Abstract
1 The effects of tubocurarine, hexamethonium and trimetaphan on the synaptic currents of rat submandibular ganglion cells have been measured at 20°C by means of a two-microelectrode voltage-clamp system. The aim was to distinguish between the receptor-blocking and channel-blocking actions of those drugs, and to test for possible selectivity of action on the `fast' and `slow' acetylcholine-operated channels.
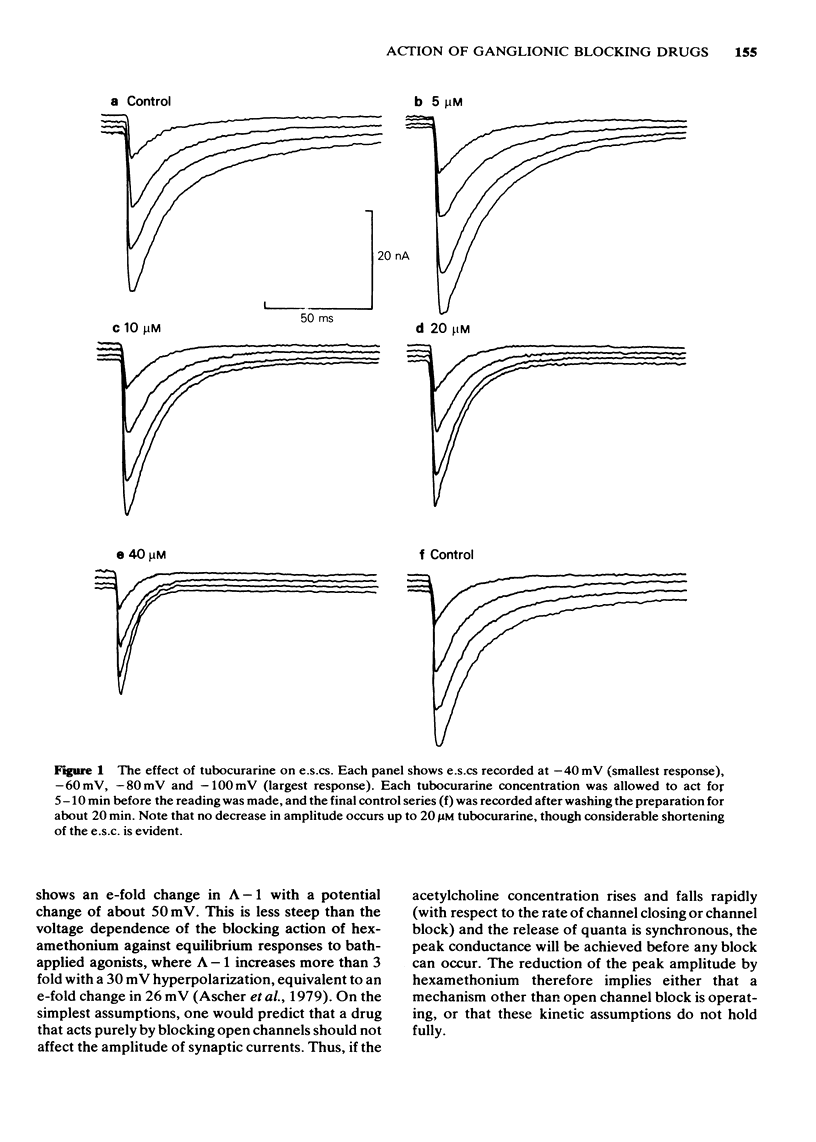
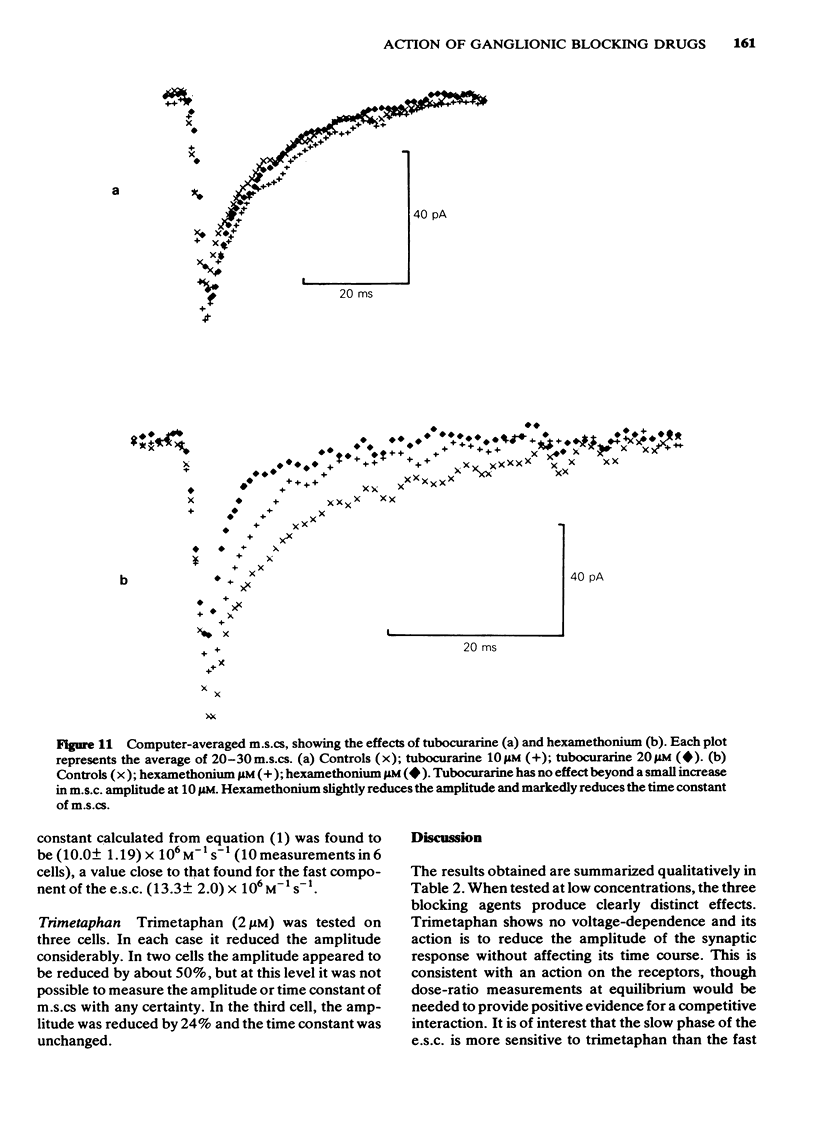
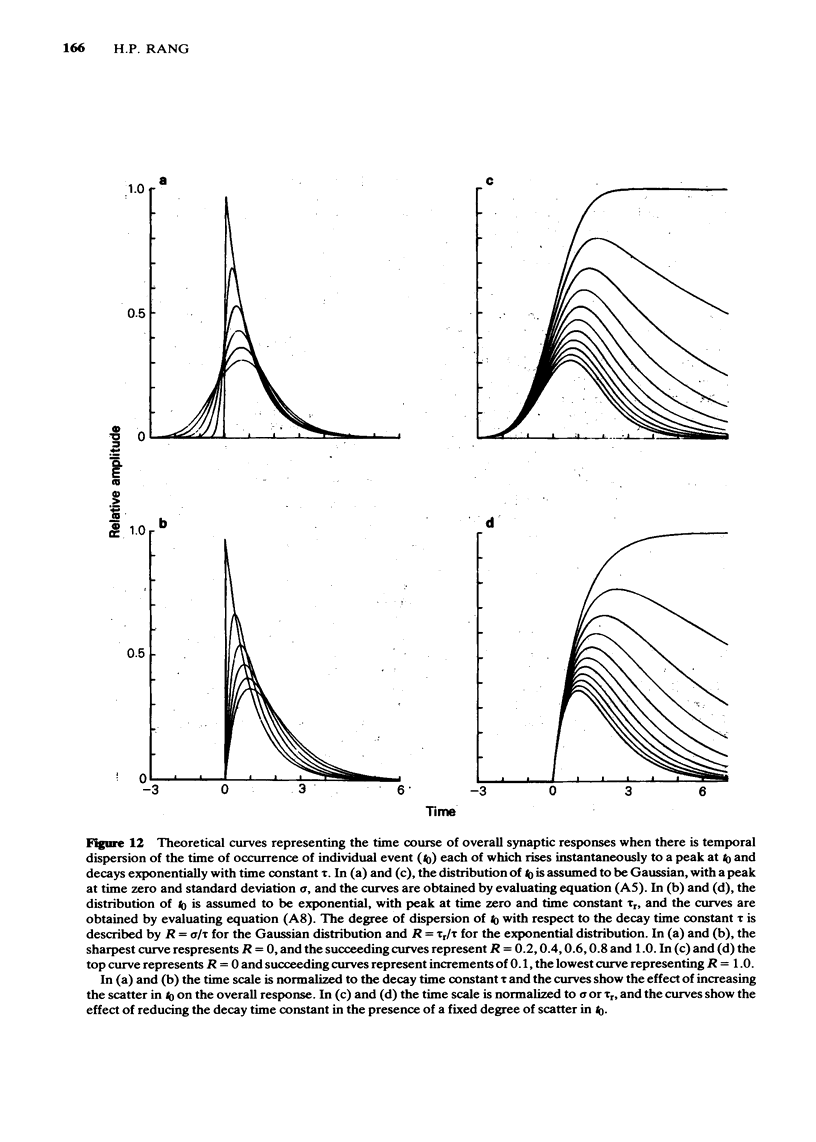
2 Tubocurarine had no effect on the amplitude of evoked synaptic currents (e.s.cs) or miniature synaptic currents (m.s.cs), except at concentrations exceeding 20 μM. The slow component of the e.s.c. was shortened by tubocurarine, this effect becoming more marked as the cell was hyperpolarized. The timecourse of m.s.cs, which have no slow component, was unaffected.
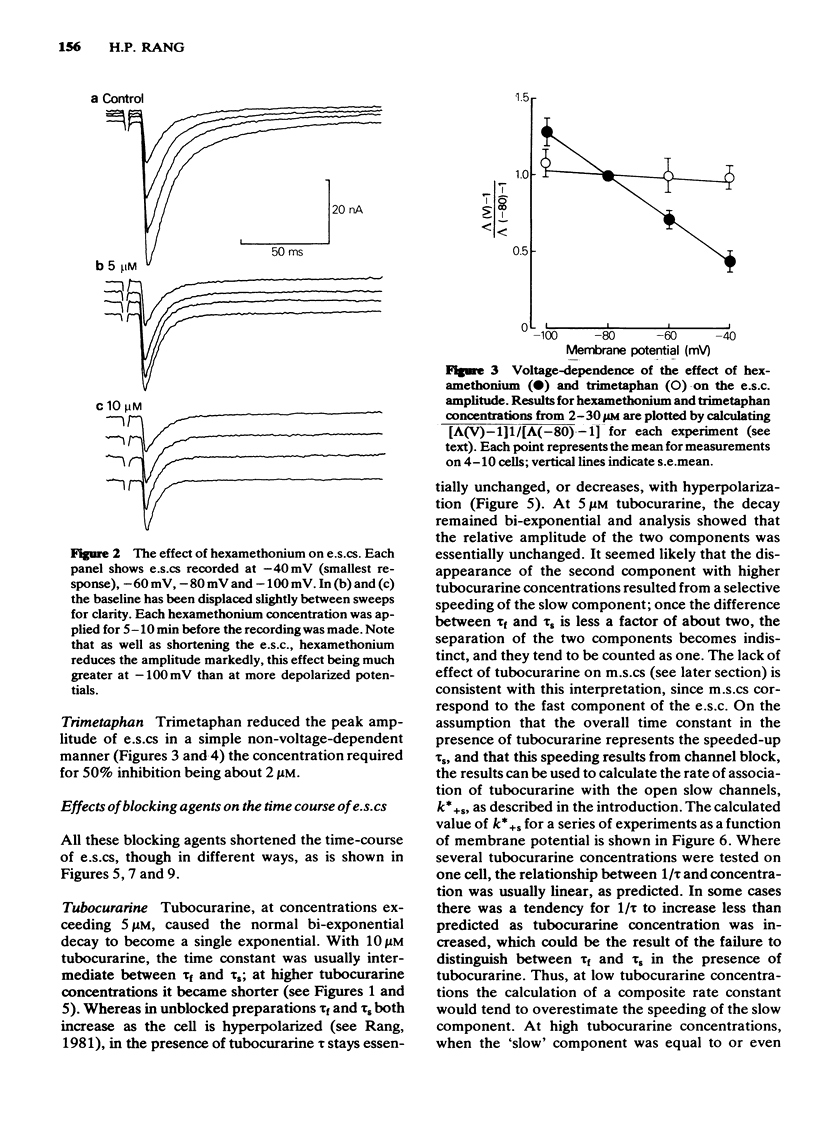
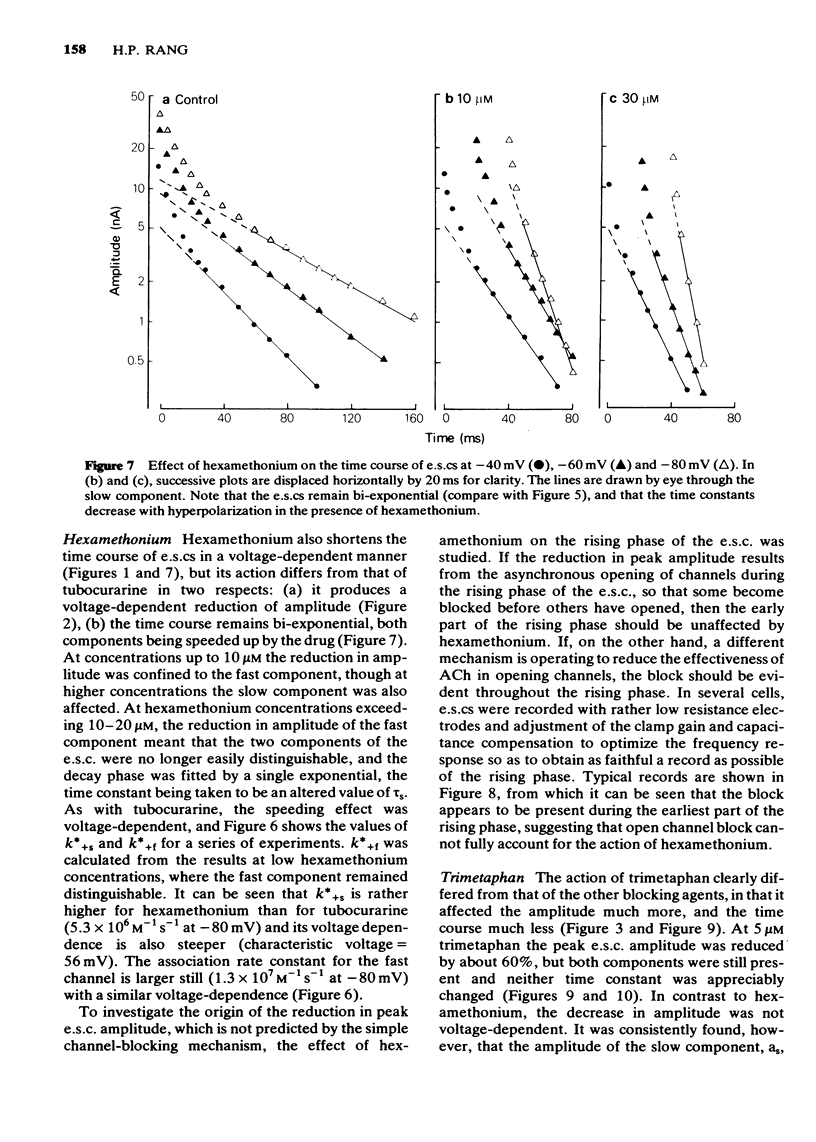
3 Hexamethonium (2-30 μM) caused a voltage-dependent reduction of e.s.c. amplitude, and voltage-dependent shortening of both fast and slow components of the e.s.c. M.s.cs were also shortened.
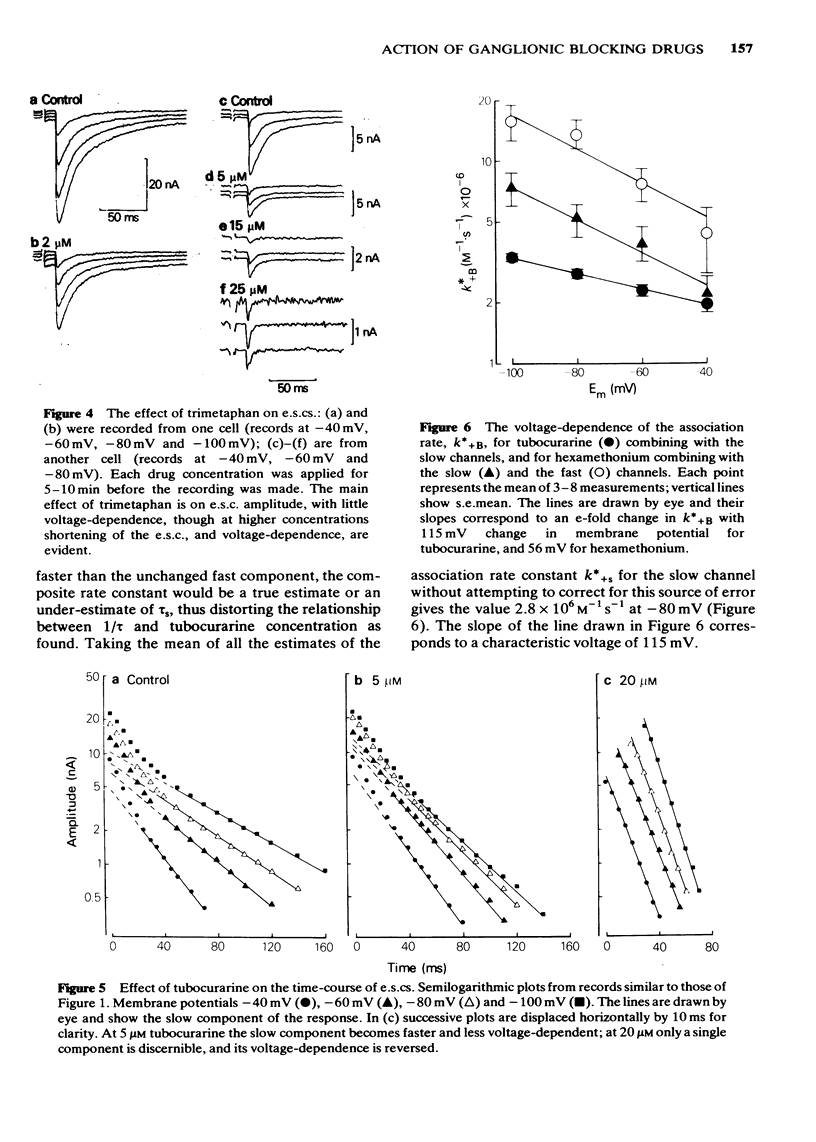
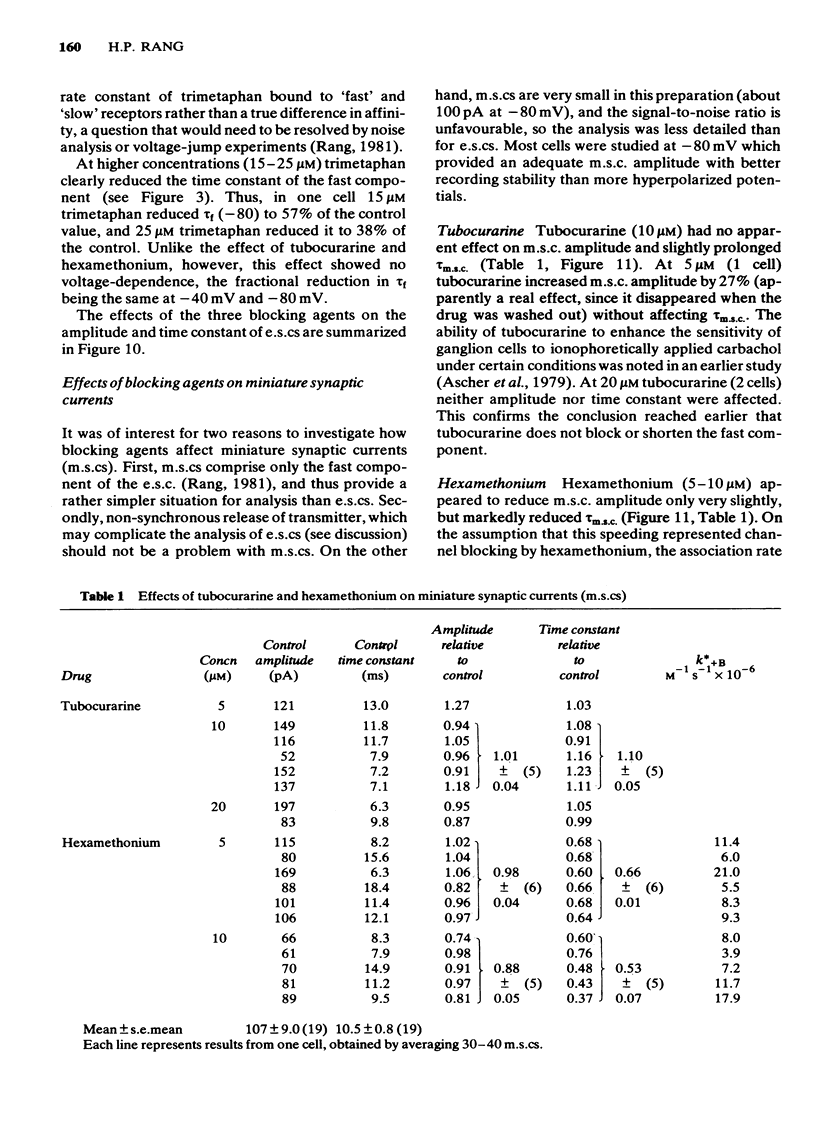
4 Trimetaphan (2-10 μM) reduced the amplitude of e.s.cs and m.s.cs. Neither component of the e.s.c. was shortened by trimetaphan; however, the slow component was reduced in amplitude more than the fast component, so that the overall duration of the e.s.c. appeared to be reduced. At higher concentrations (15-25 μM) trimetaphan clearly shortened the fast component.
5 It is concluded that tubocurarine acts selectively on the slow ionic channels, the association rate constant being 2.8 × 106 M-1 s-1 at -80 mV. Hexamethonium acts on both fast and slow channels, the association rate constants, at -80 mV, being respectively 5.3 × 106 M-1 s-1 and 1.3 × 107 M-1 s-1. With both drugs, the association rate constant increases if the cell is hyperpolarized, this effect being more pronounced with hexamethonium than with tubocurarine.
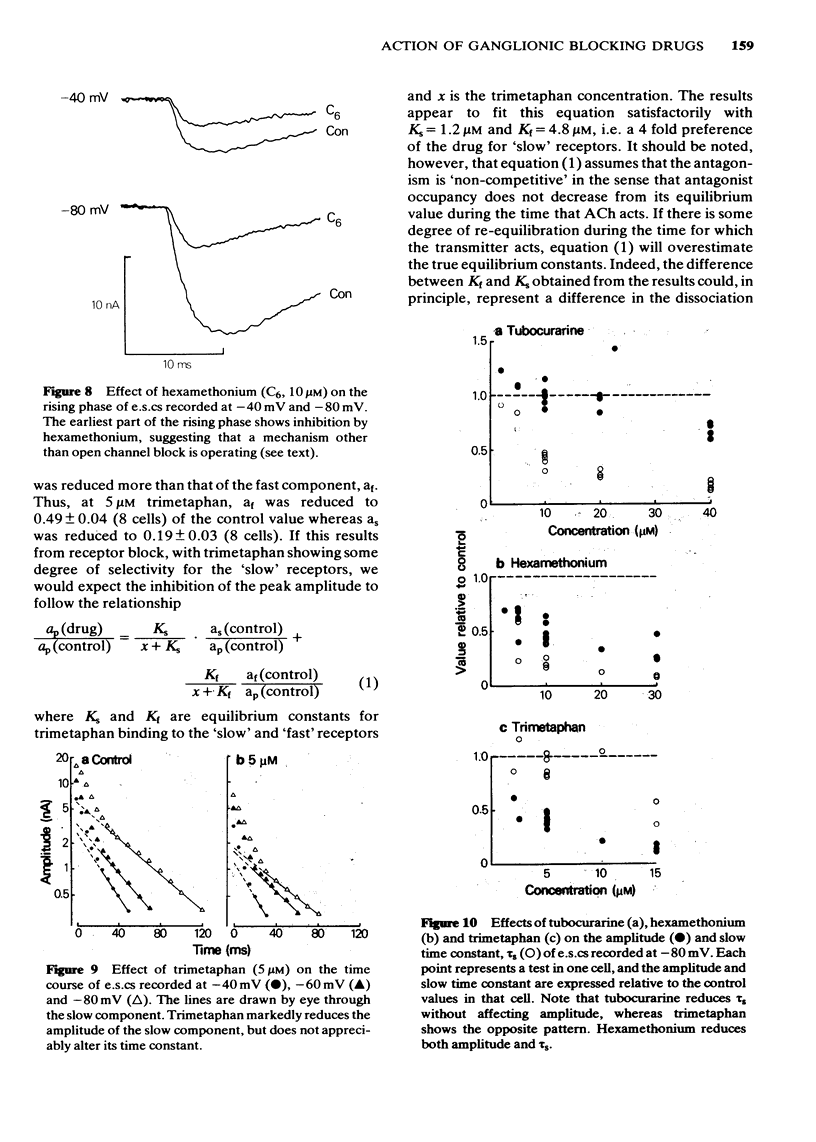
6 The marked voltage-dependent reduction of e.s.c. amplitude by hexamethonium cannot be accounted for by open channel block, and requires an additional mechanism, the nature of which is discussed.
7 Trimetaphan, at low concentrations, acts in a way consistent with receptor block, and shows a degree of selectivity for the slow component of the e.s.c.
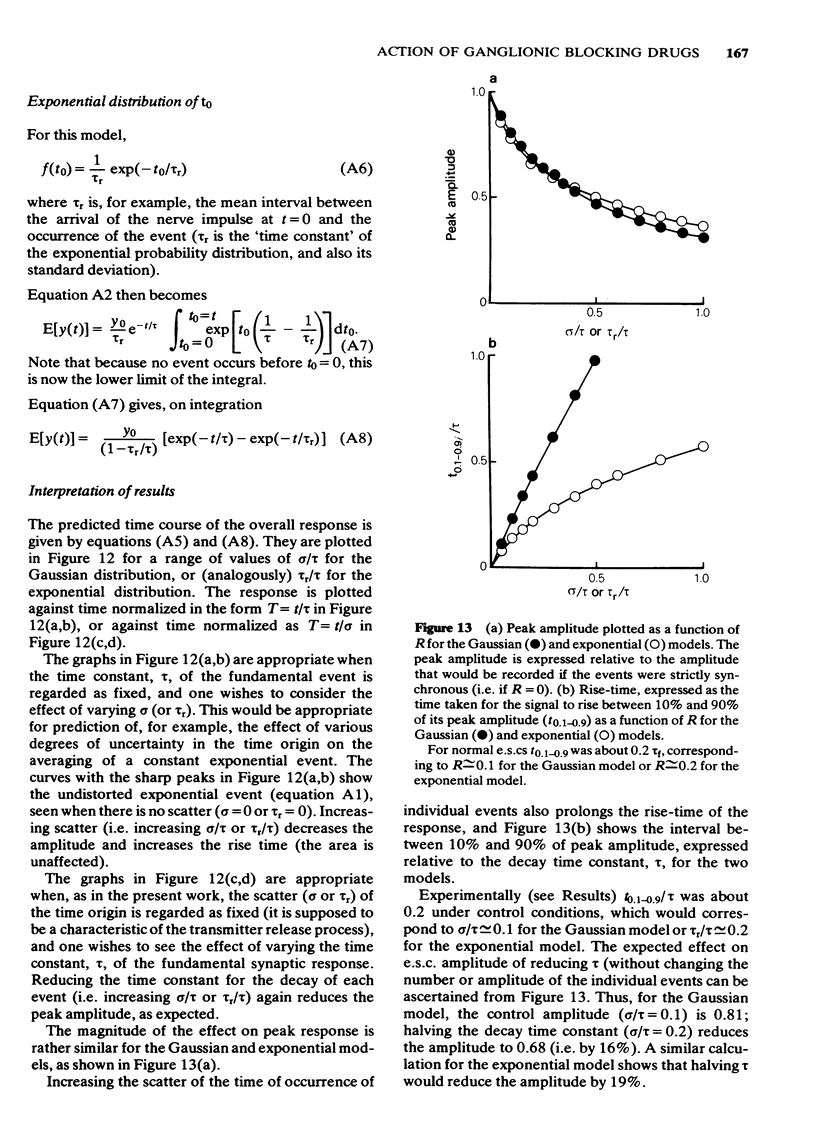
8 In an appendix, the effect of temporal dispersion of the time of opening of ionic channels on the amplitude and time-course of the composite synaptic response is analysed. It is concluded that the shortening of the time-constant of the e.s.c. decay by hexamethonium cannot, by itself, account for the drug's effect on e.s.c. amplitude.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Feltz A. Quinacrine (mepacrine) action at frog end-plate. J Physiol. 1980 Sep;306:261–281. doi: 10.1113/jphysiol.1980.sp013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Sakmann B. Decamethonium both opens and blocks endplate channels. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2994–2998. doi: 10.1073/pnas.75.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M., Albuquerque E. X., Lebeda F. J. Kinetic analysis of end plate currents altered by atropine and scopolamine. Mol Pharmacol. 1978 May;14(3):514–529. [PubMed] [Google Scholar]
- Adler M., Oliveira A. C., Albuquerque E. X., Mansour N. A., Eldefrawi A. T. Reaction of tetraethylammonium with the open and closed conformations of the acetylcholine receptor ionic channel complex. J Gen Physiol. 1979 Jul;74(1):129–152. doi: 10.1085/jgp.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Large W. A., Rang H. P. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979 Oct;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. The mode of action of antagonists of the excitatory response to acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:207–235. doi: 10.1113/jphysiol.1978.sp012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sheridan R. E. The modes of action of gallamine. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):181–203. doi: 10.1098/rspb.1981.0002. [DOI] [PubMed] [Google Scholar]
- Feltz A., Large W. A., Trautmann A. Analysis of atropine action at the frog neutromuscular junction. J Physiol. 1977 Jul;269(1):109–130. doi: 10.1113/jphysiol.1977.sp011895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flacke W., Fleisch J. H. The effect of ganglionic agonists and antagonists on the cardiac sympathetic ganglia of the dog. J Pharmacol Exp Ther. 1970 Jul;174(1):45–55. [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The antagonism between tubocurarine and substances which depolarize the motor end-plate. J Physiol. 1960 Jul;152:309–324. doi: 10.1113/jphysiol.1960.sp006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Manalis R. S. Voltage-dependent effect of curare at the frog neuromuscular junction. Nature. 1977 May 26;267(5609):366–368. doi: 10.1038/267366a0. [DOI] [PubMed] [Google Scholar]
- Marty A. Noise and relaxation studies of acetylcholine induced currents in the presence of procaine. J Physiol. 1978 May;278:237–250. doi: 10.1113/jphysiol.1978.sp012301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P. The characteristics of synaptic currents and responses to acetylcholine of rat submandibular ganglion cells. J Physiol. 1981 Feb;311:23–55. doi: 10.1113/jphysiol.1981.sp013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P. The kinetics of action of acetylcholine antagonists in smooth muscle. Proc R Soc Lond B Biol Sci. 1966 Apr 19;164(996):488–510. doi: 10.1098/rspb.1966.0045. [DOI] [PubMed] [Google Scholar]
- Ruff R. L. A quantitative analysis of local anaesthetic alteration of miniature end-plate currents and end-plate current fluctuations. J Physiol. 1977 Jan;264(1):89–124. doi: 10.1113/jphysiol.1977.sp011659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedt T. N., Albuquerque E. X., Bakry N. M., Eldefrawi M. E., Eldefrawi A. T. Voltage- and time-dependent actions of piperocaine on the ion channel of the acetylcholine receptor. Mol Pharmacol. 1979 Nov;16(3):909–921. [PubMed] [Google Scholar]
- Tsai M. C., Oliveira A. C., Albuquerque E. X., Eldefrawi M. E., Eldefrawi A. T. Mode of action of quinacrine on the acetylcholine receptor ionic channel complex. Mol Pharmacol. 1979 Sep;16(2):382–392. [PubMed] [Google Scholar]


