mycopathologia 102:87-96. [DOI] [PubMed] [Google Scholar]
- 354.Simmons, R. B., J. R. Buffington, and M. Ward. 1986. Morphology and ultrastructure of fungi in extended-wear soft contact lenses. J. Clin. Microbiol. 24:21-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Slack, J. W., R. A. Hyndiuk, G. J. Harris, and K. B. Simons. 1992. Blastomycosis of the eyelid and conjunctiva. Ophthalmic Plast. Reconstr. Surg. 8:143-149. [DOI] [PubMed] [Google Scholar]
- 356.Slomovic, M. R., R. K. Forster, and H. Gelender. 1985. Lasiodiplodia theobromae panophthalmitis. Can. J. Ophthalmol. 20:225-228. [PubMed] [Google Scholar]
- 357.Specht, C. S., K. T. Mitchell, A. E. Bauman, and M. Gupta. 1991. Ocular histoplasmosis with retinitis in a patient with acquired immunodeficiency syndrome. Ophthalmology 98:1356-1359. [DOI] [PubMed] [Google Scholar]
- 358.Reference deleted.
- 359.Sponsler, T. A., J. W. Sassani, L. N. Johnson, and J. Towfighi. 1992. Ocular invasion in mucormycosis. Surv. Ophthalmol. 36:345-350. [DOI] [PubMed] [Google Scholar]
- 360.Sridhar, M. S., P. Garg, A. K. Bansal, and S. Sharma. 2000. Fungal keratitis after in situ keratomileusis. J. Cataract Refractive Surg. 26:613-615. [DOI] [PubMed] [Google Scholar]
- 361.Sridhar, M. S., P. Garg, A. K. Bansal, and U. Gopinathan. 2000. Aspergillus flavus keratitis following laser in situ keratomileusis. Am. J. Ophthalmol. 129:802-804. [DOI] [PubMed] [Google Scholar]
- 362.Sridhar, M. S., U. Gopinathan, P. Garg, and G. N. Rao. 2001. Aspergillus fumigatus keratitis with wreath pattern infiltrates. Cornea 20:534-535 [DOI] [PubMed] [Google Scholar]
- 363.Srinivasan, R., R. Kanungo, and J. L. Goyal. 1991. Spectrum of oculomycosis in South India. Acta Ophthalmol. 69:744-749. [DOI] [PubMed] [Google Scholar]
- 364.Srinivasan, M., C. A. Gonzales, C. George, V. Cevallos, J. M. Mascarenhas, B. Asokan, J. Wilkins, G. Smolin, and J. P. Whitcher. 1997. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br. J. Ophthalmol. 81:965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Starr, M. B. 1987. Paecilomyces lilacinus keratitis: two case reports in extended-wear contact lens wearers. CLAO J. 13:95-101. [PubMed] [Google Scholar]
- 366.Stern, G. A., and M. Buttross. 1991. Use of corticosteroids in combination with antimicrobial drugs in the treatment of infectious corneal disease. Ophthalmology 98:847-853. [DOI] [PubMed] [Google Scholar]
- 367.Stevens, D. A., V. L. Kan, M. A. Judson, V. A. Morrison, S. Dummer, D. W. Denning, J. E. Bennett, T. J. Walsh, T. F. Patterson, and G. A. Pankey. 2000. Practice guidelines for diseases caused by Aspergillus. Clin. Infect. Dis. 30:696-709. [DOI] [PubMed] [Google Scholar]
- 368.Strasser, M. D., R. J. Kennedy, and R. D. Adam. 1996. Rhinocerebral mucormycosis. Therapy with amphotericin B lipid complex. Arch. Intern. Med. 156:337-339. [DOI] [PubMed] [Google Scholar]
- 369.Streeten, B. W., D. D. Rebuzzi, and D. B. Jones. 1974. Sporotrichosis of the orbital margin. Am. J. Ophthalmol. 77:750-755 [DOI] [PubMed] [Google Scholar]
- 370.Sullivan, L. J., G. Snibson, C. Joseph, and H. R. Taylor. 1994. Scedosporium prolificans sclerokeratitis. Aust. N. Z. J. Ophthalmol. 22:207-209. [DOI] [PubMed] [Google Scholar]
- 371.Sunba, M. S., and S. Y. al-Ali. 1989. The histological and the histopathological pattern of conjunctival rhinosporidiosis associated with papilloma virus infection. Histol. Histopathol. 4:257-264. [PubMed] [Google Scholar]
- 372.Sutphin, J. E., N. M. Robinson, K. R. Wilhelmus, and M. S. Osato. 1986. Improved detection of oculomycoses using induced fluorescence with Cellfluor. Ophthalmology 93:416-417. [DOI] [PubMed] [Google Scholar]
- 373.Sutton, D. A., A. W. Fothergill, and M. G. Rinaldi. 1998. Guide to clinically significant fungi. The Williams & Wilkins Co., Baltimore, Md.
- 374.Swoboda, H., and R. Ullrich. 1992. Aspergilloma in the frontal sinus expanding into the orbit. J. Clin. Pathol. 45:629-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Tabbara, K. F., and A. L. al-Jabarti. 1998. Hospital construction-associated outbreak of ocular aspergillosis after cataract surgery. Ophthalmology 105:522-526. [DOI] [PubMed] [Google Scholar]
- 376.Talbot, G. H., A. Huang, and M. Provencher. 1991. Invasive Aspergillus rhinosinusitis in patients with acute leukaemia Rev. Infect. Dis. 13:219-232. [DOI] [PubMed] [Google Scholar]
- 377.Tanure, M. A., E. J. Cohen, S. Grewal, C. J. Rapuano, and P. R. Laibson. 2000. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea 19:307-312. [DOI] [PubMed] [Google Scholar]
- 378.Tao, Y., H. E. Bazan, and N. G. Bazan. 1995. Platelet-activating factor induces the expression of the metallo-proteinases-1 and -9, but not -2 or -3 in the corneal epithelium. Investig. Ophthalmol. Visual Sci. 36:346-354. [PubMed] [Google Scholar]
- 379.Taravella, M. J., D. W. Johnson, J. G. Petty, R. B. Keyser, C. S. Foster, and B. E. Lundberg. 1997. Infectious posterior scleritis caused by Pseudallescheria boydii. Clinicopathologic findings. Ophthalmology 104:1312-1316. [DOI] [PubMed] [Google Scholar]
- 380.Thakar, M. 1994. Oral fluconazole therapy for keratomycosis. Acta Ophthalmol. Copenh. 72:765-767. [DOI] [PubMed] [Google Scholar]
- 381.Thianprasit, M., A. Chaiprasert, and P. Imwidthaya. 1996. Human pythiosis. Curr. Top. Med. Mycol. 7:43-54. [PubMed] [Google Scholar]
- 382.Thomas, M. A., and H. J. Kaplan. 1991. Surgical removal of subfoveal neovascularization in the presumed ocular histoplasmosis syndrome. Am. J. Ophthalmol. 111:1-7. [DOI] [PubMed] [Google Scholar]
- 383.Thomas, P. A. 1990. Fungi in keratitis: detection, susceptibility patterns and pathogenic mechanisms. Ph.D. thesis. Bharathidasan University, Tiruchirappalli, India.
- 384.Thomas, P. A. 1994. Mycotic keratitis: an underestimated mycosis. J. Med. Vet. Mycol. 32:235-254. [DOI] [PubMed] [Google Scholar]
- 385.Thomas, P. A., and J. Rajasekaran. 1988. Treatment of Aspergillus keratitis with imidazoles and related compounds, p. 267-279. In H. Vanden Bossche, D. W. R. Mackenzie, and G. Cauwenbergh (ed.), Aspergillus and aspergillosis. Plenum Press, Inc., New York, N.Y.
- 386.Reference deleted.
- 387.Thomas, P. A., T. Kuriakose, M. P. Kirupashanker, and V. S. Maharajan. 1991. Use of lactophenol cotton blue mounts of corneal scrapings as an aid to the diagnosis of mycotic keratitis. Diagn. Microbiol. Infect. Dis. 14:219-224. [DOI] [PubMed] [Google Scholar]
- 388.Thomas, P. A., C. M. Kalavathy, and J. Rajasekaran. 1986. Microbial keratitis: a study of 774 cases and review of the literature. J. Madras StateOphthalmol. Assoc. 23:13-21. [Google Scholar]
- 389.Thomas, P. A., C. M. Kalavathy, and P. Devanandan. 1998. Lasiodiplodia theobromae keratitis—a clinical profile. J. Tamilnadu Ophthalmol. Assoc. 39:31-32. [Google Scholar]
- 390.Thomas, P. A., D. J. Abraham, C. M. Kalavathy, and J. Rajasekaran. 1988. Oral itraconazole therapy for mycotic keratitis. Mycoses 31:271-279. [DOI] [PubMed] [Google Scholar]
- 391.Thomas, P. A., D. J. Abraham, C. M. Kalavathy, and J. Rajasekaran. 1987. Oral ketoconazole in keratomycosis. Indian J. Ophthalmol. 35:197-203. [PubMed] [Google Scholar]
- 392.Thomas, P. A., R. G. Garrison, and T. Jansen. 1991. Intrahyphal hyphae in corneal tissue from a case of keratitis due to Lasiodiplodia theobromae. J. Med. Vet. Mycol. 29:263-267. [DOI] [PubMed] [Google Scholar]
- 393.Thomas, P. A., T. Jansen, J. Van Cutsem, P. Geraldine, C. A. N. Jesudasan, and M. S. Sangamitra. 1995. Virulence factors of Lasiodiplodia theobromae in fungal keratitis. p. 3-4. In J. K. Pasricha (ed.), Indian ophthalmology today, 1995. All-India Ophthalmological Society, New Delhi, India.
- 394.Thygeson, P., and M. Okumoto. 1974. Keratomycosis: a preventable disease. Trans. Am. Acad. Ophthalmol. Otolaryngol. 78:433-439. [PubMed] [Google Scholar]
- 395.Tjebbes, G. W., J. L. van Delft, and N. J. van Haeringen. 1993. Production of lipid mediators in experimental keratitis of rabbit eye. J. Lipid Mediators 8:87-93. [PubMed] [Google Scholar]
- 396.Torres, M. A., J. Mohamed, H. Cavazos-Adame, and L. A. Martinez. 1985. Topical ketoconazole for fungal keratitis. Am. J. Ophthalmol. 100:293-298. [DOI] [PubMed] [Google Scholar]
- 397.Ukety, T. O., K. Kaimbo, A. M. Nelson, G. Moussa, R. Parys-Van-Ginderdeuren, and J. Vandepitte. 1992. Conjunctival rhinosporidiosis. Report of three cases from Zaire. Ann. Soc. Belg. Med. Trop. 72:219-223. [PubMed] [Google Scholar]
- 398.Upadhyay, M. P., P. C. D. Karmacharya, S. Koirala, N. R. Tuladhar, L. E. Bryan, G. Smolin, and J. P. Whitcher. 1991. Epidemiological characteristics, predisposing factors and aetiological diagnosis of corneal ulceration in Nepal. Am. J. Ophthalmol. 111:92-99. [DOI] [PubMed] [Google Scholar]
- 399.Vajpayee, R. B., S. K. Angra, S. Sandramouli, S. G. Honavar, and V. K. Chhabra. 1993. Laboratory diagnosis of keratomycosis: comparative evaluation of direct microscopy and culture results. Ann. Ophthalmol. 25:68-71. [PubMed] [Google Scholar]
- 400.Vajpayee, R. B., S. K. Gupta, U. Bareja, and K. Kishore. 1990. Ocular atopy and mycotic keratitis. Ann. Ophthalmol. 22:369-372. [PubMed] [Google Scholar]
- 401.Van Cauteren, H., J. Heykants, R. de Coster, and G. Cauwenbergh. 1987. Itraconazole: pharmacologic studies in animals and humans. Rev. Infect. Dis. 9:(Suppl. 1):S43-S46. [DOI] [PubMed] [Google Scholar]
- 402.Van Cutsem, J., and F. van Gerven. 1991. Activite' antifongique in vitro de l'itraconazole sur les champignons filamenteux opportunistes: traitement de la keratomycose et de la penicilliose experimentales. J. Mycol. Med. 1:10-15. [Google Scholar]
- 403.Van der Westhuijzen, A. J., F. W. Grotepass, G. Wyma and A. Padayachee. 1989. A rapidly fatal palatal ulcer: rhinocerebral mucormycosis. Oral Surg. Oral Med. Oral Pathol. 68:32-36. [DOI] [PubMed] [Google Scholar]
- 404.Vecsei, V. P., V. Huber-Spitzy, E. Arocker-Mettinger, and F. J. Steinkogler. 1994. Canaliculitis: difficulties in diagnosis, differential diagnosis and comparison between conservative and surgical treatment. Ophthalmologica 208:314-317. [DOI] [PubMed] [Google Scholar]
- 405.Velazquez, A. J., M. H. Goldstein, and W. T. Driebe. 2002. Preseptal cellulitis caused by Trichophyton (ringworm). Cornea 21:312-314. [DOI] [PubMed] [Google Scholar]
- 406.Vemuganti, G. K., P. Garg, V. Gopinathan, T. J. Naduvilath, R. K. John, R. Buddi, and G. N. Rao. 2002. Evaluation of agent and host factors in progression of mycotic keratitis. A histologic and microbiologic study of 167 corneal buttons. Opthalmology 109:1538-1546. [DOI] [PubMed] [Google Scholar]
- 407.Verma, S., and E. M. Graham. 1995. Cryptococcus presenting as cloudy choroiditis in an AIDS patient. Br. J. Ophthalmol. 79:618-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Verma, S., and S. J. Tuft. 2002. Fusarium solani keratitis following LASIK for myopia. Br. J. Ophthalmol. 86:1190-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Vida, L., and S. A. Moel. 1974. Systemic North American blastomycosis with orbital involvement. Am. J. Ophthalmol. 77:240-242. [DOI] [PubMed] [Google Scholar]
- 410.Vieira-Dias, D., C. M. Sena, F. Orefice, M. A. Tanure, and J. S. Hamdan. 1997. Ocular and concomitant cutaneous sporotrichosis. Mycoses 40:197-201. [DOI] [PubMed] [Google Scholar]
- 411.Virgile, R., H. D. Perry, B. Pardanani, K. Szabo, E. K. Rahn, J. Stone, I. Salkin, and D. M. Dixon. 1993. Human infectious corneal ulcer caused by Pythium insidiosum. Cornea 12:81-83. [DOI] [PubMed] [Google Scholar]
- 412.Reference deleted.
- 413.Vukovic, Z., A. Bobic-Radovanovic, Z. Latkovic, and Z. Radovanovic. 1995. An epidemiological investigation of the first outbreak of rhinosporidiosis in Europe. J. Trop. Med. Hyg. 98:333-337. [PubMed] [Google Scholar]
- 414.Wachsmuth, E. D. 1988. A comparison of the highly selective fluorescence staining of fungi in tissue sections with uvitex 2B and Calcofluor white M2R. Histochem. J. 20:215-221. [DOI] [PubMed] [Google Scholar]
- 415.Wagoner, M. D., I. A. Badr, and A. A. Hidayat. 1999. Chrysosporium parvum keratomycosis. Cornea 18:616-620. [PubMed] [Google Scholar]
- 416.Walker, S., S. A. N. Tailor, M. Lee, L. Louie, M. Louie, and A. E. Simor. 1998. Amphotericin B in lipid emulsion: stability, compatibility and in vitro antifungal activity. Antimicrob. Agents Chemother. 42:762-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Weissgold, D. J., S. E. Orlin, M. E. Sulewski, W. C. Frayer, and R. C. Eagle, Jr. 1998. Delayed-onset fungal keratitis after endophthalmitis. Ophthalmology 105:258-262. [DOI] [PubMed] [Google Scholar]
- 418.Wilhelmus, K. R., and D. B. Jones. 2001. Curvularia keratitis. Trans. Am. Ophthalmol. Soc. 99:111-132. [PMC free article] [PubMed] [Google Scholar]
- 419.Wilhelmus, K. R., and N. M. Robinson. 1991. Infectious crystalline keratopathy caused by Candida albicans. Am. J. Ophthalmol. 112:322-325. [DOI] [PubMed] [Google Scholar]
- 420.Wilhelmus, K. R., N. M. Robinson, and R. A. Font. 1988. Fungal keratitis in contact lens wearers. Am. J. Ophthalmol. 106:708-714. [DOI] [PubMed] [Google Scholar]
- 421.Willcox, M. D., K. N. Power, F. Stapleton, C. Leitch, N. Harmis, and D. F. Sweeny. 1997. Potential sources of bacteria that are isolated from contact lenses during wear. Optom. Vision Sci. 74:1030-1038. [DOI] [PubMed] [Google Scholar]
- 422.Wilson, L. A. 1996. Biomaterials and ocular infection, p. 215-231. In J. S. Pepose, G. N. Holland and K. R. Wilhelmus (ed.), Ocular infection and immunity. Mosby Year Book, St. Louis, Mo.
- 423.Wilson, L. A., and D. G. Ahearn. 1986. Association of fungi with extended-wear soft contact lenses. Am. J. Ophthalmol. 101:434-436. [DOI] [PubMed] [Google Scholar]
- 424.Wilson, L. A., and L. Ajello. 1998. Agents of oculomycosis: fungal infections of the eye, p. 525-567. In L. Collier, A. Balows, and A. Sussman (ed.), Topley and Wilson's microbiology and microbial infections, 9th ed., vol. 4. Arnold, London, England.
- 425.Wilson, L. A., A. D. Sawant, and D. G. Ahearn. 1991. Comparative efficacies of soft contact lens disinfectant solutions against microbial films in lens cases. Arch. Ophthalmol. 109:1155-1157. [DOI] [PubMed] [Google Scholar]
- 426.Winchester, K., W. D. Mathers, and J. E. Sutphin. 1997. Diagnosis of Aspergillus keratitis in vivo with confocal microscopy. Cornea 16:27-31. [PubMed] [Google Scholar]
- 427.Witherspoon, C. D., F. Kuhn, S. D. Owens, M. F. White, and J. A. Kimble. 1990. Endophthalmitis due to Sporothrix schenckii after penetrating ocular injury. Ann. Ophthalmol. 22:385-388. [PubMed] [Google Scholar]
- 428.Wong, T.-Y., K. G. Au Eong, W. K. Chan, and P. S. Tseng. 1996. Fusarium keratitis following the use of topical antibiotic-corticosteroid therapy in traumatised eyes. Ann. Acad. Med. Singapore 25:862-865. [PubMed] [Google Scholar]
- 429.Wong, T.-Y., T.-P. Ng, K.-S. Fong, and D. T. H. Tan. 1997. Risk factors and clinical outcome between fungal and bacterial keratitis. A comparative study. CLAO J. 23:275-281. [PubMed] [Google Scholar]
- 430.Wu, Z., H. Ying, S. Yiu, J. Irvine, and R. Smith. 2002. Fungal keratitis caused by Scedosporium apiospermum: report of 2 cases and review of treatment. Cornea 21:519-523. [DOI] [PubMed] [Google Scholar]
- 431.Xie, L., X. Dong, and W. Shi. 2001. Treatment of fungal keratitis by penetrating keratoplasty. Br. J. Ophthalmol. 85:1070-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Yamaguchi, H., S. Abe, and Y. Tokuda. 1993. Immunomodulating activity of antifungal drugs. Ann. N. Y. Acad. Sci. 685:447-457. [DOI] [PubMed] [Google Scholar]
- 433.Yau, T. H., P. M. Rivera-Velazquez, A. S. Mark, A. S. Cytryn, C. S. Levy, B. M. Shmookler and M. P. Kolsky. 1996. Unilateral optic neuritis caused by Histoplasma capsulatum in a patient with the acquired immunodeficiency syndrome. Am. J. Ophthalmol. 121:324-326. [DOI] [PubMed] [Google Scholar]
- 434.Yee, R. W., C. J. Cheng, S. Meenakshi, T. M. Ludden, J. E. Wallace, and M. G. Rinaldi. 1997. Ocular penetration and pharmacokinetics of topical fluconazole. Cornea 16:64-71. [PubMed] [Google Scholar]
- 435.Yohai, R. A., J. D. Bullock, A. A. Aziz, and R. J. Markert. 1994. Survival factors in rhino-orbital-cerebral mucormycosis. Surv. Ophthalmol. 39:3-22. [DOI] [PubMed] [Google Scholar]
- 436.Yoshizumi, M. O., and A. R. Banihashemi. 1988. Experimental intravitreal ketoconazole in DMSO. Retina 8:210-215. [DOI] [PubMed] [Google Scholar]
- 437.Zapater, R. C., and E. J. Albesi. 1979. Corneal microsporidiosis. A review and report on a case. Ophthalmologica 178:142-147. [DOI] [PubMed] [Google Scholar]
- 438.Zhu, W. S., K. Wojdyla, K. Donlon, P. A. Thomas, and H. I. Eberle. 1990. Extracellular proteases of Aspergillus flavus. Diagn. Microbiol. Infect. Dis. 13:491-497. [DOI] [PubMed] [Google Scholar]