Abstract
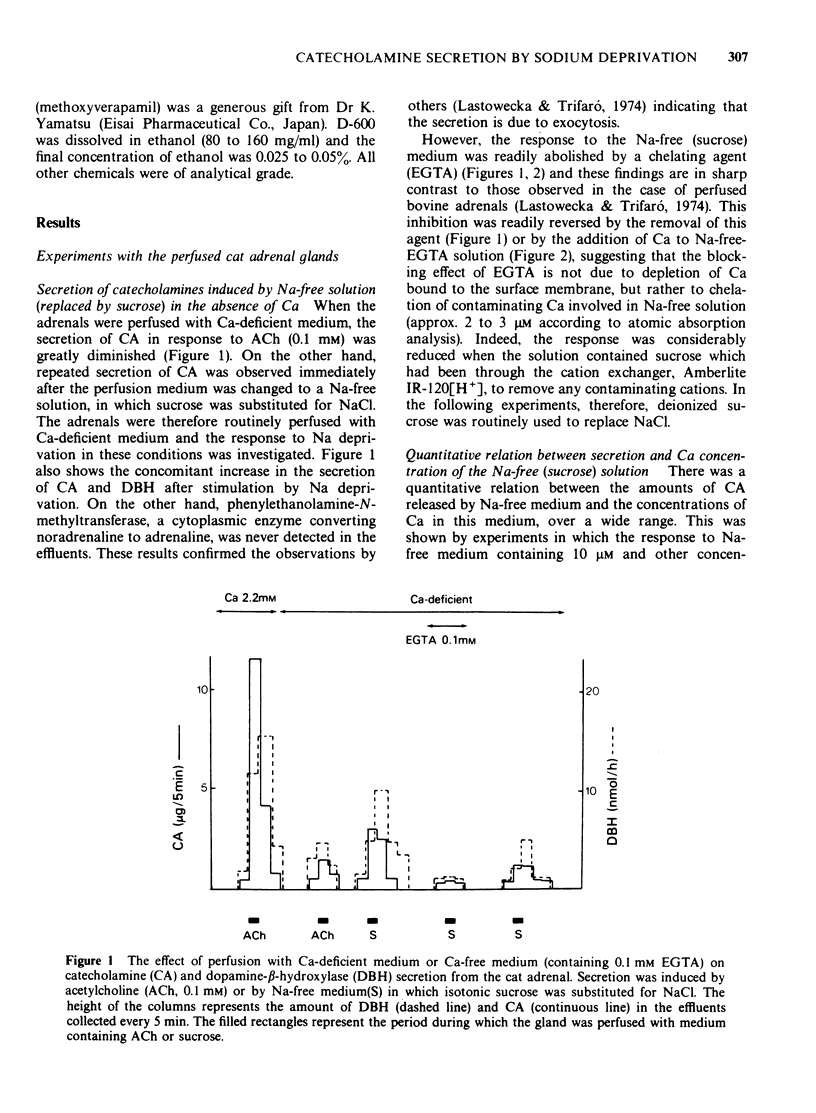
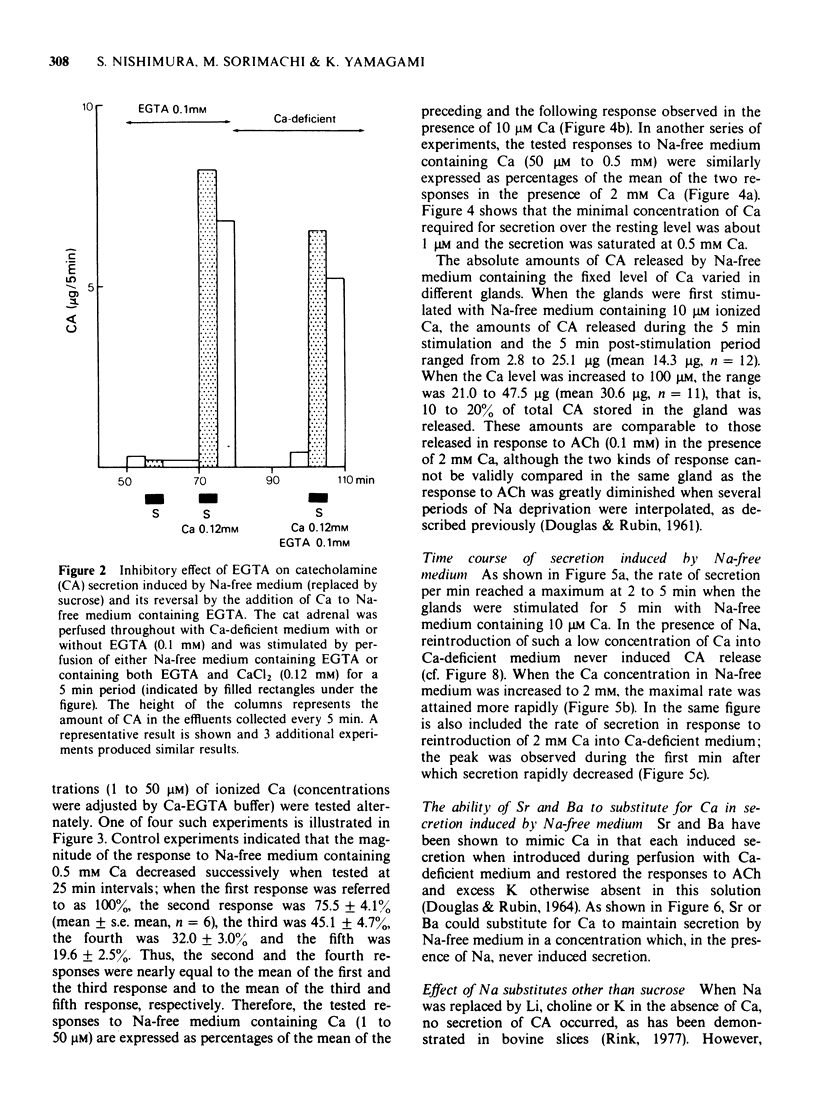
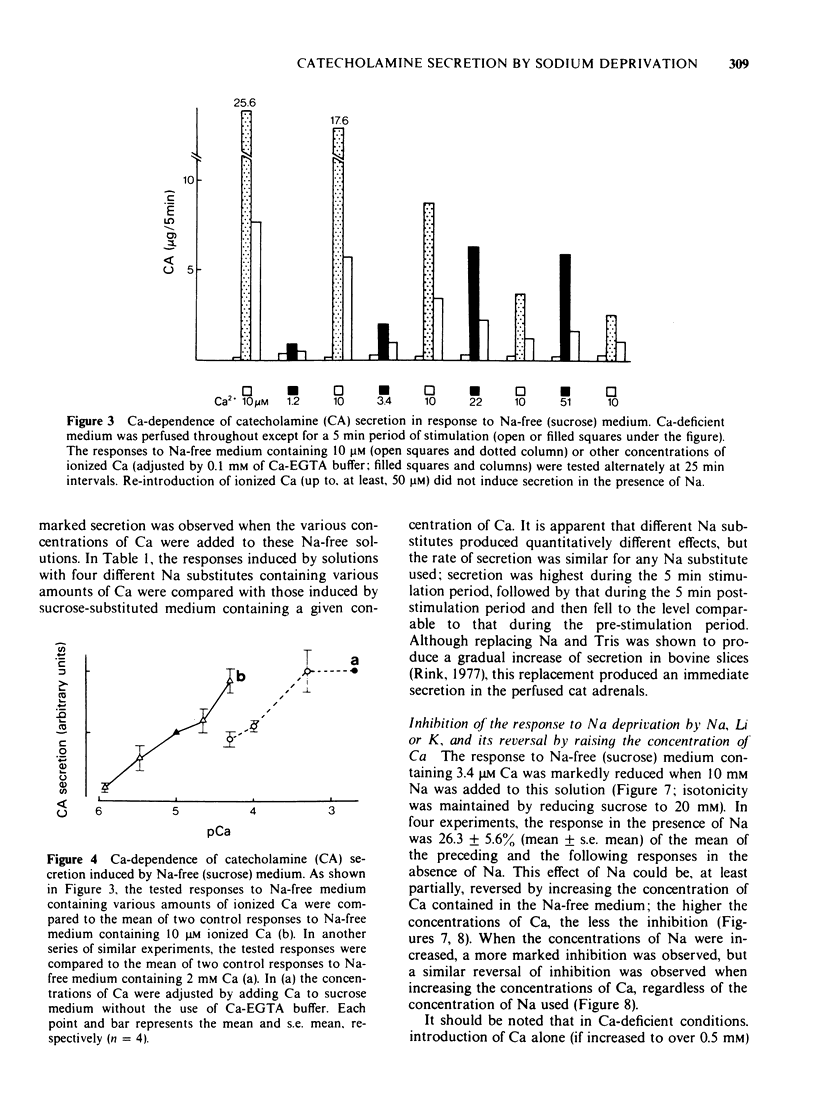
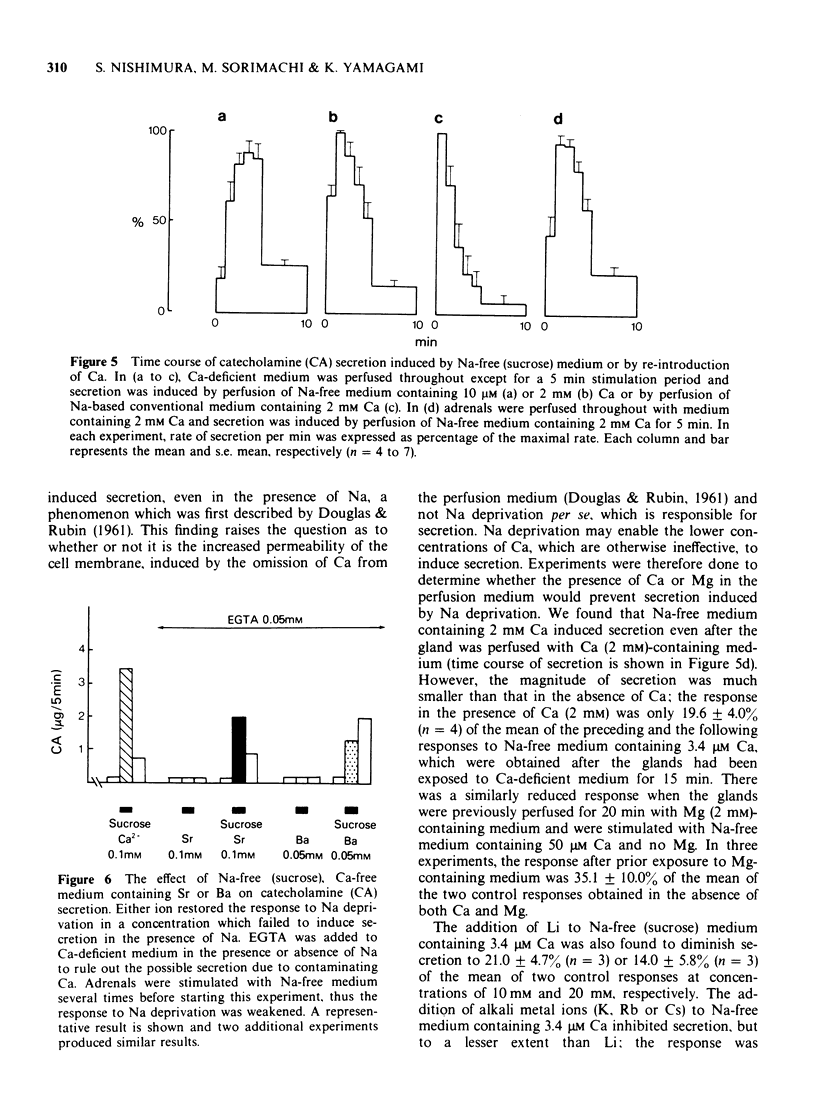
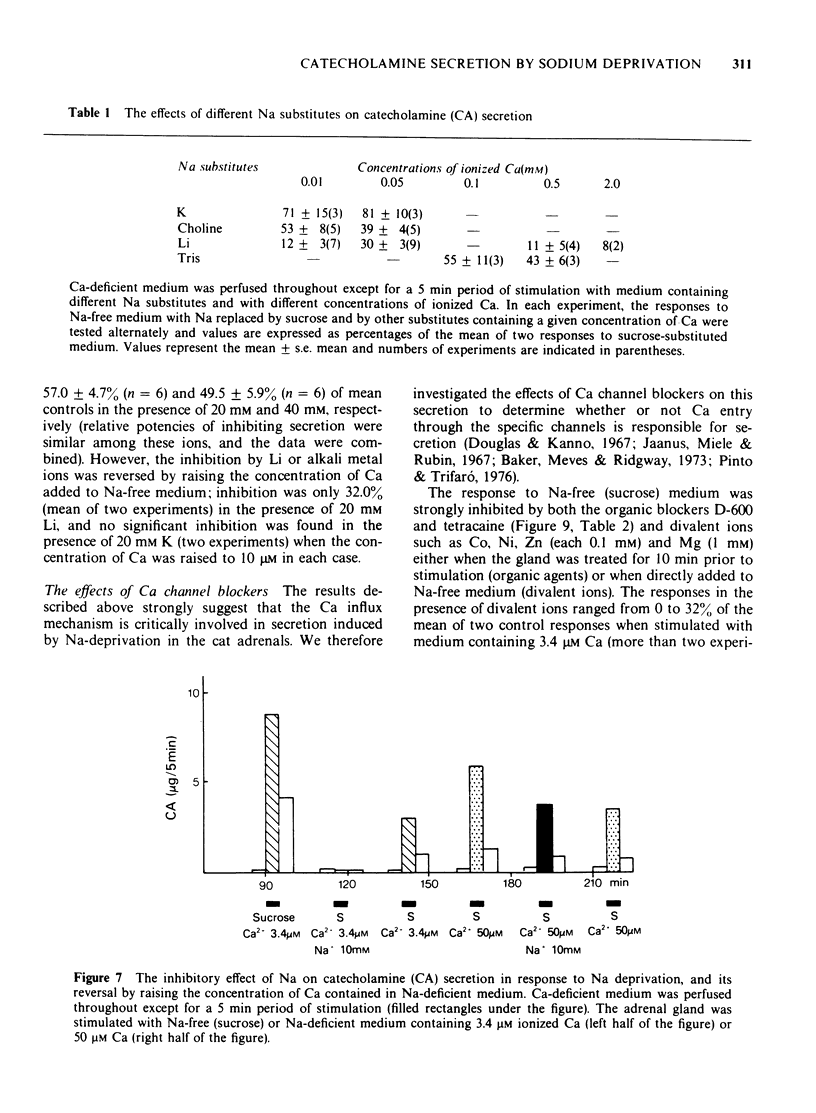
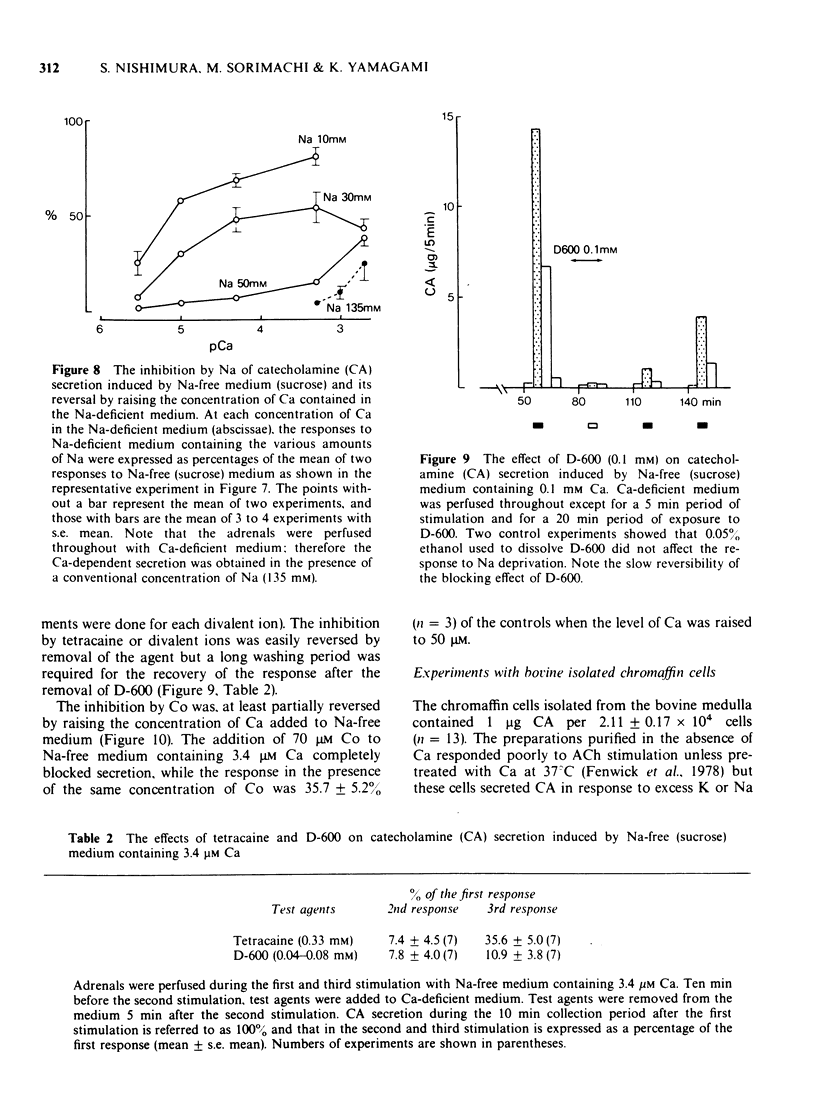
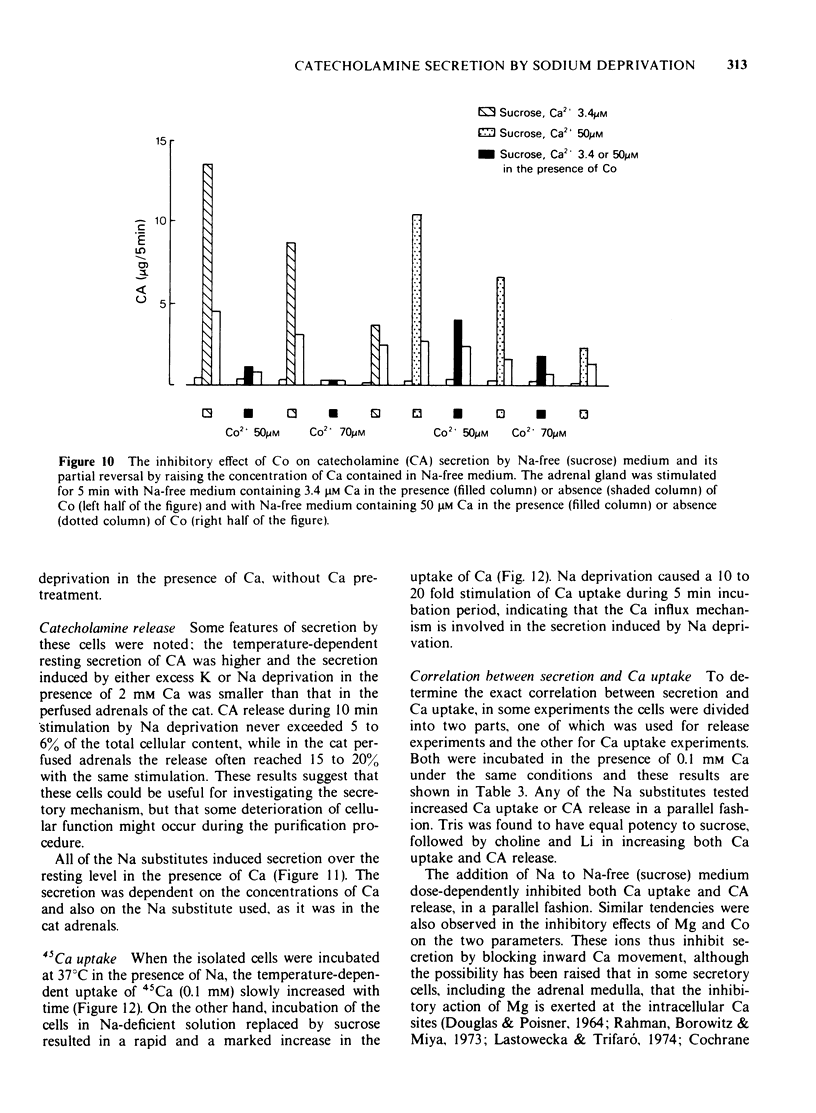
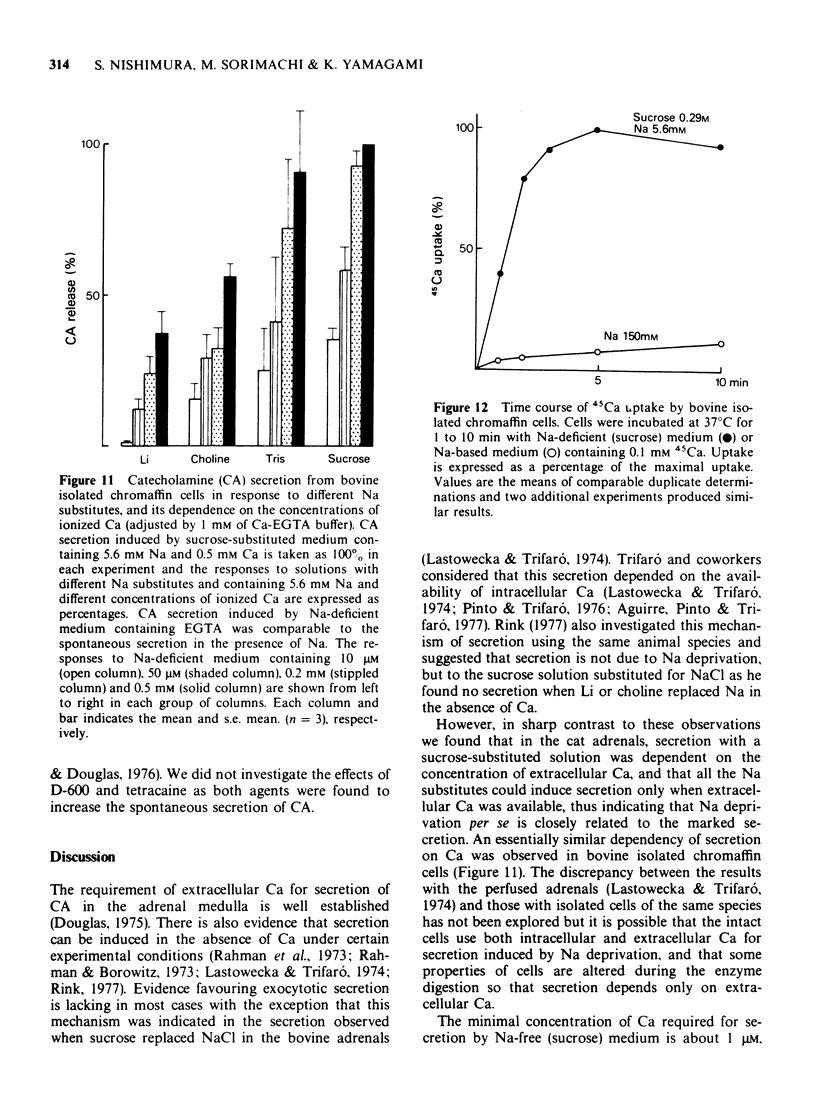
1 Cat adrenal glands were perfused with Ca-deficient medium and secretion of catecholamines (CA) was induced by perfusion with Na-free medium in which NaCl was replaced by an osmotically equivalent amount of sucrose. 2 Release of CA and dopamine-beta-hydroxylase (DBH), but not that of phenylethanolamine-N-methyltransferase, was concomitantly found in the effluents when the adrenals were stimulated, indicating that secretion was due to exocytosis. 3 Secretion of CA induced by Na-free (sucrose) medium was dependent on the concentration of Ca and was saturated at 0.5 mM of Ca. 4 Sr or Ba substituted for Ca in maintaining secretion by Na-free (sucrose) medium. 5 The addition of Na, Li or alkali metal ions to Na-free (sucrose) medium containing Ca reduced the response to a variable extent but this inhibition was reversed by raising the concentration of Ca in the Na-free medium. 6 All of the Na substitutes used induced secretion only when this medium contained Ca. However, different Na substitutes released different amounts of CA; sucrose was most effective, K, Tris and choline were moderately and Li least effective. 7 Secretion of CA by Na-free (sucrose) medium was strongly inhibited by D-600, tetracaine or divalent cations such as Co, Ni, Zn and Mg. The inhibition by Co was partially reversed by raising the concentration of Ca in the Na-free medium. 8 Secretion of CA from bovine isolated chromaffin cells was induced by Na-deficient (sucrose) medium and was dependent on the concentrations of ionized Ca involved. 9 All the Na substitutes tested increased secretion of CA and 45Ca uptake, in a parallel fashion. 10 A correlation between secretion and 45Ca uptake was found under various experimental manipulations which reduced secretion of CA. 11 These results demonstrated that unlike the perfused bovine adrenals, the Ca influx mechanism is essential for secretion by Na deprivation in the perfused cat adrenals as it is in bovine isolated chromaffin cells. 12 It is suggested that Na deprivation increases Ca entry through the Ca channels by eliminating the competition between Na and Ca, and possibly by activating Ca influx linked with Na efflux.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Aguirre J., Pinto J. E., Trifaró J. M. Calcium movements during the release of catecholamines from the adrenal medulla: effects of methoxyverapamil and external cations. J Physiol. 1977 Jul;269(2):371–394. doi: 10.1113/jphysiol.1977.sp011907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature. 1978 Dec 7;276(5688):620–622. doi: 10.1038/276620a0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Biales B., Dichter M., Tischler A. Electrical excitability of cultured adrenal chromaffin cells. J Physiol. 1976 Nov;262(3):743–753. doi: 10.1113/jphysiol.1976.sp011618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane D. E., Douglas W. W. Histamine release by exocytosis from rat mast cells on reduction of extracellular sodium: a secretory response inhibited by calcium, strontium, barium or magnesium. J Physiol. 1976 May;257(2):433–448. doi: 10.1113/jphysiol.1976.sp011377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. CALCIUM MOVEMENT IN THE NEUROHYPOPHYSIS OF THE RAT AND ITS RELATION TO THE RELEASE OF VASOPRESSIN. J Physiol. 1964 Jul;172:19–30. doi: 10.1113/jphysiol.1964.sp007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. THE EFFECTS OF ALKALINE EARTHS AND OTHER DIVALENT CATIONS ON ADRENAL MEDULLARY SECRETION. J Physiol. 1964 Dec;175:231–241. doi: 10.1113/jphysiol.1964.sp007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells: effect of ions. J Physiol. 1970 Sep;210(2):265–275. doi: 10.1113/jphysiol.1970.sp009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T. The effect of amethocaine on acetylcholine-induced depolarization and catecholamine secretion in the adrenal chromaffin cell. Br J Pharmacol Chemother. 1967 Aug;30(3):612–619. doi: 10.1111/j.1476-5381.1967.tb02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Casteels R. Sodium and calcium interactions in vascular smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1979 Jul;74(1):57–70. doi: 10.1085/jgp.74.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Fajdiga P. B., Howe N. B., Livett B. G. Functional and morphological characterization of isolated bovine adrenal medullary cells. J Cell Biol. 1978 Jan;76(1):12–30. doi: 10.1083/jcb.76.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimblet E. G., Marney A. F., Bonsnes R. W. Determination of calcium and magnesium in serum, urine, diet, and stool by atomic absorption spectrophotometry. Clin Chem. 1967 Mar;13(3):204–214. [PubMed] [Google Scholar]
- Griffey M. A., Conaway H. H., Whitney J. E. Insulin secretion induced by Na+ deprivation. Endocrinology. 1974 Nov;95(5):1469–1472. doi: 10.1210/endo-95-5-1469. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaanus S. D., Miele E., Rubin R. P. The analysis of the inhibitory effect of anaesthetics and propranolol on adreno-medullary secretion evoked by calcium or acetylcholine. Br J Pharmacol Chemother. 1967 Oct;31(2):319–330. doi: 10.1111/j.1476-5381.1967.tb02001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The permeability of frog muscle fibres to lithium ions. J Physiol. 1959 Oct;147:626–638. doi: 10.1113/jphysiol.1959.sp006265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T., Cochrane D. E., Douglas W. W. Exocytosis (secretory granule extrusion) induced by injection of calcium into mast cells. Can J Physiol Pharmacol. 1973 Dec;51(12):1001–1004. doi: 10.1139/y73-153. [DOI] [PubMed] [Google Scholar]
- Lambert A. E., Henquin J. C., Malvaux P. Cationic environment and dynamics of insulin secretion. I. Effect of low concentrations of sodium. Endocrinology. 1974 Oct;95(4):1069–1077. doi: 10.1210/endo-95-4-1069. [DOI] [PubMed] [Google Scholar]
- Lastowecka A., Trifaró J. M. The effect of sodium and calcium ions on the release of catecholamines from the adrenal medulla: sodium deprivation induces release by exocytosis in the absence of extracellular calcium. J Physiol. 1974 Feb;236(3):681–705. doi: 10.1113/jphysiol.1974.sp010460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Molinoff P. B., Weinshilboum R., Axelrod J. A sensitive enzymatic assay for dopamine- -hydroxylase. J Pharmacol Exp Ther. 1971 Sep;178(3):425–431. [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto J. E., Trifaró J. M. The different effects of D-600 (methoxyverapamil) on the release of adrenal catecholamines induced by acetylcholine, high potassium or sodium deprivation. Br J Pharmacol. 1976 May;57(1):127–132. doi: 10.1111/j.1476-5381.1976.tb07662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahwan R. G., Borowitz J. L. Mechanisms of stimulus-secretion coupling in adrenal medulla. J Pharm Sci. 1973 Dec;62(12):1911–1923. doi: 10.1002/jps.2600621202. [DOI] [PubMed] [Google Scholar]
- Rahwan R. G., Borowitz J. L., Miya T. S. The role of intracellular calcium in catecholamine secretion from the bovine adrenal medulla. J Pharmacol Exp Ther. 1973 Jan;184(1):106–118. [PubMed] [Google Scholar]
- Rink T. J. The influence of sodium on calcium movements and catecholamine release in thin slices of bovine adrenal medulla. J Physiol. 1977 Apr;266(2):297–325. doi: 10.1113/jphysiol.1977.sp011769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorimachi M., Yoshida K. Exocytotic release of catecholamines and dopamine-beta-hydroxylase from the perfused adrenal gland of the rabbit and cat. Br J Pharmacol. 1979 Jan;65(1):117–125. doi: 10.1111/j.1476-5381.1979.tb17340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


