Abstract
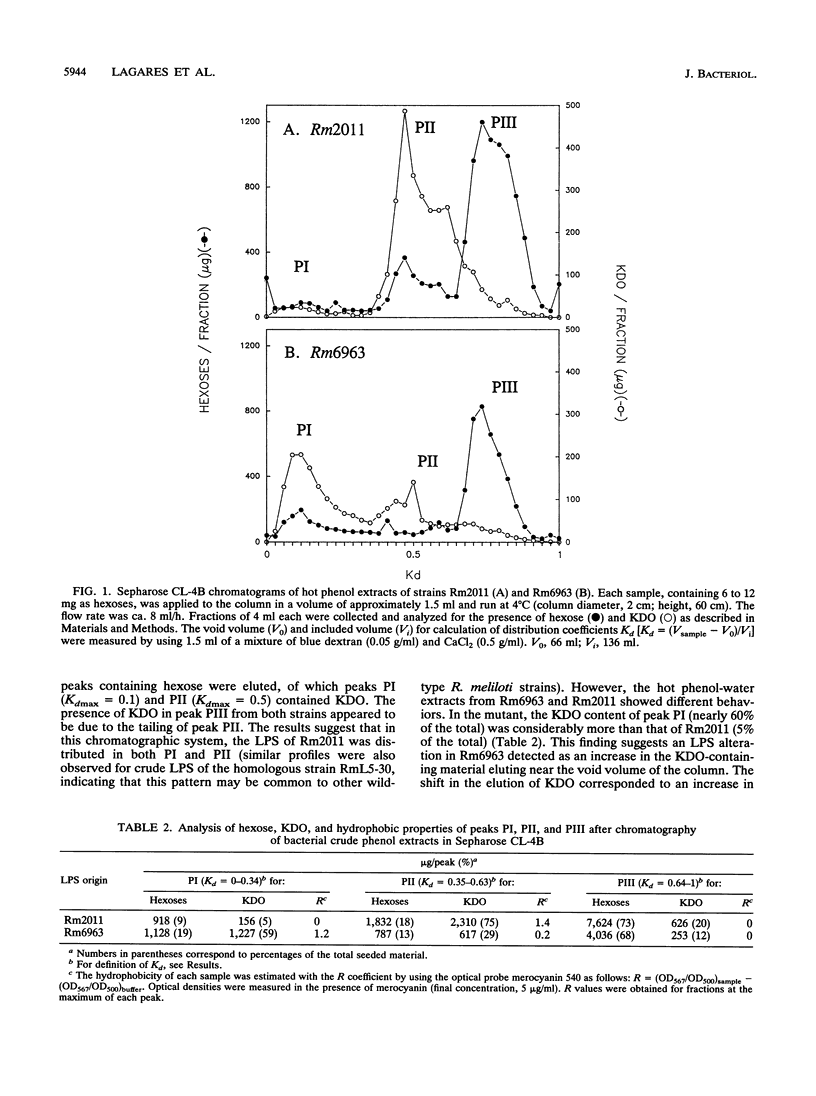
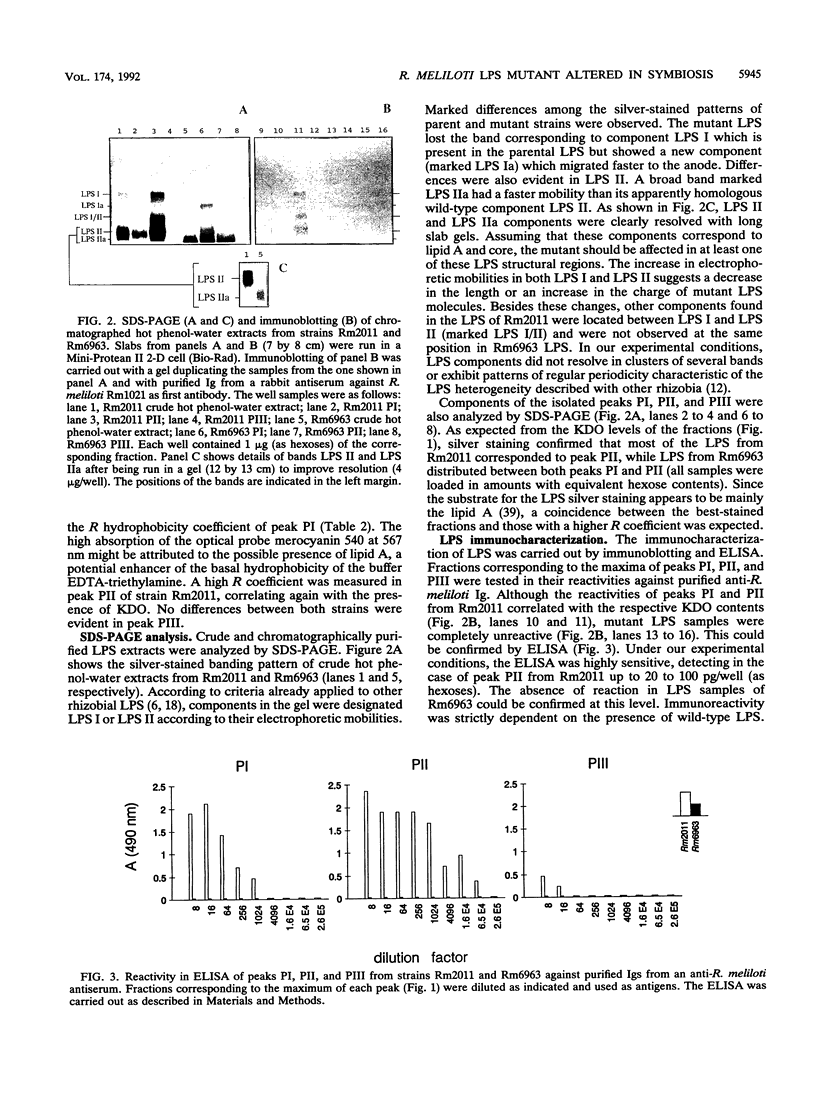
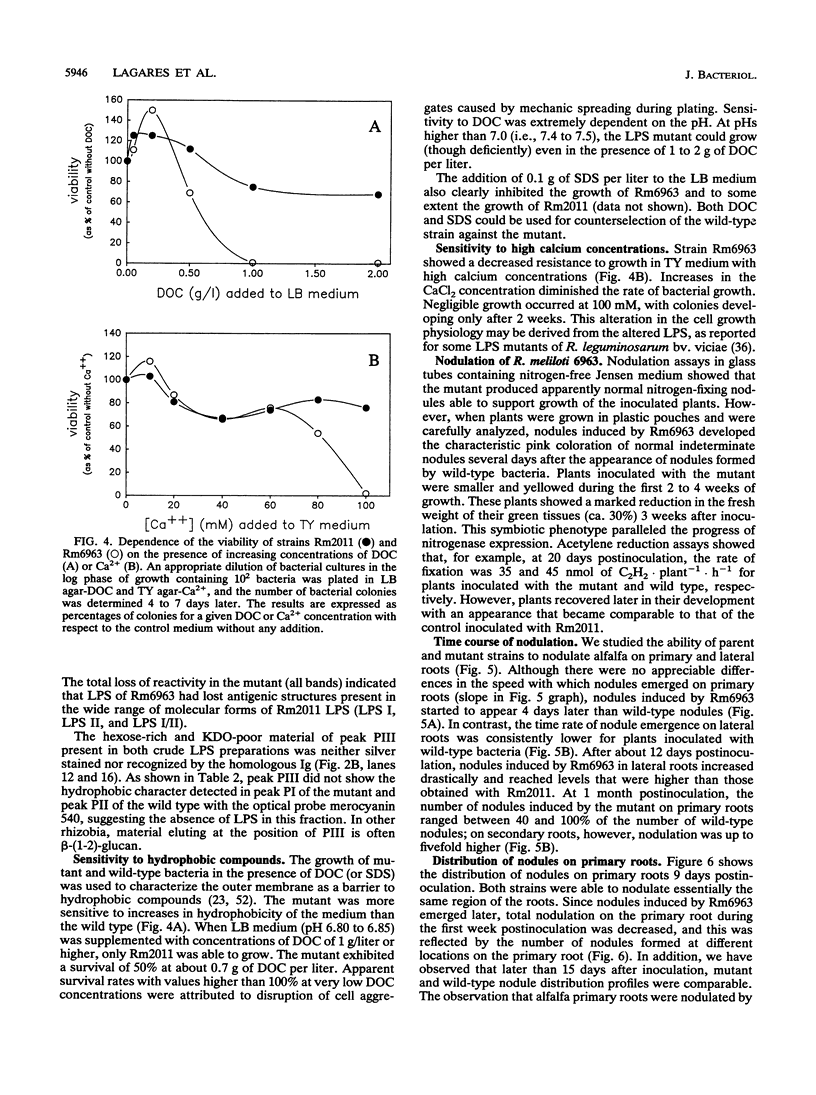
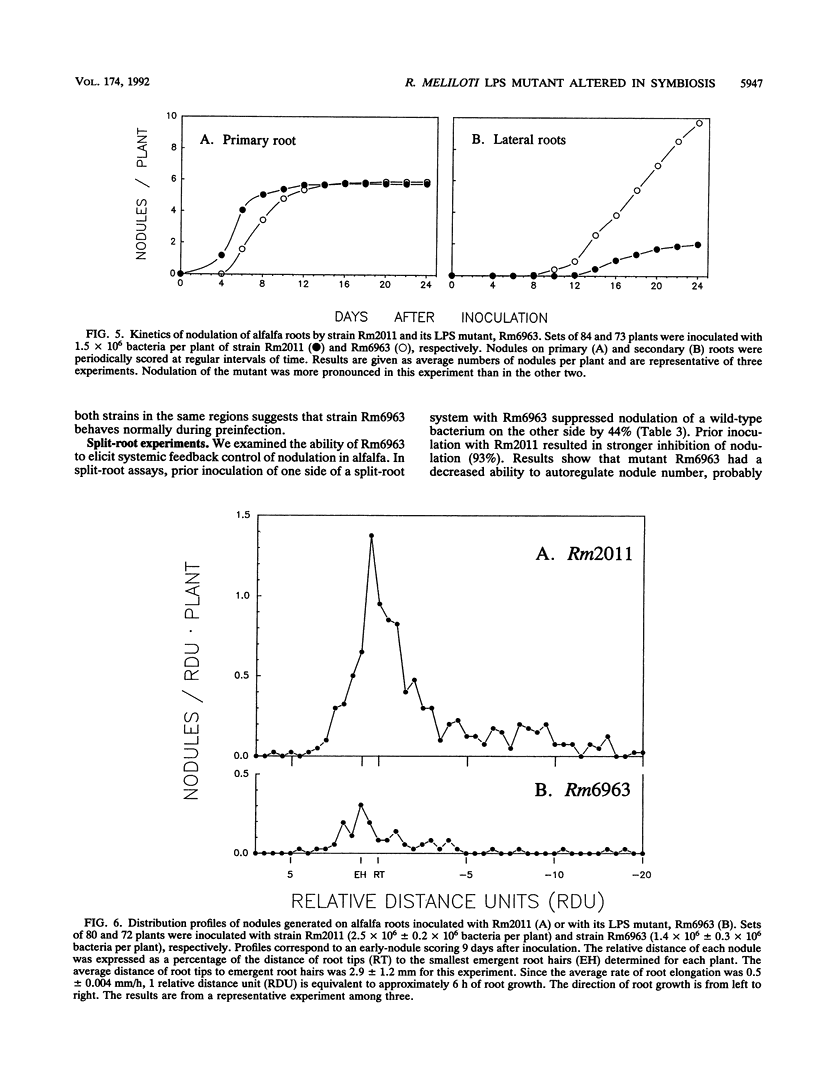
A transposon Tn5-induced mutant of Rhizobium meliloti Rm2011, designated Rm6963, showed a rough colony morphology on rich and minimal media and an altered lipopolysaccharide (LPS). Major differences from the wild-type LPS were observed in (i) hexose and 2-keto-3-deoxyoctonate elution profiles of crude phenol extracts chromatographed in Sepharose CL-4B, (ii) silver-stained sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis patterns of crude and purified LPS fractions, and (iii) immunoreactivities otherwise present in purified LPS of the parental strain Rm2011. In addition, Rm6963 lost the ability to grow in Luria-Bertani medium containing the hydrophobic compounds sodium deoxycholate or SDS and showed a decrease in survival in TY medium supplemented with high calcium concentrations. The mutant also had altered symbiotic properties. Rm6963 formed nodules that fixed nitrogen but showed a delayed or even reduced ability to nodulate the primary root of alfalfa without showing changes in the position of nodule distribution profiles along the roots. Furthermore, 2 to 3 weeks after inoculation, plants nodulated by Rm6963 were smaller than control plants inoculated with wild-type bacteria in correlation with a transient decrease in nitrogen fixation. In most experiments, the plants recovered later by expressing a full nitrogen-fixing phenotype and developing an abnormally high number of small nodules in lateral roots after 1 month. Rm6963 was also deficient in the ability to compete for nodulation. In coinoculation experiments with equal bacterial numbers of both mutant and wild-type rhizobia, only the parent was recovered from the uppermost root nodules. A strain ratio of approximately 100 to 1 favoring the mutant was necessary to obtain an equal ratio (1:1) of nodule occupancy. These results show that alterations in Rm6963 which include LPS changes lead to an altered symbiotic phenotype during the association with alfalfa that affects the timing of nodule emergence, the progress of nitrogen fixation, and the strain competitiveness for nodulation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beattie G. A., Clayton M. K., Handelsman J. Quantitative comparison of the laboratory and field competitiveness of Rhizobium leguminosarum biovar phaseoli. Appl Environ Microbiol. 1989 Nov;55(11):2755–2761. doi: 10.1128/aem.55.11.2755-2761.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Mills K. K., Crist D. K., Evans W. R., Bauer W. D. Effects of culture age on symbiotic infectivity of Rhizobium japonicum. J Bacteriol. 1983 Jan;153(1):443–451. doi: 10.1128/jb.153.1.443-451.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink B. A., Miller J., Carlson R. W., Noel K. D. Expression of Rhizobium leguminosarum CFN42 genes for lipopolysaccharide in strains derived from different R. leguminosarum soil isolates. J Bacteriol. 1990 Feb;172(2):548–555. doi: 10.1128/jb.172.2.548-555.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano Anollés G., Favelukes G. Quantitation of adsorption of rhizobia in low numbers to small legume roots. Appl Environ Microbiol. 1986 Aug;52(2):371–376. doi: 10.1128/aem.52.2.371-376.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G., Lagares A., Bauer W. D. Rhizobium meliloti exopolysaccharide Mutants Elicit Feedback Regulation of Nodule Formation in Alfalfa. Plant Physiol. 1990 Feb;92(2):368–374. doi: 10.1104/pp.92.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W. Heterogeneity of Rhizobium lipopolysaccharides. J Bacteriol. 1984 Jun;158(3):1012–1017. doi: 10.1128/jb.158.3.1012-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W. Heterogeneity of Rhizobium lipopolysaccharides. J Bacteriol. 1984 Jun;158(3):1012–1017. doi: 10.1128/jb.158.3.1012-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Sanders R. E., Napoli C., Albersheim P. Host-Symbiont Interactions: III. Purification and Partial Characterization of Rhizobium Lipopolysaccharides. Plant Physiol. 1978 Dec;62(6):912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Shatters R., Duh J. L., Turnbull E., Hanley B., Rolfe B. G., Djordjevic M. A. The Isolation and Partial Characterization of the Lipopolysaccharides from Several Rhizobium trifolii Mutants Affected in Root Hair Infection. Plant Physiol. 1987 Jun;84(2):421–427. doi: 10.1104/pp.84.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Yadav M. Isolation and partial characterization of the extracellular polysaccharides and lipopolysaccharides from fast-growing Rhizobium japonicum USDA 205 and its Nod- mutant, HC205, which lacks the symbiotic plasmid. Appl Environ Microbiol. 1985 Nov;50(5):1219–1224. doi: 10.1128/aem.50.5.1219-1224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion M., Bhat U. R., Reuhs B., Carlson R. W. Isolation and characterization of the lipopolysaccharides from Bradyrhizobium japonicum. J Bacteriol. 1990 Apr;172(4):1725–1731. doi: 10.1128/jb.172.4.1725-1731.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cava J. R., Elias P. M., Turowski D. A., Noel K. D. Rhizobium leguminosarum CFN42 genetic regions encoding lipopolysaccharide structures essential for complete nodule development on bean plants. J Bacteriol. 1989 Jan;171(1):8–15. doi: 10.1128/jb.171.1.8-15.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty A. K., Zurkowski W., Shine J., Rolfe B. G. Symbiotic nitrogen fixation: molecular cloning of Rhizobium genes involved in exopolysaccharide synthesis and effective nodulation. J Mol Appl Genet. 1982;1(6):585–596. [PubMed] [Google Scholar]
- Clark M. F., Adams A. N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol. 1977 Mar;34(3):475–483. doi: 10.1099/0022-1317-34-3-475. [DOI] [PubMed] [Google Scholar]
- Clover R. H., Kieber J., Signer E. R. Lipopolysaccharide mutants of Rhizobium meliloti are not defective in symbiosis. J Bacteriol. 1989 Jul;171(7):3961–3967. doi: 10.1128/jb.171.7.3961-3967.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Truchet G. L., Hollingsworth R. I., Hrabak E. M., Pankratz H. S., Philip-Hollingsworth S., Salzwedel J. L., Chapman K., Appenzeller L., Squartini A. Rhizobium lipopolysaccharide modulates infection thread development in white clover root hairs. J Bacteriol. 1991 Sep;173(17):5371–5384. doi: 10.1128/jb.173.17.5371-5384.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Finan T. M., Hartweig E., LeMieux K., Bergman K., Walker G. C., Signer E. R. General transduction in Rhizobium meliloti. J Bacteriol. 1984 Jul;159(1):120–124. doi: 10.1128/jb.159.1.120-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Geremia R. A., Cavaignac S., Zorreguieta A., Toro N., Olivares J., Ugalde R. A. A Rhizobium meliloti mutant that forms ineffective pseudonodules in alfalfa produces exopolysaccharide but fails to form beta-(1----2) glucan. J Bacteriol. 1987 Feb;169(2):880–884. doi: 10.1128/jb.169.2.880-884.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosen-de Roo L., de Maagd R. A., Lugtenberg B. J. Antigenic changes in lipopolysaccharide I of Rhizobium leguminosarum bv. viciae in root nodules of Vicia sativa subsp. nigra occur during release from infection threads. J Bacteriol. 1991 May;173(10):3177–3183. doi: 10.1128/jb.173.10.3177-3183.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Signal exchange in plant-microbe interactions. Microbiol Rev. 1986 Jun;50(2):193–225. doi: 10.1128/mr.50.2.193-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. R. Rhizobium genetics. Annu Rev Genet. 1989;23:483–506. doi: 10.1146/annurev.ge.23.120189.002411. [DOI] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Noel K. D., Vandenbosch K. A., Kulpaca B. Mutations in Rhizobium phaseoli that lead to arrested development of infection threads. J Bacteriol. 1986 Dec;168(3):1392–1401. doi: 10.1128/jb.168.3.1392-1401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priefer U. B. Genes involved in lipopolysaccharide production and symbiosis are clustered on the chromosome of Rhizobium leguminosarum biovar viciae VF39. J Bacteriol. 1989 Nov;171(11):6161–6168. doi: 10.1128/jb.171.11.6161-6168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnoky P., Petrovics G., Kereszt A., Grosskopf E., Ha D. T., Bánfalvi Z., Kondorosi A. Rhizobium meliloti lipopolysaccharide and exopolysaccharide can have the same function in the plant-bacterium interaction. J Bacteriol. 1990 Sep;172(9):5450–5458. doi: 10.1128/jb.172.9.5450-5458.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., MacAlister T., Costerton J. W., Cheng K. J. Permeability of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium to antibiotics, lysozyme, and other agents. Can J Microbiol. 1974 Aug;20(8):1135–1145. doi: 10.1139/m74-176. [DOI] [PubMed] [Google Scholar]
- Sindhu S. S., Brewin N. J., Kannenberg E. L. Immunochemical analysis of lipopolysaccharides from free-living and endosymbiotic forms of Rhizobium leguminosarum. J Bacteriol. 1990 Apr;172(4):1804–1813. doi: 10.1128/jb.172.4.1804-1813.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey G., So J. S., Roth L. E., Lakshmi SK B., Carlson R. W. A lipopolysaccharide mutant of Bradyrhizobium japonicum that uncouples plant from bacterial differentiation. Mol Plant Microbe Interact. 1991 Jul-Aug;4(4):332–340. doi: 10.1094/mpmi-4-332. [DOI] [PubMed] [Google Scholar]
- Sturm S., Fortnagel P., Timmis K. N. Immunoblotting procedure for the analysis of electrophoretically-fractionated bacterial lipopolysaccharide. Arch Microbiol. 1984 Dec;140(2-3):198–201. doi: 10.1007/BF00454926. [DOI] [PubMed] [Google Scholar]
- Tao H., Brewin N. J., Noel K. D. Rhizobium leguminosarum CFN42 lipopolysaccharide antigenic changes induced by environmental conditions. J Bacteriol. 1992 Apr;174(7):2222–2229. doi: 10.1128/jb.174.7.2222-2229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Williams M. N., Hollingsworth R. I., Brzoska P. M., Signer E. R. Rhizobium meliloti chromosomal loci required for suppression of exopolysaccharide mutations by lipopolysaccharide. J Bacteriol. 1990 Nov;172(11):6596–6598. doi: 10.1128/jb.172.11.6596-6598.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. N., Hollingsworth R. I., Klein S., Signer E. R. The symbiotic defect of Rhizobium meliloti exopolysaccharide mutants is suppressed by lpsZ+, a gene involved in lipopolysaccharide biosynthesis. J Bacteriol. 1990 May;172(5):2622–2632. doi: 10.1128/jb.172.5.2622-2632.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson P., Mattocks K., Schlegel R. A. Merocyanine 540, a fluorescent probe sensitive to lipid packing. Biochim Biophys Acta. 1983 Jul 27;732(2):387–393. doi: 10.1016/0005-2736(83)90055-x. [DOI] [PubMed] [Google Scholar]
- de Maagd R. A., Rao A. S., Mulders I. H., Goosen-de Roo L., van Loosdrecht M. C., Wijffelman C. A., Lugtenberg B. J. Isolation and characterization of mutants of Rhizobium leguminosarum bv. viciae 248 with altered lipopolysaccharides: possible role of surface charge or hydrophobicity in bacterial release from the infection thread. J Bacteriol. 1989 Feb;171(2):1143–1150. doi: 10.1128/jb.171.2.1143-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]




