Abstract
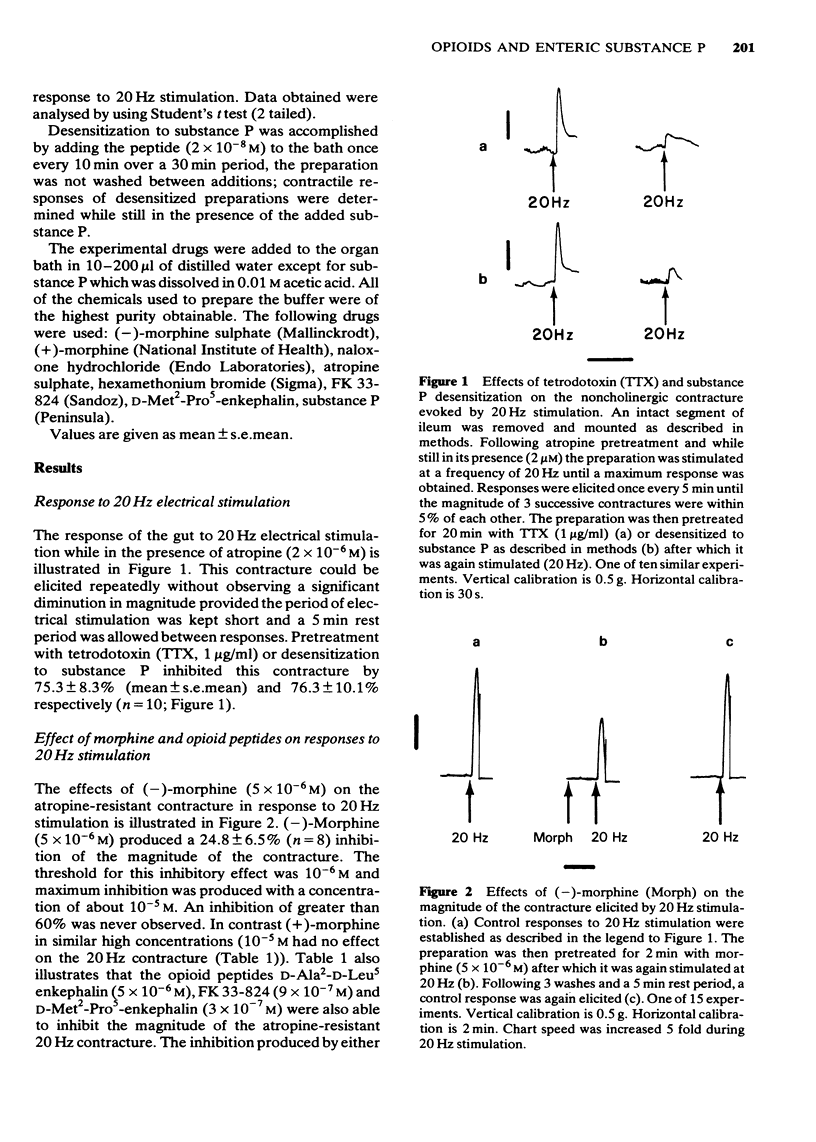
1 Experiments were carried out to determine whether opiates and opioid peptides could affect noncholinergic excitatory responses of the isolated guinea-pig ileum. 2 Transmural field stimulation (10-20 Hz) of an atropine pretreated, intact segment of gut produced a contracture that could be elicited repeatedly without significant variation in magnitude. 3 This noncholinergic contracture was significantly reduced 75.3 +/- 8.3% (mean +/- s.e. mean) by tetrodotoxin (TTX; 1 microgram/ml) and by desensitizing the preparation to substance P (76.3 +/- 10.1%). 4 Morphine (5 x 10(-6) M) as well as the opioid peptides D-Ala2, N-Phe4, Met-(0)-01 (FK 33-824; 9 x 10(-7) M), D-Met2-Pro5 enkephalin (3 x 10(-7) M) and D-Ala2-D-Leu5-enkephalin (5 x 10(-6) M) inhibited the magnitude of the noncholinergic contracture but did not alter contractile responses to exogenous substance P (4 x 10(-11) M--4 x 10(-10) M). 5 Pretreatment with the nicotinic receptor blocker, hexamethonium (10(-5)--10(-4) M) reduced by about 35% the magnitude of the atropine-resistant contracture but did not affect inhibitory responses to morphine or opioid peptides. Thus the inhibition produced by morphine on the 20 Hz contracture does not involve a nicotinic cholinergic mechanism. 6 Naloxone pretreatment (10(-6) M) in the presence of hexamethonium (10(-5)--10(-4) M) enhanced the magnitude of the noncholinergic contracture without affecting responses to exogenous substance P (4 x 10(-11)--4 x 10(-10) M). 7 These data suggest that substance P is the main, if not the sole, mediator of the atropine-resistant 20 Hz contracture and indicate further that exogenous as well as endogenous opioids can modulate the release of this enteric peptide.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
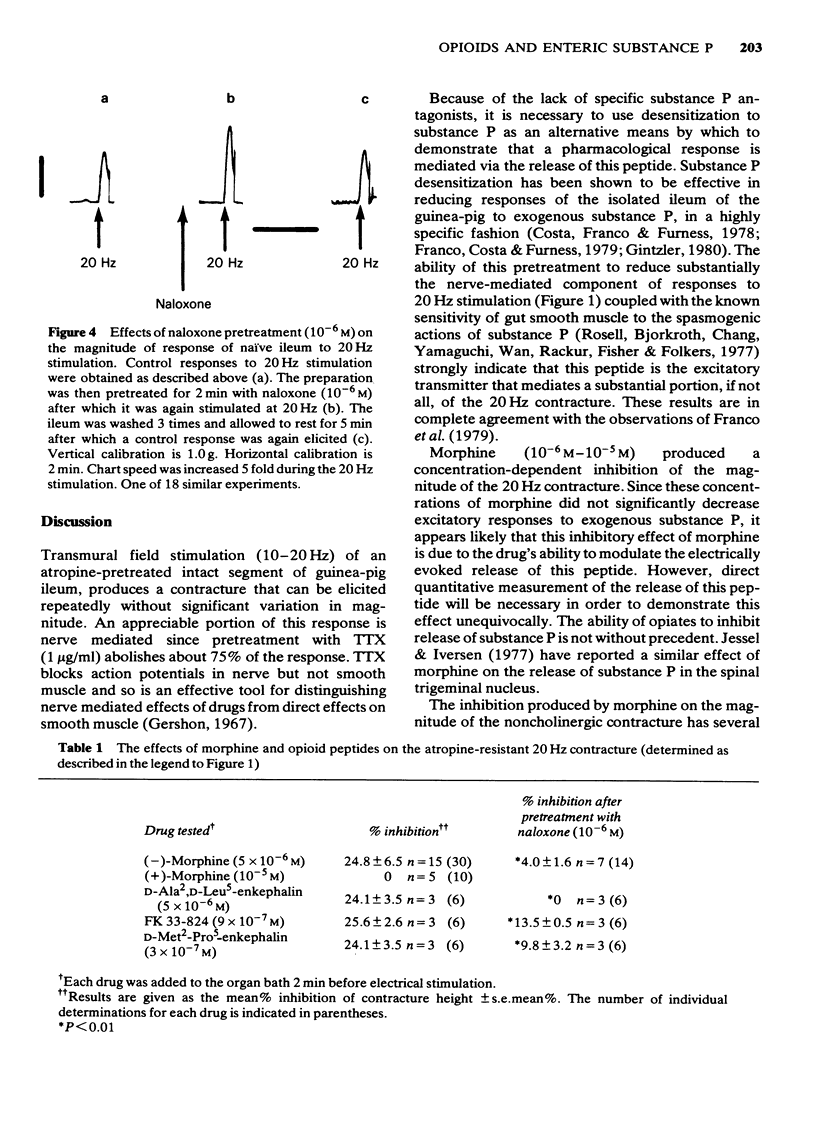
- Dreyfus C. F., Bornstein M. B. Synthesis of serotonin by neurons of the myenteric plexus in situ and in organotypic tissue culture. Brain Res. 1977 Jun 3;128(1):125–139. doi: 10.1016/0006-8993(77)90240-2. [DOI] [PubMed] [Google Scholar]
- Dreyfus C. F., Sherman D. L., Gershon M. D. Uptake of serotonin by intrinsic neurons of the myenteric plexus grown in organotypic tissue culture. Brain Res. 1977 Jun 3;128(1):109–123. doi: 10.1016/0006-8993(77)90239-6. [DOI] [PubMed] [Google Scholar]
- Franco R., Costa M., Furness J. B. Evidence for the release of endogenous substance P from intestinal nerves. Naunyn Schmiedebergs Arch Pharmacol. 1979 Apr;306(3):195–201. doi: 10.1007/BF00507103. [DOI] [PubMed] [Google Scholar]
- Gershon M. D. Effects of tetrodotoxin on innervated smooth muscle preparations. Br J Pharmacol Chemother. 1967 Mar;29(3):259–279. doi: 10.1111/j.1476-5381.1967.tb01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gintzler A. R. Serotonin participation in gut withdrawal from opiates. J Pharmacol Exp Ther. 1979 Oct;211(1):7–12. [PubMed] [Google Scholar]
- Gintzler A. R. Substance P involvement in the expression of gut dependence on opiates. Brain Res. 1980 Jan 20;182(1):224–228. doi: 10.1016/0006-8993(80)90850-1. [DOI] [PubMed] [Google Scholar]
- Goldstein A., Schulz R. Morphine-tolerant longitudinal muscle strip from guinea-pig ileum. Br J Pharmacol. 1973 Aug;48(4):655–666. doi: 10.1111/j.1476-5381.1973.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature. 1977 Aug 11;268(5620):549–551. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- Kosterlitz H. W., Waterfield A. A. In vitro models in the study of structure-activity relationships of narcotic analgesics. Annu Rev Pharmacol. 1975;15:29–47. doi: 10.1146/annurev.pa.15.040175.000333. [DOI] [PubMed] [Google Scholar]
- Leslie F. M., Chavkin C., Cox B. M. Opioid binding properties of brain and peripheral tissues: evidence for heterogeneity in opioid ligand binding sites. J Pharmacol Exp Ther. 1980 Aug;214(2):395–402. [PubMed] [Google Scholar]
- Lord J. A., Waterfield A. A., Hughes J., Kosterlitz H. W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977 Jun 9;267(5611):495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Martin W. R., Eades C. G., Thompson J. A., Huppler R. E., Gilbert P. E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976 Jun;197(3):517–532. [PubMed] [Google Scholar]
- Nilsson G., Larsson L. I., Håkanson R., Brodin E., Pernow B., Sundler F. Localization of substance P-like immunoreactivity in mouse gut. Histochemistry. 1975;43(1):97–99. doi: 10.1007/BF00490158. [DOI] [PubMed] [Google Scholar]
- PATON W. D. The action of morphine and related substances on contraction and on acetylcholine output of coaxially stimulated guinea-pig ileum. Br J Pharmacol Chemother. 1957 Mar;12(1):119–127. doi: 10.1111/j.1476-5381.1957.tb01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M. Immunocytochemical localization of substance P in mammalian intestine. Histochemistry. 1975;41(4):373–375. doi: 10.1007/BF00490081. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Dreyfus C. F., Gershon M. D., Hökfelt T., Elde R. P., Nilsson G., Said S., Goldstein M. VIP-, enkephalin-, substance P- and somatostatin-like immunoreactivity in neurons intrinsic to the intestine: immunohistochemical evidence from organotypic tissue cultures. Brain Res. 1978 Oct 27;155(2):239–248. doi: 10.1016/0006-8993(78)91020-x. [DOI] [PubMed] [Google Scholar]
- Schulz R., Herz A. Aspects of opiate dependence in the myenteric plexus of the guinea-pig. Life Sci. 1976 Oct 15;19(8):1117–1127. doi: 10.1016/0024-3205(76)90246-0. [DOI] [PubMed] [Google Scholar]


