Abstract
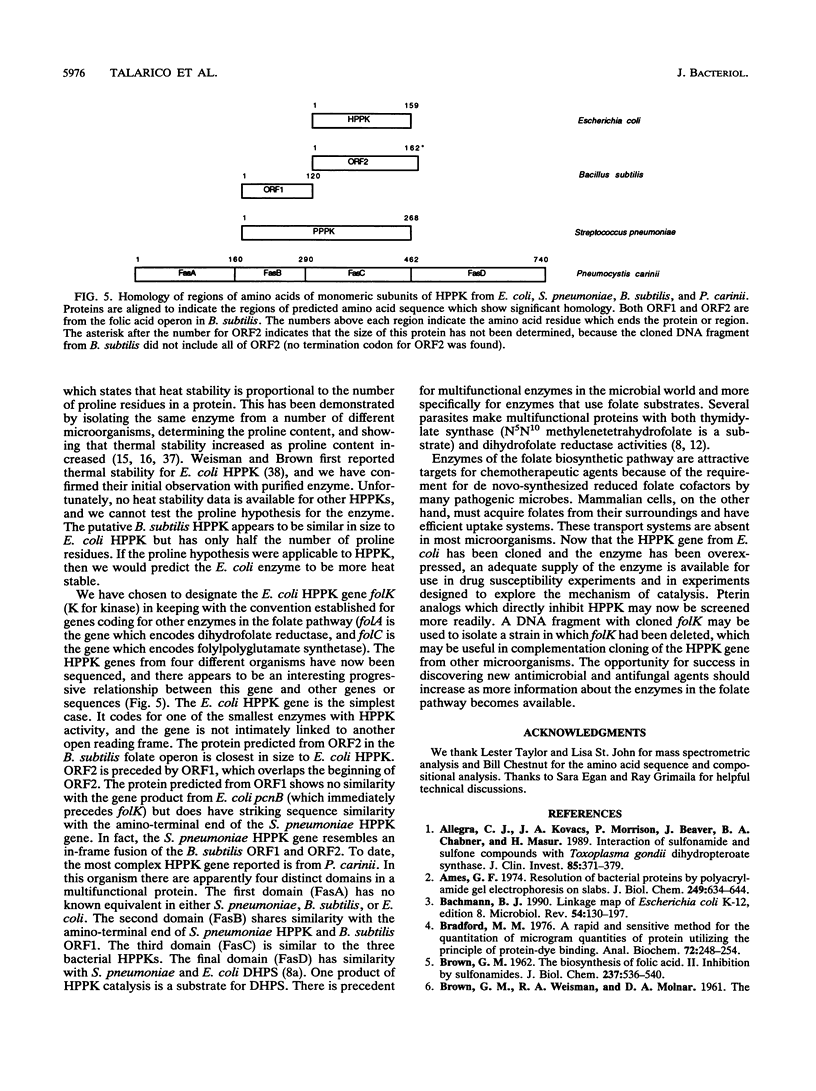
The gene coding for the Escherichia coli enzyme 7,8-dihydro-6-hydroxymethylpterin-pyrophosphokinase has been cloned and sequenced. This gene, designated folK, codes for a protein of 159 amino acids, including an amino-terminal methionine. The protein was overexpressed in E. coli MC4100 by cloning the gene behind the lacUV5 promoter in a high-copy-number plasmid. The enzyme was purified to homogeneity. Amino-terminal analysis of the purified protein showed that the amino-terminal methionine had been removed. The compositional molecular mass (17,945 Da) was identical to the molecular mass determined by mass spectrometry. The enzyme was observed to have a large number of proline residues and migrated anomalously in sodium dodecyl sulfate-polyacrylamide gels, with an apparent molecular mass of 23,000 Da.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegra C. J., Boarman D., Kovacs J. A., Morrison P., Beaver J., Chabner B. A., Masur H. Interaction of sulfonamide and sulfone compounds with Toxoplasma gondii dihydropteroate synthase. J Clin Invest. 1990 Feb;85(2):371–379. doi: 10.1172/JCI114448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- BROWN G. M. The biosynthesis of folic acid. II. Inhibition by sulfonamides. J Biol Chem. 1962 Feb;237:536–540. [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burchall J. J., Hitchings G. H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965 Sep;1(2):126–136. [PubMed] [Google Scholar]
- Bzik D. J., Li W. B., Horii T., Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn J. B., Mann M., Meng C. K., Wong S. F., Whitehouse C. M. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989 Oct 6;246(4926):64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- Ferone R. The enzymic synthesis of dihydropteroate and dihydrofolate by Plasmodium berghei. J Protozool. 1973 Aug;20(3):459–464. doi: 10.1111/j.1550-7408.1973.tb00926.x. [DOI] [PubMed] [Google Scholar]
- GRIFFIN M. J., BROWN G. M. THE BIOSYNTHESIS OF FOLIC ACID. III. ENZYMATIC FORMATION OF DIHYDROFOLIC ACID FROM DIHYDROPTEROIC ACID AND OF TETRAHYDROPTEROYLPOLYGLUTAMIC ACID COMPOUNDS FROM TETRAHYDROFOLIC ACID. J Biol Chem. 1964 Jan;239:310–316. [PubMed] [Google Scholar]
- Grumont R., Washtien W. L., Caput D., Santi D. V. Bifunctional thymidylate synthase-dihydrofolate reductase from Leishmania tropica: sequence homology with the corresponding monofunctional proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5387–5391. doi: 10.1073/pnas.83.15.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. J. Growth and initiation of protein synthesis in Escherichia coli in the presence of trimethoprim. J Bacteriol. 1973 Apr;114(1):309–322. doi: 10.1128/jb.114.1.309-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirel P. H., Schmitter M. J., Dessen P., Fayat G., Blanquet S. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8247–8251. doi: 10.1073/pnas.86.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Yamada H., Nakamura S., Imoto T. Purification, amino acid sequence, and some properties of rabbit kidney lysozyme. J Biochem. 1990 Feb;107(2):236–241. doi: 10.1093/oxfordjournals.jbchem.a123032. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Liu J. D., Parkinson J. S. Genetics and sequence analysis of the pcnB locus, an Escherichia coli gene involved in plasmid copy number control. J Bacteriol. 1989 Mar;171(3):1254–1261. doi: 10.1128/jb.171.3.1254-1261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P., Espinosa M., Greenberg B., Lacks S. A. Sulfonamide resistance in Streptococcus pneumoniae: DNA sequence of the gene encoding dihydropteroate synthase and characterization of the enzyme. J Bacteriol. 1987 Sep;169(9):4320–4326. doi: 10.1128/jb.169.9.4320-4326.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P., Greenberg B., Lacks S. A. DNA sequence of folate biosynthesis gene sulD, encoding hydroxymethyldihydropterin pyrophosphokinase in Streptococcus pneumoniae, and characterization of the enzyme. J Bacteriol. 1990 Sep;172(9):4766–4774. doi: 10.1128/jb.172.9.4766-4774.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March J. B., Colloms M. D., Hart-Davis D., Oliver I. R., Masters M. Cloning and characterization of an Escherichia coli gene, pcnB, affecting plasmid copy number. Mol Microbiol. 1989 Jul;3(7):903–910. doi: 10.1111/j.1365-2958.1989.tb00239.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okinaka O., Iwai K. The biosynthesis of folic acid compounds in plants. IV. Purification and properties of the dihydropteroate-synthesizing enzyme from pea seedlings. J Vitaminol (Kyoto) 1970 Sep;16(3):201–209. doi: 10.5925/jnsv1954.16.201. [DOI] [PubMed] [Google Scholar]
- Ortiz P. J., Hotchkiss R. D. The enzymatic synthesis of dihydrofolate and dihydropteroate in cell-free preparations from wild-type and sulfonamide-resistant pneumococcus. Biochemistry. 1966 Jan;5(1):67–74. doi: 10.1021/bi00865a010. [DOI] [PubMed] [Google Scholar]
- Richey D. P., Brown G. M. The biosynthesis of folic acid. IX. Purification and properties of the enzymes required for the formation of dihydropteroic acid. J Biol Chem. 1969 Mar 25;244(6):1582–1592. [PubMed] [Google Scholar]
- Roland S., Ferone R., Harvey R. J., Styles V. L., Morrison R. W. The characteristics and significance of sulfonamides as substrates for Escherichia coli dihydropteroate synthase. J Biol Chem. 1979 Oct 25;254(20):10337–10345. [PubMed] [Google Scholar]
- SHIOTA T., DISRAELY M. N., MCCANN M. P. THE ENZYMATIC SYNTHESIS OF FOLATE-LIKE COMPOUNDS FROM HYDROXYMETHYLDIHYDROPTERIDINE PYROPHOSPHATE. J Biol Chem. 1964 Jul;239:2259–2266. [PubMed] [Google Scholar]
- SHIOTA T., DISRAELY M. N. The enzymic synthesis of dihydrofolate from 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine and p-aminobenzoylglutamate by extracts of Lactobacillus plantarum. Biochim Biophys Acta. 1961 Sep 30;52:467–473. doi: 10.1016/0006-3002(61)90404-8. [DOI] [PubMed] [Google Scholar]
- Slock J., Stahly D. P., Han C. Y., Six E. W., Crawford I. P. An apparent Bacillus subtilis folic acid biosynthetic operon containing pab, an amphibolic trpG gene, a third gene required for synthesis of para-aminobenzoic acid, and the dihydropteroate synthase gene. J Bacteriol. 1990 Dec;172(12):7211–7226. doi: 10.1128/jb.172.12.7211-7226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico T. L., Dev I. K., Dallas W. S., Ferone R., Ray P. H. Purification and partial characterization of 7,8-dihydro-6-hydroxymethylpterin-pyrophosphokinase and 7,8-dihydropteroate synthase from Escherichia coli MC4100. J Bacteriol. 1991 Nov;173(21):7029–7032. doi: 10.1128/jb.173.21.7029-7032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe F., Dyer M., Scaife J. G., Darby G., Stammers D. K., Delves C. J. The multifunctional folic acid synthesis fas gene of Pneumocystis carinii appears to encode dihydropteroate synthase and hydroxymethyldihydropterin pyrophosphokinase. Gene. 1992 Mar 15;112(2):213–218. doi: 10.1016/0378-1119(92)90378-3. [DOI] [PubMed] [Google Scholar]
- WEISMAN R. A., BROWN G. M. THE BIOSYNTHESIS OF FOLIC ACID. V. CHARACTERISTICS OF THE ENZYME SYSTEM THAT CATALYZES THE SYNTHESIS OF DIHYDROPTEROIC ACID. J Biol Chem. 1964 Jan;239:326–331. [PubMed] [Google Scholar]
- Watanabe K., Chishiro K., Kitamura K., Suzuki Y. Proline residues responsible for thermostability occur with high frequency in the loop regions of an extremely thermostable oligo-1,6-glucosidase from Bacillus thermoglucosidasius KP1006. J Biol Chem. 1991 Dec 25;266(36):24287–24294. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]