Abstract
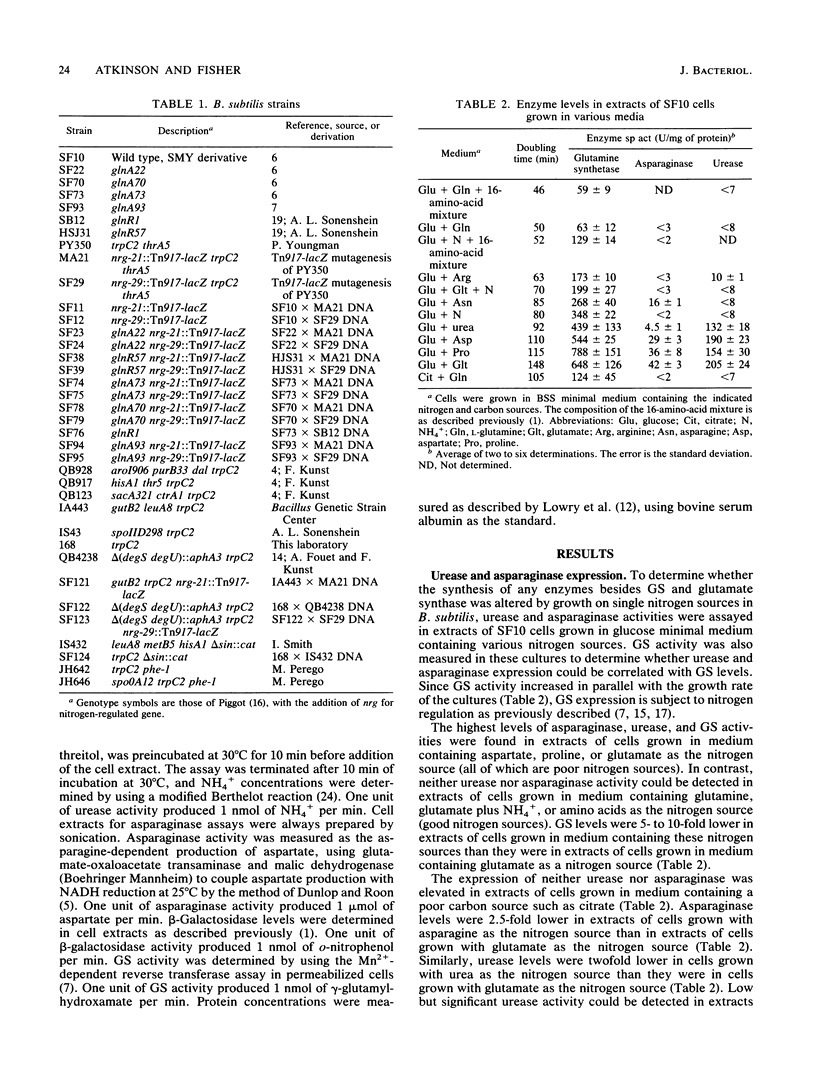
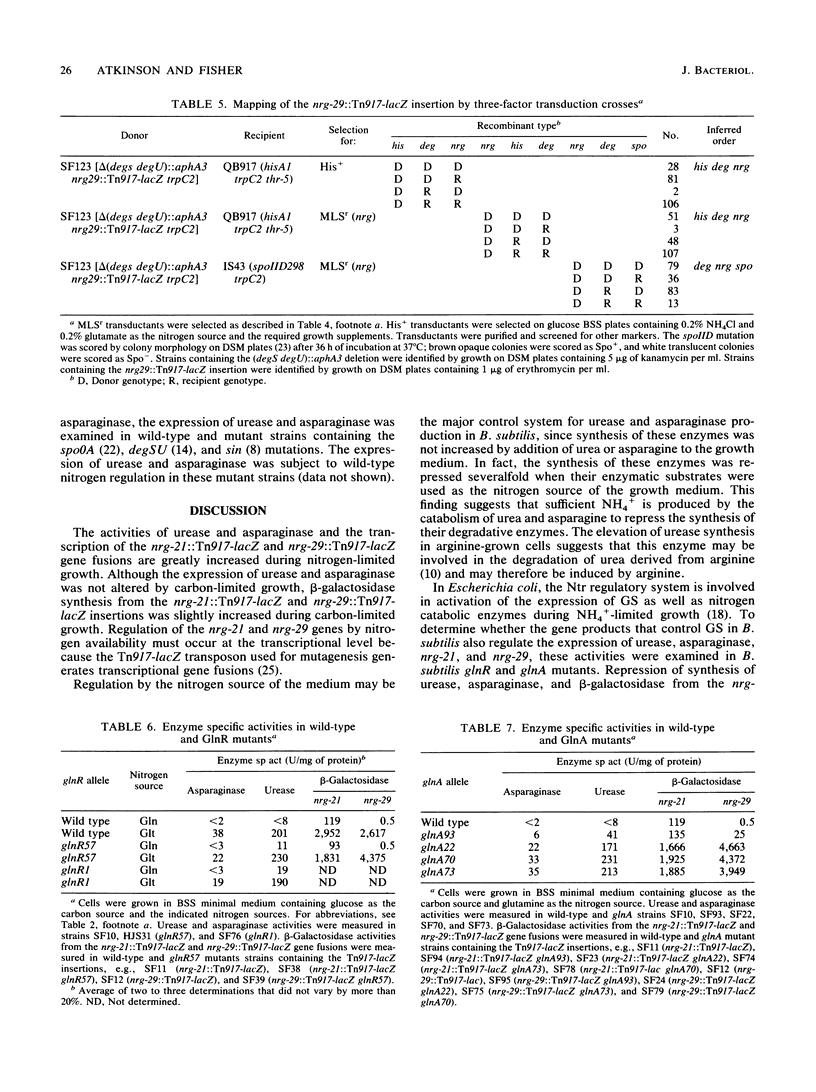
The levels of urease and asparaginase were elevated 25- and 20-fold, respectively, in extracts of Bacillus subtilis cells grown in medium containing nitrogen sources that are poor sources of ammonium (NH4+) compared with the levels seen in extracts of cells grown in medium containing nitrogen sources that are good sources of NH4+. To determine whether a collection of genes whose expression responds to nitrogen availability could be isolated, a library of Tn917-lacZ insertions was screened for nitrogen-regulated beta-galactosidase expression. Two fusion strains were identified. beta-Galactosidase expression was 26- and 4,000-fold higher, respectively, in the nrg-21::Tn917-lacZ and the nrg-29::Tn917-lacZ insertion strains during NH4(+)-restricted growth than during growth on nitrogen sources that are good sources of NH4+. PBS1 transduction analysis showed that the nrg-21::Tn917-lacZ insertion mapped between gutB and purB and that the nrg-29::Tn917-lacZ insertion mapped between degSU and spoIID. The repression of expression of these four gene products during growth on good sources of NH4+ required the wild-type glutamine synthetase protein but not the glutamine synthetase regulatory protein, GlnR.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. R., Wray L. V., Jr, Fisher S. H. Regulation of histidine and proline degradation enzymes by amino acid availability in Bacillus subtilis. J Bacteriol. 1990 Sep;172(9):4758–4765. doi: 10.1128/jb.172.9.4758-4765.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedonder R. A., Lepesant J. A., Lepesant-Kejzlarová J., Billault A., Steinmetz M., Kunst F. Construction of a kit of reference strains for rapid genetic mapping in Bacillus subtilis 168. Appl Environ Microbiol. 1977 Apr;33(4):989–993. doi: 10.1128/aem.33.4.989-993.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop P. C., Roon R. J. L-Asparaginase of Saccharomyces cerevisiae: an extracellular Enzyme. J Bacteriol. 1975 Jun;122(3):1017–1024. doi: 10.1128/jb.122.3.1017-1024.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. H., Sonenshein A. L. Bacillus subtilis glutamine synthetase mutants pleiotropically altered in glucose catabolite repression. J Bacteriol. 1984 Feb;157(2):612–621. doi: 10.1128/jb.157.2.612-621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. H., Sonenshein A. L. Glutamine-requiring mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1977 Dec 7;79(3):987–995. doi: 10.1016/0006-291x(77)91207-4. [DOI] [PubMed] [Google Scholar]
- Gaur N. K., Dubnau E., Smith I. Characterization of a cloned Bacillus subtilis gene that inhibits sporulation in multiple copies. J Bacteriol. 1986 Nov;168(2):860–869. doi: 10.1128/jb.168.2.860-869.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden K. J., Bernlohr R. W. Nitrogen catabolite repression of the L-asparaginase of Bacillus licheniformis. J Bacteriol. 1985 Nov;164(2):938–940. doi: 10.1128/jb.164.2.938-940.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. R., Baumberg S. Arginine hydroxamate-resistant mutants of Bacillus subtilis with altered control of arginine metabolism. J Gen Microbiol. 1977 May;100(1):177–188. doi: 10.1099/00221287-100-1-177. [DOI] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Msadek T., Kunst F., Henner D., Klier A., Rapoport G., Dedonder R. Signal transduction pathway controlling synthesis of a class of degradative enzymes in Bacillus subtilis: expression of the regulatory genes and analysis of mutations in degS and degU. J Bacteriol. 1990 Feb;172(2):824–834. doi: 10.1128/jb.172.2.824-834.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F. L., Coote J. G. Glutamine synthetase and glutamate synthase activities during growth and sporulation in Bacillus subtilis. J Gen Microbiol. 1979 Jun;112(2):373–377. doi: 10.1099/00221287-112-2-373. [DOI] [PubMed] [Google Scholar]
- Rebello J. L., Strauss N. Regulation of synthesis of glutamine synthase in Bacillus subtilis. J Bacteriol. 1969 May;98(2):683–688. doi: 10.1128/jb.98.2.683-688.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H. J., Brown S. W., Hirschi K. D., Nomellini J. F., Sonenshein A. L. Regulation of Bacillus subtilis glutamine synthetase gene expression by the product of the glnR gene. J Mol Biol. 1989 Nov 5;210(1):51–63. doi: 10.1016/0022-2836(89)90290-8. [DOI] [PubMed] [Google Scholar]
- Schreier H. J., Smith T. M., Bernlohr R. W. Regulation of nitrogen catabolic enzymes in Bacillus spp. J Bacteriol. 1982 Aug;151(2):971–975. doi: 10.1128/jb.151.2.971-975.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H. J., Sonenshein A. L. Altered regulation of the glnA gene in glutamine synthetase mutants of Bacillus subtilis. J Bacteriol. 1986 Jul;167(1):35–43. doi: 10.1128/jb.167.1.35-43.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein A. L., Cami B., Brevet J., Cote R. Isolation and characterization of rifampin-resistant and streptolydigin-resistant mutants of Bacillus subtilis with altered sporulation properties. J Bacteriol. 1974 Oct;120(1):253–265. doi: 10.1128/jb.120.1.253-265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B. L., Shobe C. R. Single-step purification of urease by affinity chromatography. Can J Microbiol. 1974 Apr;20(4):623–630. doi: 10.1139/m74-095. [DOI] [PubMed] [Google Scholar]



