Abstract
To investigate the effect of chromosomal mutation on the synthesis of rfe-dependent Escherichia coli O9 lipopolysaccharide (LPS), the cloned E. coli O9 rfb gene was introduced into Salmonella typhimurium strains defective in various genes involved in the synthesis of LPS. When E. coli O9 rfb was introduced into S. typhimurium strains possessing defects in rfb or rfc, they synthesized E. coli O9 LPS on their cell surfaces. The rfe-defective mutant of S. typhimurium synthesized only very small amounts of E. coli O9 LPS after the introduction of E. coli O9 rfb. These results confirmed the widely accepted idea that the biosynthesis of E. coli O9-specific polysaccharide does not require rfc but requires rfe. By using an rfbT mutant of the E. coli O9 rfb gene, the mechanism of transfer of the synthesized E. coli O9-specific polysaccharide from antigen carrier lipid to the R-core of S. typhimurium was investigated. The rfbT mutant of the E. coli O9 rfb gene failed to direct the synthesis of E. coli O9 LPS in the rfc mutant strain of S. typhimurium, in which rfaL and rfbT functions are intact, but directed the synthesis of the precursor. Because the intact E. coli O9 rfb gene directed the synthesis of E. coli O9 LPS in the same strain, it was suggested that the rfaL product of S. typhimurium and rfbT product of E. coli O9 cooperate to synthesize E. coli O9 LPS in S. typhimurium.
Full text
PDF
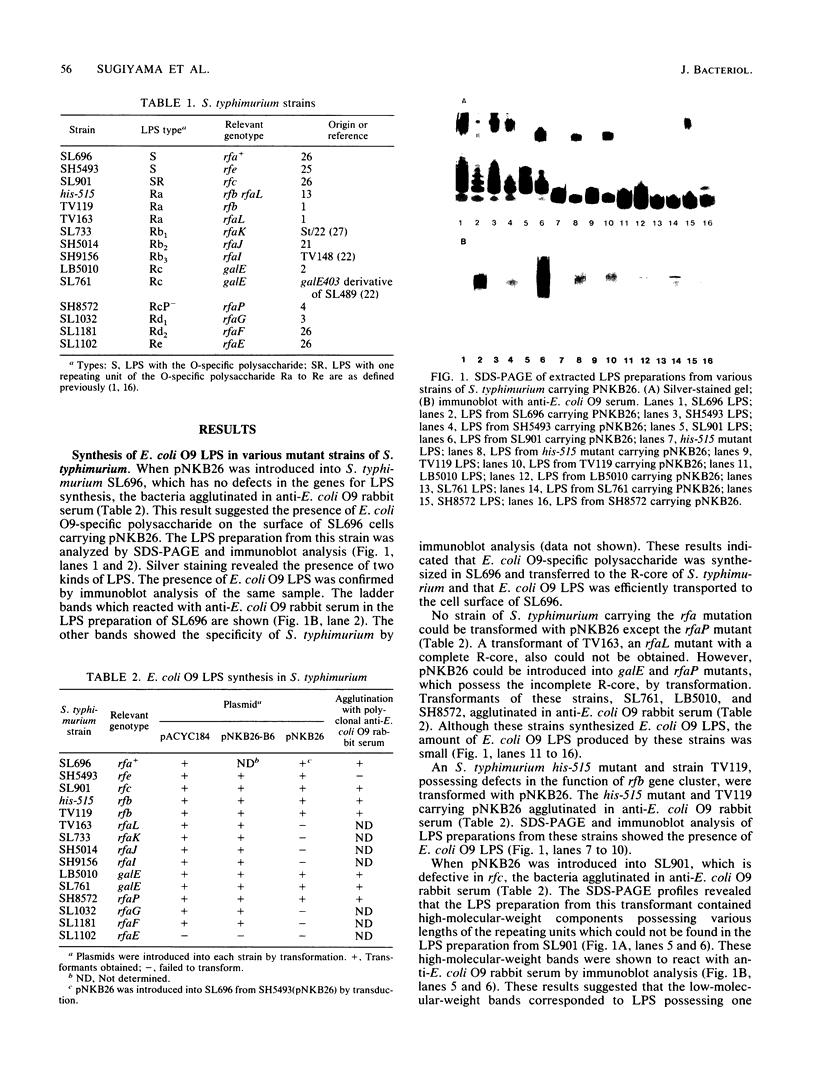

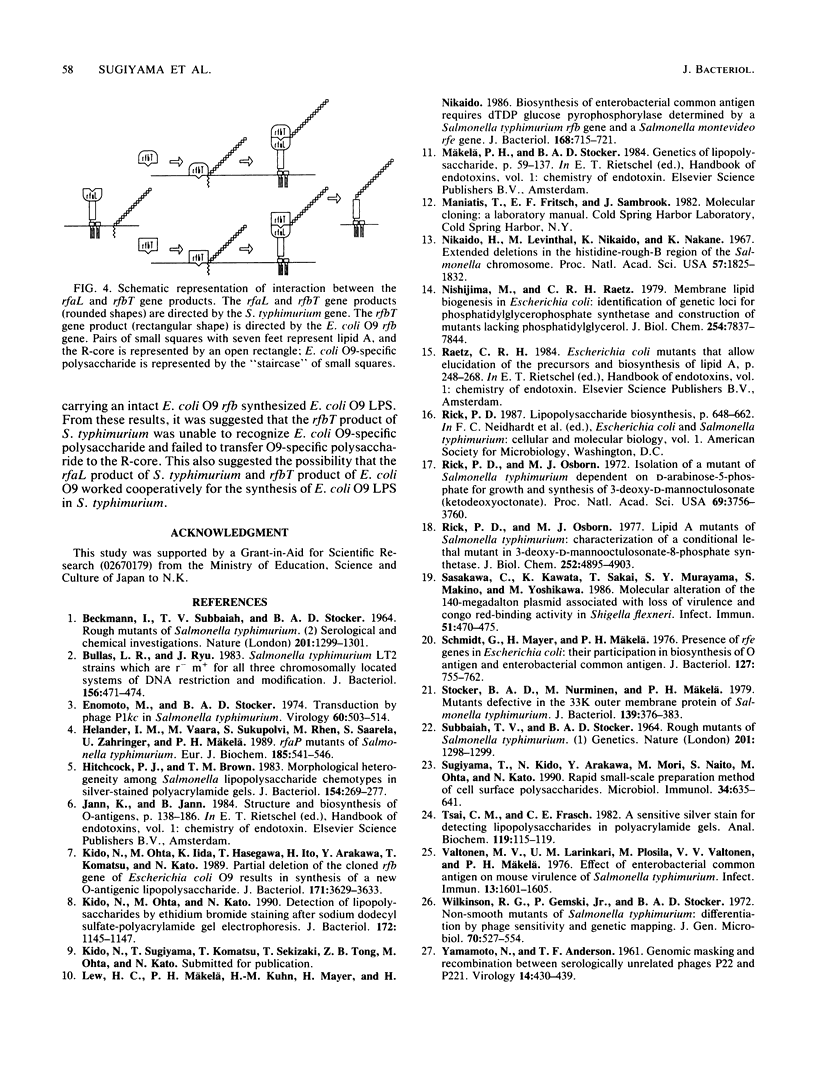
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKMANN I., SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. II. SEROLOGICAL AND CHEMICAL INVESTIGATIONS. Nature. 1964 Mar 28;201:1299–1301. doi: 10.1038/2011299a0. [DOI] [PubMed] [Google Scholar]
- Bullas L. R., Ryu J. I. Salmonella typhimurium LT2 strains which are r- m+ for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983 Oct;156(1):471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M., Stocker B. A. Transduction by phage P1kc in Salmonella typhimurium. Virology. 1974 Aug;60(2):503–514. doi: 10.1016/0042-6822(74)90344-4. [DOI] [PubMed] [Google Scholar]
- Helander I. M., Vaara M., Sukupolvi S., Rhen M., Saarela S., Zähringer U., Mäkelä P. H. rfaP mutants of Salmonella typhimurium. Eur J Biochem. 1989 Nov 20;185(3):541–546. doi: 10.1111/j.1432-1033.1989.tb15147.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido N., Ohta M., Iida K., Hasegawa T., Ito H., Arakawa Y., Komatsu T., Kato N. Partial deletion of the cloned rfb gene of Escherichia coli O9 results in synthesis of a new O-antigenic lipopolysaccharide. J Bacteriol. 1989 Jul;171(7):3629–3633. doi: 10.1128/jb.171.7.3629-3633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido N., Ohta M., Kato N. Detection of lipopolysaccharides by ethidium bromide staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Bacteriol. 1990 Feb;172(2):1145–1147. doi: 10.1128/jb.172.2.1145-1147.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew H. C., Mäkelä P. H., Kuhn H. M., Mayer H., Nikaido H. Biosynthesis of enterobacterial common antigen requires dTDPglucose pyrophosphorylase determined by a Salmonella typhimurium rfb gene and a Salmonella montevideo rfe gene. J Bacteriol. 1986 Nov;168(2):715–721. doi: 10.1128/jb.168.2.715-721.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Levinthal M., Nikaido K., Nakane K. Extended deletions in the histidine-rough-B region of the Salmonella chromosome. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1825–1832. doi: 10.1073/pnas.57.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima M., Raetz C. R. Membrane lipid biogenesis in Escherichia coli: identification of genetic loci for phosphatidylglycerophosphate synthetase and construction of mutants lacking phosphatidylglycerol. J Biol Chem. 1979 Aug 25;254(16):7837–7844. [PubMed] [Google Scholar]
- Rick P. D., Osborn M. J. Isolation of a mutant of Salmonella typhimurium dependent on D-arabinose-5-phosphate for growth and synthesis of 3-deoxy-D-mannoctulosonate (ketodeoxyoctonate). Proc Natl Acad Sci U S A. 1972 Dec;69(12):3756–3760. doi: 10.1073/pnas.69.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick P. D., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Characterization of a conditional lethal mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4895–4903. [PubMed] [Google Scholar]
- SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. I. GENETICS. Nature. 1964 Mar 28;201:1298–1299. doi: 10.1038/2011298a0. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Kamata K., Sakai T., Murayama S. Y., Makino S., Yoshikawa M. Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect Immun. 1986 Feb;51(2):470–475. doi: 10.1128/iai.51.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Mayer H., Mäkelä P. H. Presence of rfe genes in Escherichia coli: their participation in biosynthesis of O antigen and enterobacterial common antigen. J Bacteriol. 1976 Aug;127(2):755–762. doi: 10.1128/jb.127.2.755-762.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker B. A., Nurminen M., Mäkelä P. H. Mutants defective in the 33K outer membrane protein of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):376–383. doi: 10.1128/jb.139.2.376-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Kido N., Arakawa Y., Mori M., Naito S., Ohta M., Kato N. Rapid small-scale preparation method of cell surface polysaccharides. Microbiol Immunol. 1990;34(7):635–641. doi: 10.1111/j.1348-0421.1990.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Valtonen M. V., Larinkari U. M., Plosila M., Valtonen V. V., Mäkelä P. H. Effect of enterobacterial common antigen on mouse virulence of Salmonella typhimurium. Infect Immun. 1976 Jun;13(6):1601–1605. doi: 10.1128/iai.13.6.1601-1605.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO N., ANDERSON T. F. Genomic masking and recombination between serologically unrelated phages P22 and P221. Virology. 1961 Aug;14:430–439. doi: 10.1016/0042-6822(61)90334-8. [DOI] [PubMed] [Google Scholar]