Abstract
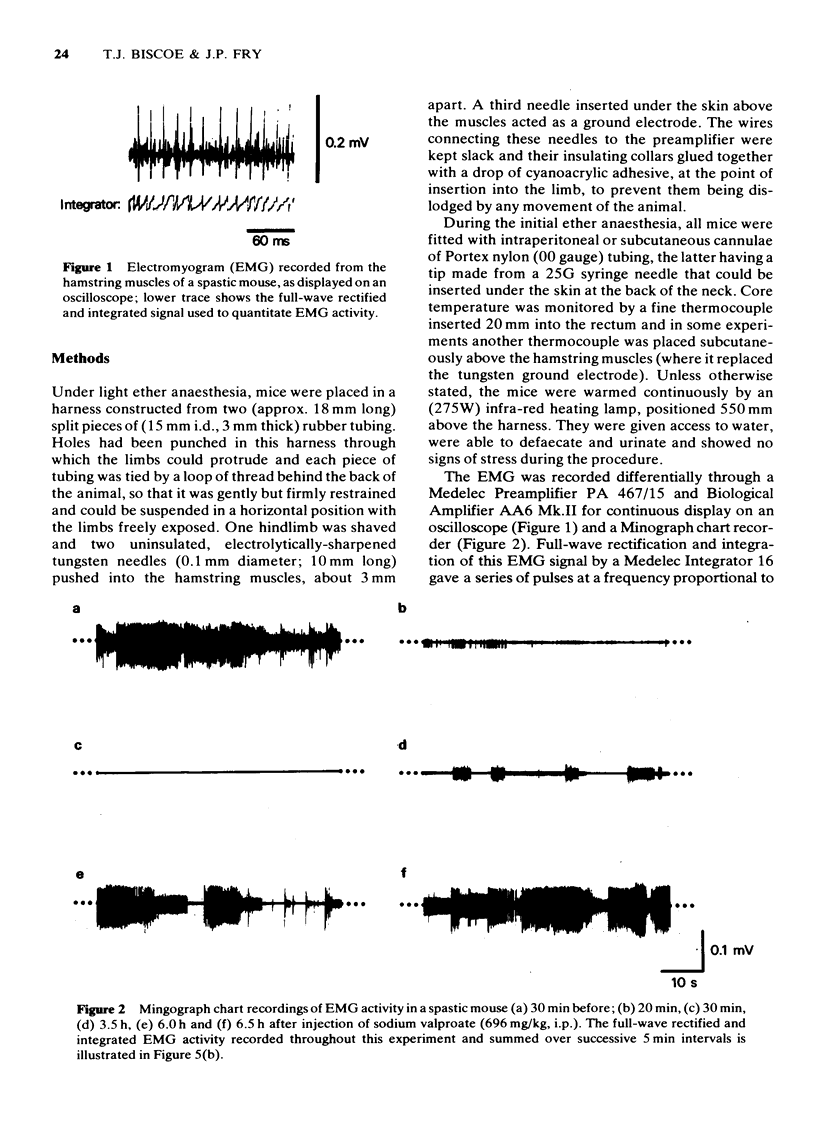
1 Full-wave rectification and integration of the EMG signal recorded from the hamstring muscles of the spastic mouse was used to evaluate the actions of a variety of drugs on the muscle rigidity of these mutants, animals in which no histological lesion has yet been found. 2 Profound and long-lasting muscle relaxant responses were consistently observed upon the injection of diazepam (2 mg/kg, i.p.) and flunitrazepam (2 mg/kg, i.p.). Such responses were always greater than those obtained upon injection of 40% (v/v) propylene glycol (10 ml/kg) alone, the vehicle for the benzodiazepines. 3 The muscle relaxant action of a low dose (0.25 mg/kg i.p.) of the benzodiazepine Roll-6896 was not shared by the same dose of its enantiomer Roll-6893. 4 Profound and long-lasting muscle relaxation was caused by sodium valproate (696 mg/kg, i.p.). Consistent muscle relaxant responses were also observed upon the injection of pentobarbitone (30 mg/kg, i.p.), but not phenobarbitone (30 mg/kg, i.p.). 5 Other drugs that had little or no detectable effect on the muscle rigidity of the spastic mouse included diphenylhydantoin (30 mg/kg, i.p.) and bromocriptine (10 mg/kg, s.c.) while, in some animals, benztropine (2 mg/kg, i.p.) and baclofen (10 mg/kg, i.p.) increased muscle rigidity. 6 The development of full muscle relaxant responses to flunitrazepam (2 mg/kg, i.p.) and to sodium valproate (696 mg/kg, i.p.) was shown to depend upon mild warming of the animals with radiant heat, a procedure which can increase muscle spindle afferent input to the spinal cord. 7 The results suggest a hyperactivity of stretch reflexes in the spastic mouse, ameliorated selectively by those drugs that enhance the GABA-mediated presynaptic inhibition of such pathways.
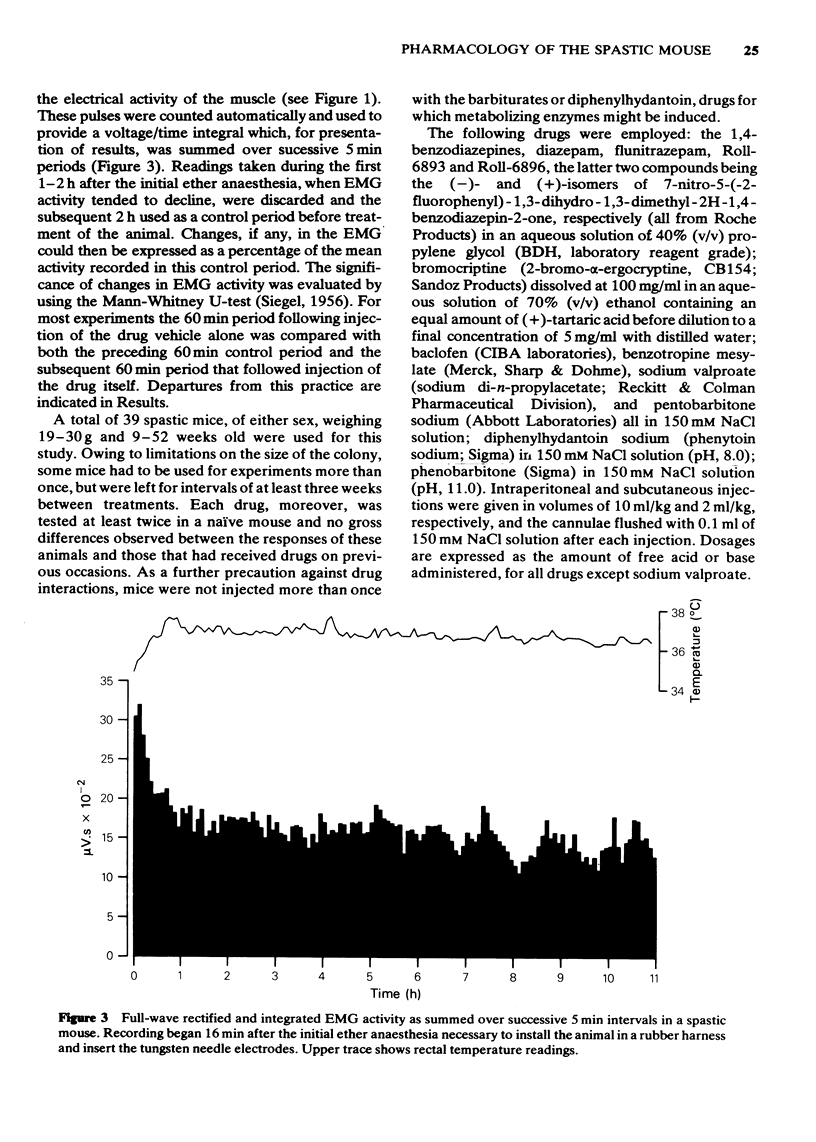
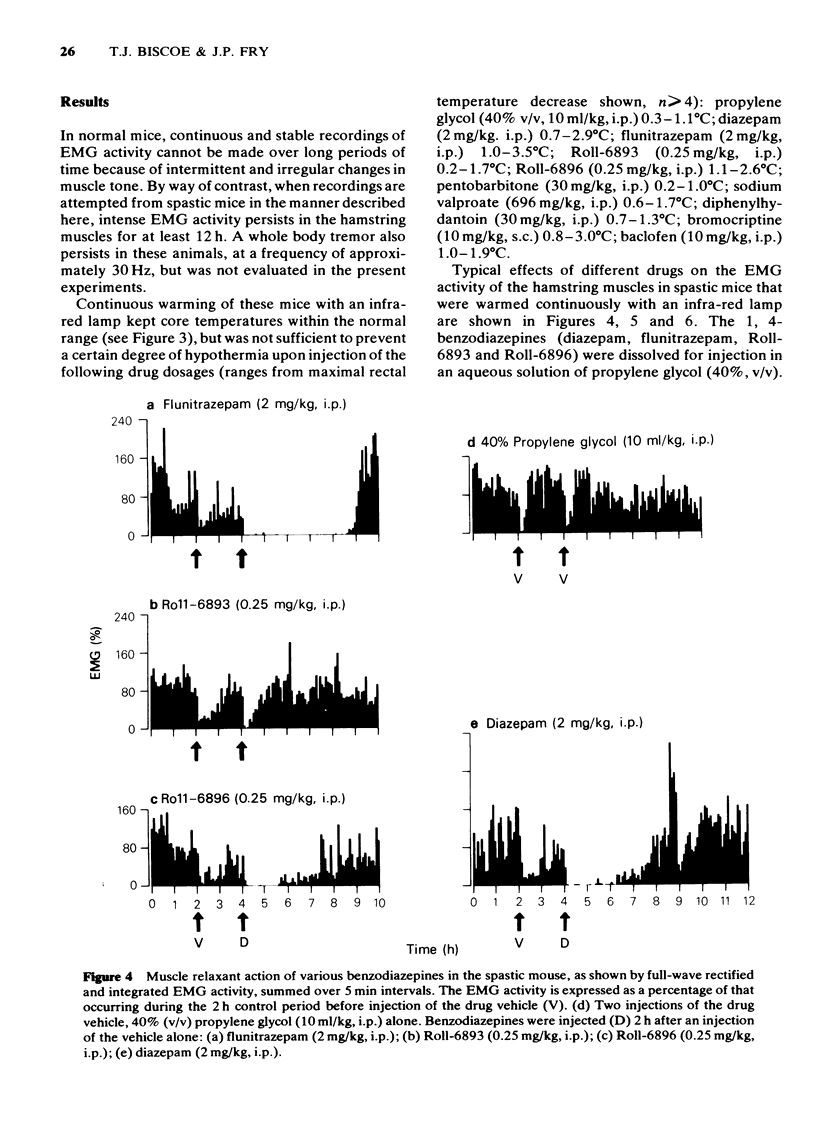
Full text
PDF
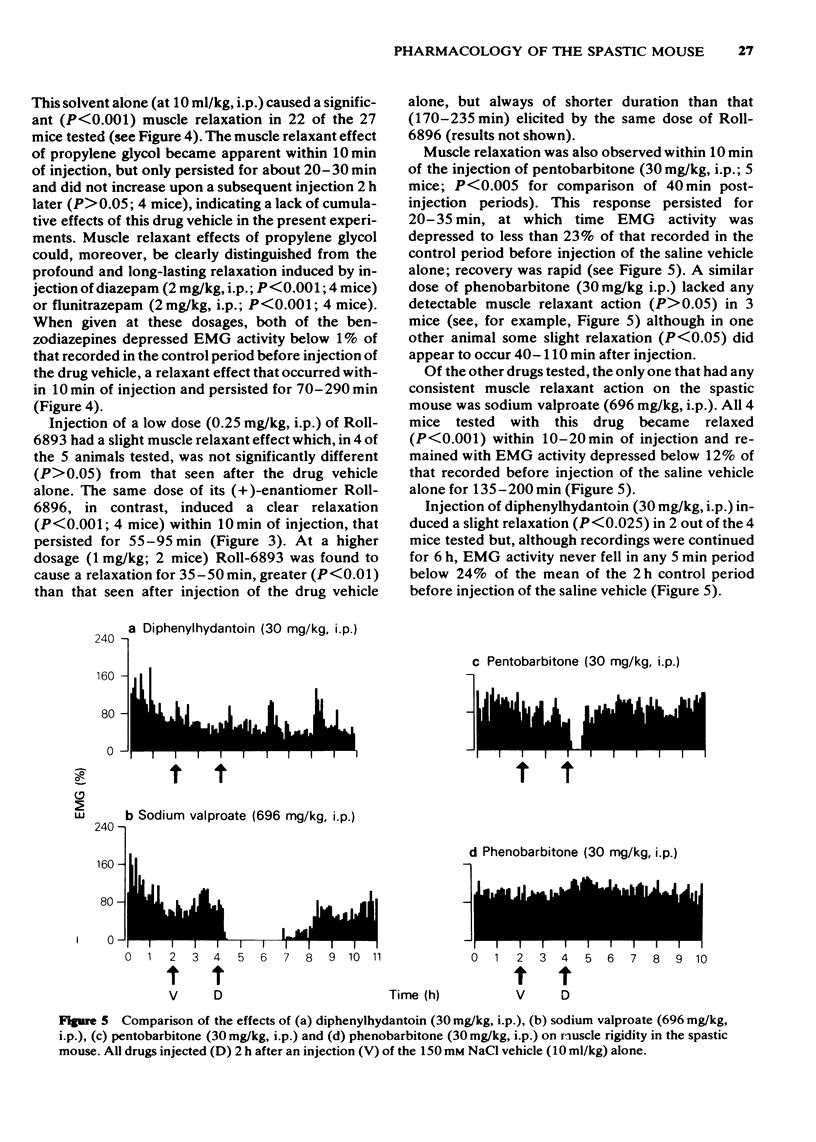






Selected References
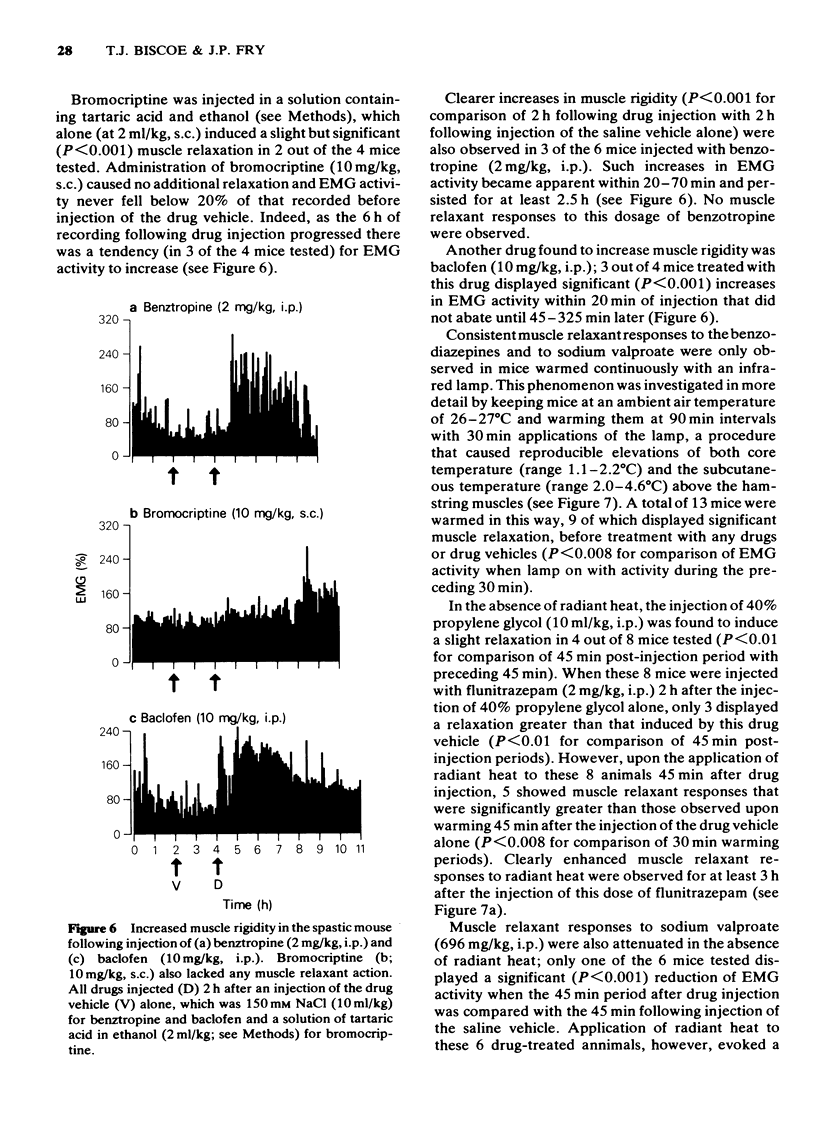
These references are in PubMed. This may not be the complete list of references from this article.
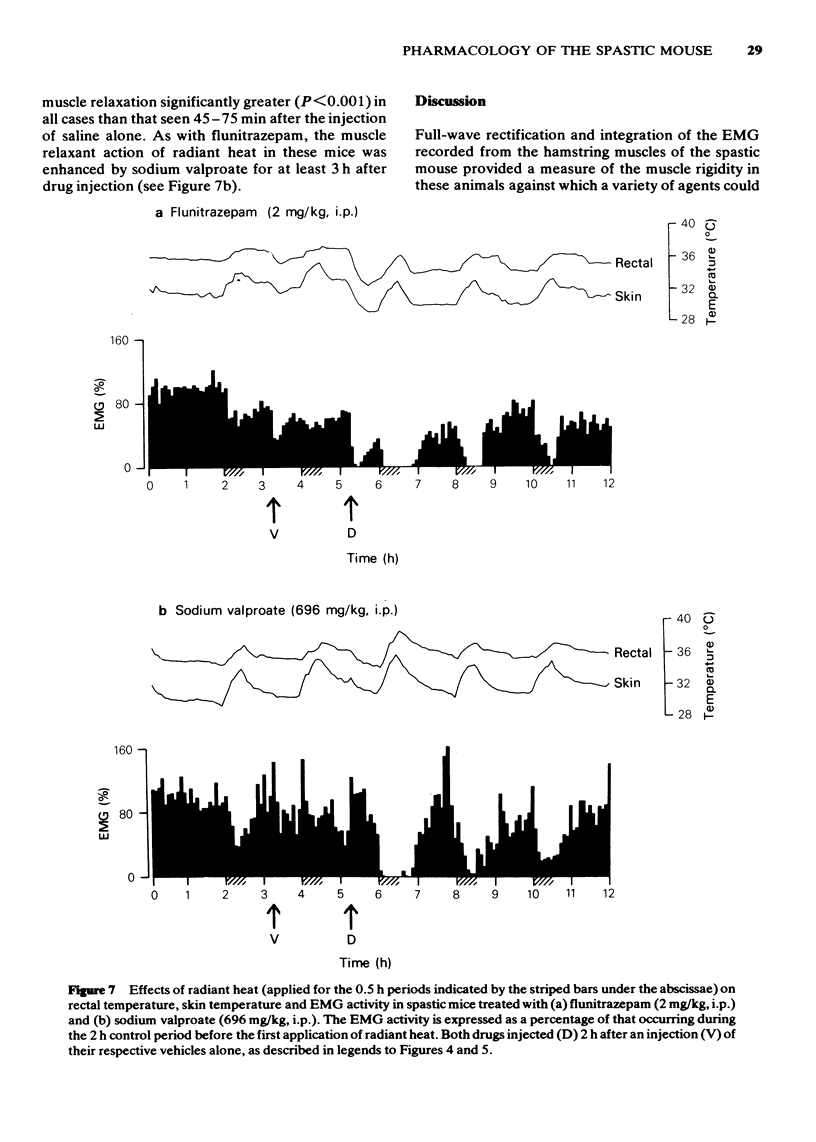
- AHMED A., MARSHALL P. B. Relationship between anti-acetylcholine and anti-Tremorine activity in anti-parkinsonian and related drugs. Br J Pharmacol Chemother. 1962 Apr;18:247–254. doi: 10.1111/j.1476-5381.1962.tb01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anlezark G., Horton R. W., Meldrium B. S., Sawaya C. B. Anticonvulsant action of ethanolamine-O-sulphate and di-n-propylacetate and the metabolism of gamma-aminobutyric acid (GABA) in mice with audiogenic seizures. Biochem Pharmacol. 1976 Feb 15;25(4):413–417. doi: 10.1016/0006-2952(76)90343-9. [DOI] [PubMed] [Google Scholar]
- Arvidsson J., Jurna I., Steg G. Striatal and spinal lesions eliminating reserpine and physostigmine rigidity. Life Sci. 1967 Oct 1;6(19):2017–2020. doi: 10.1016/0024-3205(67)90219-6. [DOI] [PubMed] [Google Scholar]
- Arvidsson J., Roos B. E., Steg G. Reciprocal effects on alpha- and gamma-motoneurones of drugs influencing monoaminergic and cholinergic transmission. Acta Physiol Scand. 1966 Jul-Aug;67(3):398–404. doi: 10.1111/j.1748-1716.1966.tb03326.x. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Pentobarbitone pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:355–372. doi: 10.1113/jphysiol.1978.sp012388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmayer W., Danielczyk W., Weiler G. Zur Objektivierbarkeit des myotonolytischen Effektes eines Aminobuttersäurederivates (CIBA 34647-Ba) Wien Med Wochenschr. 1967 Jan 7;117(1):7–9. [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- CHAI C. K., ROBERTS E., SIDMAN R. L. Influence of aminooxyacetic acid, a gamma-aminobutyrate transaminase inhibitor, on hereditary spastic defect in the mouse. Proc Soc Exp Biol Med. 1962 Mar;109:491–495. doi: 10.3181/00379727-109-27245. [DOI] [PubMed] [Google Scholar]
- Calne D. B., Teychenne P. F., Claveria L. E., Eastman R., Greenacre J. K., Petrie A. Bromocriptine in Parkinsonism. Br Med J. 1974 Nov 23;4(5942):442–444. doi: 10.1136/bmj.4.5942.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrodi H., Fuxe K., Hökfelt T., Lidbrink P., Ungerstedt U. Effect of ergot drugs on central catecholamine neurons: evidence for a stimulation of central dopamine neurons. J Pharm Pharmacol. 1973 May;25(5):409–412. doi: 10.1111/j.2042-7158.1973.tb10037.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971 Sep 10;32(1):69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Game C. J., Johnston G. A., McCulloch R. M. Central effects of beta-(para-chlorophenyl)-gamma-aminobutyric acid. Brain Res. 1974 Apr 26;70(3):493–499. doi: 10.1016/0006-8993(74)90257-1. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Lodge D., Brand S. J. GABA and spinal afferent terminal excitability in the cat. Brain Res. 1977 Jul 15;130(2):360–363. doi: 10.1016/0006-8993(77)90283-9. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Lodge D., Johnston G. A., Brand S. J. Central actions of benzodiazepines. Brain Res. 1976 Dec 17;118(2):344–347. doi: 10.1016/0006-8993(76)90723-x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. The synaptic excitation of Renshaw cells. Exp Brain Res. 1966;2(1):81–96. doi: 10.1007/BF00234362. [DOI] [PubMed] [Google Scholar]
- Cutting D. A., Jordan C. C. Alternative approaches to analgesia: baclofen as a model compound. Br J Pharmacol. 1975 Jun;54(2):171–179. doi: 10.1111/j.1476-5381.1975.tb06926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. The action of beta-phenyl-GABA derivatives on neurones of the cat cerebral cortex. Brain Res. 1974 Apr 26;70(3):501–505. doi: 10.1016/0006-8993(74)90258-3. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., MAGNI F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol. 1961 Nov;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., KOZAK W., MAGNI F. Dorsal root reflexes of muscle group I afferent fibres. J Physiol. 1961 Nov;159:128–146. doi: 10.1113/jphysiol.1961.sp006797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESPLIN D. W. Effects of diphenylhydantoin on synaptic transmission in cat spinal cord and stellate ganglion. J Pharmacol Exp Ther. 1957 Jul;120(3):301–323. [PubMed] [Google Scholar]
- Gallager D. W., Mallorga P., Tallman J. F. Interaction of diphenylhydantoin and benzodiazepines in the CNS. Brain Res. 1980 May 5;189(1):209–220. doi: 10.1016/0006-8993(80)90018-9. [DOI] [PubMed] [Google Scholar]
- Johnson A. M., Loew D. M., Vigouret J. M. Stimulant properties of bromocriptine on central dopamine receptors in comparison to apomorphine, (+)-amphetamine and L-DOPA. Br J Pharmacol. 1976 Jan;56(1):59–68. doi: 10.1111/j.1476-5381.1976.tb06959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. A. Neuropharmacology of amino acid inhibitory transmitters. Annu Rev Pharmacol Toxicol. 1978;18:269–289. doi: 10.1146/annurev.pa.18.040178.001413. [DOI] [PubMed] [Google Scholar]
- Julien R. M. Cerebellar involvement in the antiepileptic action of diazepam. Neuropharmacology. 1972 Sep;11(5):683–691. doi: 10.1016/0028-3908(72)90076-7. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Barker J. L. Different actions of anticonvulsant and anesthetic barbiturates revealed by use of cultured mammalian neurons. Science. 1978 May 19;200(4343):775–777. doi: 10.1126/science.205953. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Bergey G. K. Valproic acid augments GABA-mediated postsynaptic inhibition in cultured mammalian neurons. Brain Res. 1979 Jul 20;170(3):558–562. doi: 10.1016/0006-8993(79)90975-2. [DOI] [PubMed] [Google Scholar]
- Mense S. Effects of temperature on the discharges of muscle spindles and tendon organs. Pflugers Arch. 1978 May 18;374(2):159–166. doi: 10.1007/BF00581297. [DOI] [PubMed] [Google Scholar]
- Miyahara J. T., Esplin D. W., Zablocka B. Differential effects of depressant drugs on presynaptic inhibition. J Pharmacol Exp Ther. 1966 Oct;154(1):119–127. [PubMed] [Google Scholar]
- Montarolo P. G., Raschi F., Strata P. Interactions between benzodiazepines and GABA in the cerebellar cortex. Brain Res. 1979 Feb 23;162(2):358–362. doi: 10.1016/0006-8993(79)90298-1. [DOI] [PubMed] [Google Scholar]
- Möhler H., Okada T. Benzodiazepine receptor: demonstration in the central nervous system. Science. 1977 Nov 25;198(4319):849–851. doi: 10.1126/science.918669. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Eccles J. C., Oshima T., Rubia F. Prolongation of hippocampal inhibitory postsynaptic potentials by barbiturates. Nature. 1975 Dec 18;258(5536):625–627. doi: 10.1038/258625a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Presynaptic action of barbiturates in the frog spinal cord. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1460–1463. doi: 10.1073/pnas.72.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe R., Sharman D. F., Vogt M. Effect of drugs used in psychoses on cerebral dopamine metabolism. Br J Pharmacol. 1970 Feb;38(2):287–304. doi: 10.1111/j.1476-5381.1970.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierau F. K., Zimmermann P. Action of a GABA-derivative on postsynaptic potentials and membrane properties of cats' spinal motoneurones. Brain Res. 1973 May 17;54:376–380. doi: 10.1016/0006-8993(73)90064-4. [DOI] [PubMed] [Google Scholar]
- Polc P., Haefely W. Effects of two benzodiazepines, phenobarbitone, and baclofen on synaptic transmission in the cat cuneate nucleus. Naunyn Schmiedebergs Arch Pharmacol. 1976 Aug;294(2):121–131. doi: 10.1007/BF00507844. [DOI] [PubMed] [Google Scholar]
- Polc P., Möhler H., Haefely W. The effect of diazepam on spinal cord activities: possible sites and mechanisms of action. Naunyn Schmiedebergs Arch Pharmacol. 1974;284(4):319–337. doi: 10.1007/BF00504702. [DOI] [PubMed] [Google Scholar]
- Potashner S. J. Baclofen: effects on amino acid release. Can J Physiol Pharmacol. 1978 Feb;56(1):150–154. doi: 10.1139/y78-019. [DOI] [PubMed] [Google Scholar]
- Raabe W., Ayala G. F. Diphenylhydantoin increases cortical postsynaptic inhibition. Brain Res. 1976 Apr 9;105(3):597–601. doi: 10.1016/0006-8993(76)90611-9. [DOI] [PubMed] [Google Scholar]
- Raabe W., Gumnit R. J. Anticonvulsant action of diazepam: increase of cortical postsynaptic inhibition. Epilepsia. 1977 Mar;18(1):117–120. doi: 10.1111/j.1528-1157.1977.tb05594.x. [DOI] [PubMed] [Google Scholar]
- SWINYARD E. A., BROWN W. C., GOODMAN L. S. Comparative assays of antiepileptic drugs in mice and rats. J Pharmacol Exp Ther. 1952 Nov;106(3):319–330. [PubMed] [Google Scholar]
- Sastry B. R. Gamma-Aminobutyric acid and primary afferent depolarization in feline spinal cord. Can J Physiol Pharmacol. 1979 Oct;57(10):1157–1167. doi: 10.1139/y79-171. [DOI] [PubMed] [Google Scholar]
- Schlosser W. Action of diazepam on the spinal cord. Arch Int Pharmacodyn Ther. 1971 Nov;194(1):93–102. [PubMed] [Google Scholar]
- Schmidt R. F. Presynaptic inhibition in the vertebrate central nervous system. Ergeb Physiol. 1971;63:20–101. doi: 10.1007/BFb0047741. [DOI] [PubMed] [Google Scholar]
- Schmidt R. F., Vogel M. E., Zimmermann M. Die Wirkung von Diazepam auf die präsynaptische Hemmung und andere Rückenmarksreflexe. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1967;258(1):69–82. [PubMed] [Google Scholar]
- Squires R. F., Brastrup C. Benzodiazepine receptors in rat brain. Nature. 1977 Apr 21;266(5604):732–734. doi: 10.1038/266732a0. [DOI] [PubMed] [Google Scholar]
- Steiner F. A., Felix D. Antagonistic effects of GABA and benzodiazepines on vestibular and cerebellar neurones. Nature. 1976 Mar 25;260(5549):346–347. doi: 10.1038/260346a0. [DOI] [PubMed] [Google Scholar]
- Stratten W. P., Barnes C. D. Diazepam and presynaptic inhibition. Neuropharmacology. 1971 Nov;10(6):685–696. doi: 10.1016/0028-3908(71)90083-9. [DOI] [PubMed] [Google Scholar]


