Abstract
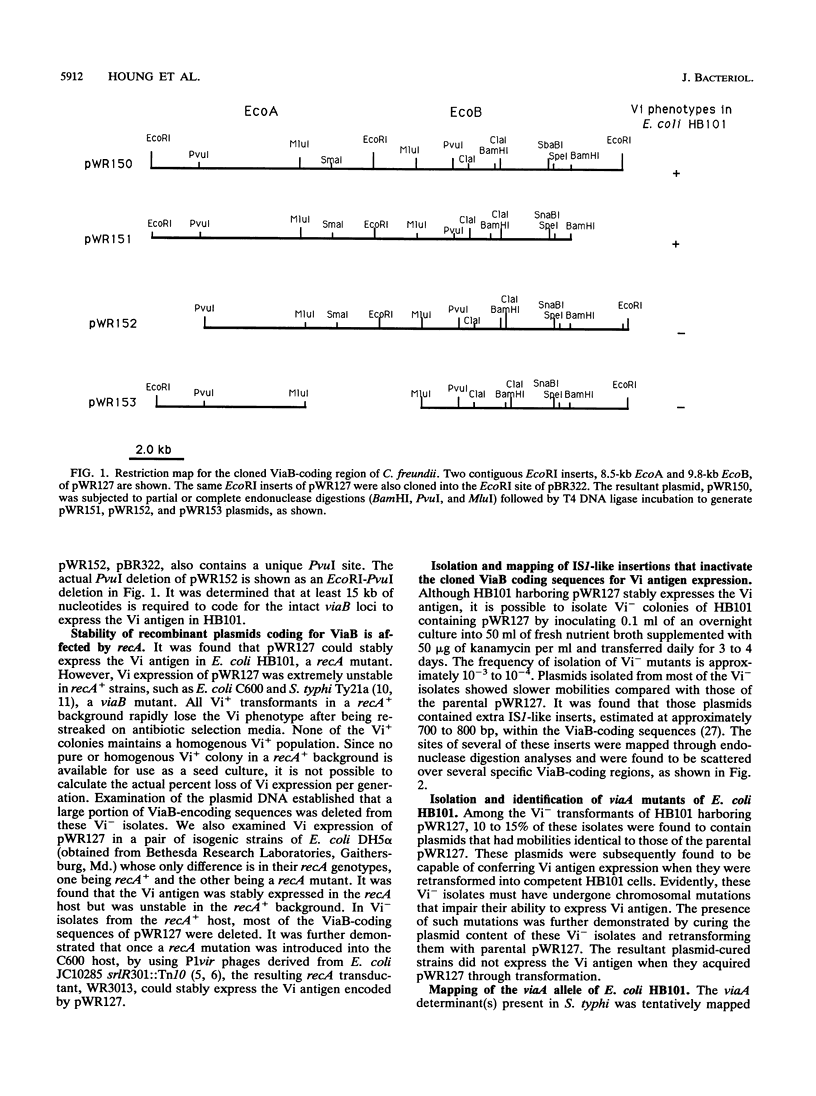
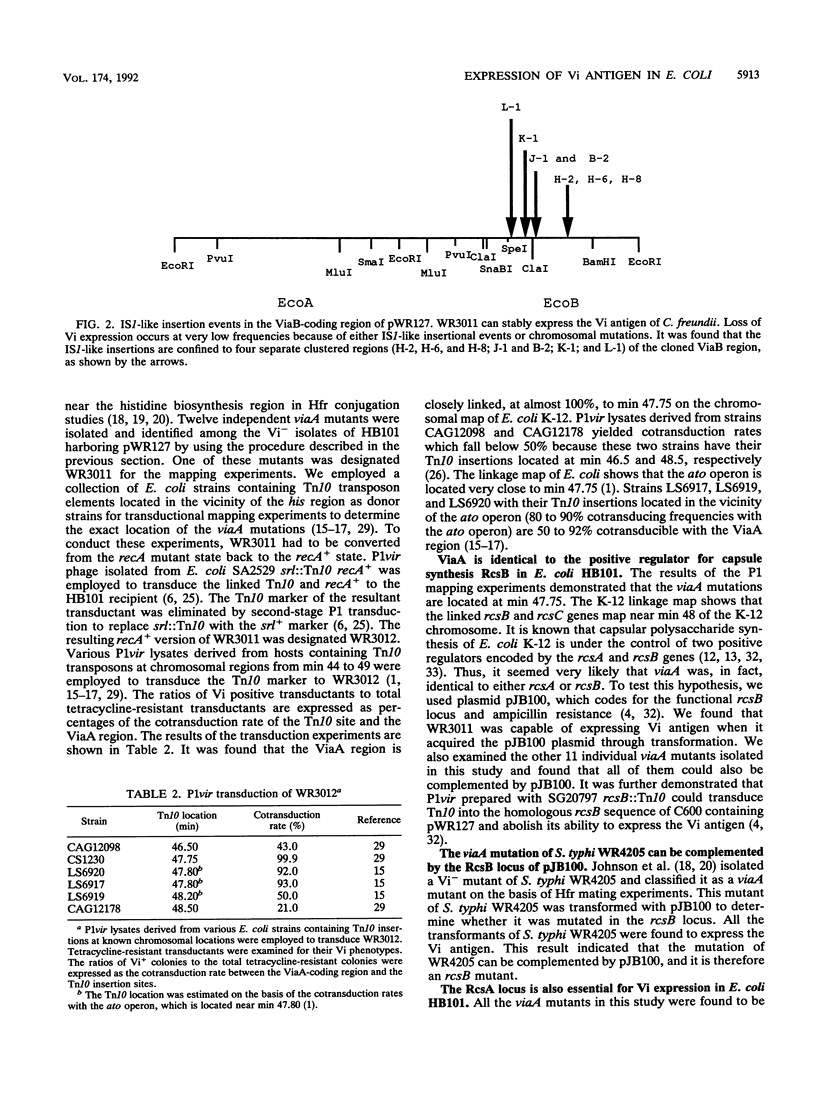
The Vi antigen in Salmonella typhi is stably expressed and may act to protect the strain against the defensive system of the host. Citrobacter freundii, not usually a common human pathogen, also expresses the Vi antigen but expresses it unstably, exhibiting a reversible transition between the Vi+ and Vi- states. Two widely separated chromosomal regions, ViaA and ViaB, are needed for Vi synthesis. Escherichia coli K-12 harboring a functional ViaB plasmid can also express Vi antigen, but the cloned ViaB sequence can only be stably maintained and expressed in recA hosts. Vi- derivatives arise either through IS1-like insertional events occurring in ViaB sequences or by chromosomal mutations at the ViaA region. P1vir mapping indicates that the ViaA mutations are located at min 47.75 on the E. coli chromosome. All the spontaneous viaA mutants isolated from E. coli and S. typhi were identified as rcsB mutants by complementation tests using plasmid pJB100. Introduction of rcsA::Tn10 into E. coli harboring functional ViaB sequences eliminates the expression of Vi antigen. These results indicate that Vi antigen synthesis is regulated by the same regulatory proteins involved in colanic acid synthesis in E. coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron L. S., Kopecko D. J., McCowen S. M., Snellings N. J., Johnson E. M., Reid W. C., Life C. A. Genetic and molecular studies of the regulation of atypical citrate utilization and variable Vi antigen expression in enteric bacteria. Basic Life Sci. 1982;19:175–194. doi: 10.1007/978-1-4684-4142-0_16. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill J. A., Quinlan-Walshe C., Gottesman S. Fine-structure mapping and identification of two regulators of capsule synthesis in Escherichia coli K-12. J Bacteriol. 1988 Jun;170(6):2599–2611. doi: 10.1128/jb.170.6.2599-2611.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Construction of an Hfr strain useful for transferring recA mutations between Escherichia coli strains. J Bacteriol. 1980 Jul;143(1):529–530. doi: 10.1128/jb.143.1.529-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Deletions generated by the transposon Tn10 in the srl recA region of the Escherichia coli K-12 chromosome. Genetics. 1979 Oct;93(2):321–343. doi: 10.1093/genetics/93.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels E. M., Schneerson R., Egan W. M., Szu S. C., Robbins J. B. Characterization of the Salmonella paratyphi C Vi polysaccharide. Infect Immun. 1989 Oct;57(10):3159–3164. doi: 10.1128/iai.57.10.3159-3164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R., Füer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975 May;131(5):553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- Gilman R. H., Hornick R. B., Woodard W. E., DuPont H. L., Snyder M. J., Levine M. M., Libonati J. P. Evaluation of a UDP-glucose-4-epimeraseless mutant of Salmonella typhi as a liver oral vaccine. J Infect Dis. 1977 Dec;136(6):717–723. doi: 10.1093/infdis/136.6.717. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Trisler P., Torres-Cabassa A. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J Bacteriol. 1985 Jun;162(3):1111–1119. doi: 10.1128/jb.162.3.1111-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Ezaki T., Li N., Yamamoto H. Molecular cloning of the ViaB region of Salmonella typhi. FEMS Microbiol Lett. 1991 Dec 15;69(1):53–56. doi: 10.1016/0378-1097(91)90645-q. [DOI] [PubMed] [Google Scholar]
- JOHNSON E. M., KRAUSKOPF B., BARON L. S. GENETIC MAPPING OF VI AND SOMATIC ANTIGENIC DETERMINANTS IN SALMONELLA. J Bacteriol. 1965 Aug;90:302–308. doi: 10.1128/jb.90.2.302-308.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins L. S., Nunn W. D. Genetic and molecular characterization of the genes involved in short-chain fatty acid degradation in Escherichia coli: the ato system. J Bacteriol. 1987 Jan;169(1):42–52. doi: 10.1128/jb.169.1.42-52.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins L. S., Nunn W. D. Regulation of the ato operon by the atoC gene in Escherichia coli. J Bacteriol. 1987 May;169(5):2096–2102. doi: 10.1128/jb.169.5.2096-2102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Baron L. S. Genetic transfer of the Vi antigen from Salmonella typhosa to Escherichia coli. J Bacteriol. 1969 Jul;99(1):358–359. doi: 10.1128/jb.99.1.358-359.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Krauskopf B., Baron L. S. Genetic analysis of the ViA-his chromosomal region in Salmonella. J Bacteriol. 1966 Nov;92(5):1457–1463. doi: 10.1128/jb.92.5.1457-1463.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolyva S., Waxin H., Popoff M. Y. The Vi antigen of Salmonella typhi: molecular analysis of the viaB locus. J Gen Microbiol. 1992 Feb;138(2):297–304. doi: 10.1099/00221287-138-2-297. [DOI] [PubMed] [Google Scholar]
- LANDY M. The visual identification of V and W form colonies in Salmonella cultures. Public Health Rep. 1950 Jul 28;65(30):950–951. [PubMed] [Google Scholar]
- McEntee K. Specialized transduction of recA by bacteriophage lambda. Virology. 1976 Mar;70(1):221–222. doi: 10.1016/0042-6822(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Ou J. T., Baron L. S., Rubin F. A., Kopecko D. J. Specific insertion and deletion of insertion sequence 1-like DNA element causes the reversible expression of the virulence capsular antigen Vi of Citrobacter freundii in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4402–4405. doi: 10.1073/pnas.85.12.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin F. A., Kopecko D. J., Noon K. F., Baron L. S. Development of a DNA probe to detect Salmonella typhi. J Clin Microbiol. 1985 Oct;22(4):600–605. doi: 10.1128/jcm.22.4.600-605.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellings N. J., Johnson E. M., Baron L. S. Genetic basis of Vi antigen expression in Salmonella paratyphi C. J Bacteriol. 1977 Jul;131(1):57–62. doi: 10.1128/jb.131.1.57-62.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellings N. J., Johnson E. M., Kopecko D. J., Collins H. H., Baron L. S. Genetic regulation of variable Vi antigen expression in a strain of Citrobacter freundii. J Bacteriol. 1981 Feb;145(2):1010–1017. doi: 10.1128/jb.145.2.1010-1017.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout V., Gottesman S. RcsB and RcsC: a two-component regulator of capsule synthesis in Escherichia coli. J Bacteriol. 1990 Feb;172(2):659–669. doi: 10.1128/jb.172.2.659-669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout V., Torres-Cabassa A., Maurizi M. R., Gutnick D., Gottesman S. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J Bacteriol. 1991 Mar;173(5):1738–1747. doi: 10.1128/jb.173.5.1738-1747.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trisler P., Gottesman S. lon transcriptional regulation of genes necessary for capsular polysaccharide synthesis in Escherichia coli K-12. J Bacteriol. 1984 Oct;160(1):184–191. doi: 10.1128/jb.160.1.184-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



