Abstract
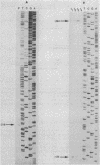
Transcripts of the Clostridium thermocellum endoglucanase genes celF and celD, encoding endoglucanases F and D, respectively, were characterized. The size of the mRNAs was about 2.35 kb for celF and 2.1 kb for celD, indicating monocistronic transcription of both genes. A unique 5' end, located 218 bp upstream from the initiation codon, was found for celF mRNA. No convincing homology could be identified between the sequence upstream from the celF 5' end and other procaryotic promoters. Two 5' ends, located 124 and 294 bp upstream from the initiation codon, were mapped for celD mRNA. The -10 and the -35 sequences preceding the ATG-distal 5' end of celD mRNA were homologous to the consensus sequence of Bacillus subtilis sigma 43 promoters. The sequence upstream from the ATG-proximal 5' end had some similarity with the -10 sequence of B. subtilis sigma 28 promoters. During growth on cellobiose, the 5' end of celD transcripts was found predominantly at the -124 site during the late exponential phase but almost exclusively at the -294 site during the early stationary phase. The kinetics of appearance of celA, celC, celD, and celF mRNA was followed by dot blot analysis. Transcripts of celA, celD, and celF were detected during late exponential and early stationary phase. In contrast, the celC transcript was detected almost exclusively during early stationary phase. Since growth was limited by the availability of cellobiose, the results suggest that the genes are regulated by a mechanism analogous to catabolite repression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beguin P., Cornet P., Millet J. Identification of the endoglucanase encoded by the celB gene of Clostridium thermocellum. Biochimie. 1983 Aug-Sep;65(8-9):495–500. doi: 10.1016/s0300-9084(83)80131-x. [DOI] [PubMed] [Google Scholar]
- Béguin P., Cornet P., Aubert J. P. Sequence of a cellulase gene of the thermophilic bacterium Clostridium thermocellum. J Bacteriol. 1985 Apr;162(1):102–105. doi: 10.1128/jb.162.1.102-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P., Rocancourt M., Chebrou M. C., Aubert J. P. Mapping of mRNA encoding endoglucanase A from Clostridium thermocellum. Mol Gen Genet. 1986 Feb;202(2):251–254. doi: 10.1007/BF00331645. [DOI] [PubMed] [Google Scholar]
- Doi R. H., Wang L. F. Multiple procaryotic ribonucleic acid polymerase sigma factors. Microbiol Rev. 1986 Sep;50(3):227–243. doi: 10.1128/mr.50.3.227-243.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gilman M. Z., Chamberlin M. J. Developmental and genetic regulation of Bacillus subtilis genes transcribed by sigma 28-RNA polymerase. Cell. 1983 Nov;35(1):285–293. doi: 10.1016/0092-8674(83)90231-3. [DOI] [PubMed] [Google Scholar]
- Glatron M. F., Rapoport G. Biosynthesis of the parasporal inclusion of Bacillus thuringiensis: half-life of its corresponding messenger RNA. Biochimie. 1972;54(10):1291–1301. doi: 10.1016/s0300-9084(72)80070-1. [DOI] [PubMed] [Google Scholar]
- Greenberg N. M., Warren R. A., Kilburn D. G., Miller R. C., Jr Regulation and initiation of cenB transcripts of Cellulomonas fimi. J Bacteriol. 1987 Oct;169(10):4674–4677. doi: 10.1128/jb.169.10.4674-4677.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg N. M., Warren R. A., Kilburn D. G., Miller R. C., Jr Regulation, initiation, and termination of the cenA and cex transcripts of Cellulomonas fimi. J Bacteriol. 1987 Feb;169(2):646–653. doi: 10.1128/jb.169.2.646-653.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräbnitz F., Rücknagel K. P., Seiss M., Staudenbauer W. L. Nucleotide sequence of the Clostridium thermocellum bgIB gene encoding thermostable beta-glucosidase B: homology to fungal beta-glucosidases. Mol Gen Genet. 1989 May;217(1):70–76. doi: 10.1007/BF00330944. [DOI] [PubMed] [Google Scholar]
- Grépinet O., Béguin P. Sequence of the cellulase gene of Clostridium thermocellum coding for endoglucanase B. Nucleic Acids Res. 1986 Feb 25;14(4):1791–1799. doi: 10.1093/nar/14.4.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grépinet O., Chebrou M. C., Béguin P. Nucleotide sequence and deletion analysis of the xylanase gene (xynZ) of Clostridium thermocellum. J Bacteriol. 1988 Oct;170(10):4582–4588. doi: 10.1128/jb.170.10.4582-4588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grépinet O., Chebrou M. C., Béguin P. Purification of Clostridium thermocellum xylanase Z expressed in Escherichia coli and identification of the corresponding product in the culture medium of C. thermocellum. J Bacteriol. 1988 Oct;170(10):4576–4581. doi: 10.1128/jb.170.10.4576-4581.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J., Hazlewood G. P., Barker P. J., Gilbert H. J. Conserved reiterated domains in Clostridium thermocellum endoglucanases are not essential for catalytic activity. Gene. 1988 Sep 15;69(1):29–38. doi: 10.1016/0378-1119(88)90375-7. [DOI] [PubMed] [Google Scholar]
- Joliff G., Béguin P., Aubert J. P. Nucleotide sequence of the cellulase gene celD encoding endoglucanase D of Clostridium thermocellum. Nucleic Acids Res. 1986 Nov 11;14(21):8605–8613. doi: 10.1093/nar/14.21.8605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. S., Wilson D. B. Transcription of the celE gene in Thermomonospora fusca. J Bacteriol. 1988 Sep;170(9):3838–3842. doi: 10.1128/jb.170.9.3838-3842.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E., Wilson D. B. Regulation of beta-1,4-Endoglucanase Synthesis in Thermomonospora fusca. Appl Environ Microbiol. 1987 Jun;53(6):1352–1357. doi: 10.1128/aem.53.6.1352-1357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. D., Bear D. G., Litchman B. L., von Hippel P. H. RNA sequence and secondary structure requirements for rho-dependent transcription termination. Nucleic Acids Res. 1985 May 24;13(10):3739–3754. doi: 10.1093/nar/13.10.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser B., Gilkes N. R., Kilburn D. G., Warren R. A., Miller R. C., Jr Purification and characterization of endoglucanase C of Cellulomonas fimi, cloning of the gene, and analysis of in vivo transcripts of the gene. Appl Environ Microbiol. 1989 Oct;55(10):2480–2487. doi: 10.1128/aem.55.10.2480-2487.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petre J., Longin R., Millet J. Purification and properties of an endo-beta-1,4-glucanase from Clostridium thermocellum. Biochimie. 1981 Jul;63(7):629–639. doi: 10.1016/s0300-9084(81)80061-2. [DOI] [PubMed] [Google Scholar]
- Pétré D., Millet J., Longin R., Béguin P., Girard H., Aubert J. P. Purification and properties of the endoglucanase C of Clostridium thermocellum produced in Escherichia coli. Biochimie. 1986 May;68(5):687–695. doi: 10.1016/s0300-9084(86)80162-6. [DOI] [PubMed] [Google Scholar]
- Robson L. M., Chambliss G. H. Endo-beta-1,4-glucanase gene of Bacillus subtilis DLG. J Bacteriol. 1987 May;169(5):2017–2025. doi: 10.1128/jb.169.5.2017-2025.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz W. H., Gräbnitz F., Staudenbauer W. L. Properties of a Clostridium thermocellum Endoglucanase Produced in Escherichia coli. Appl Environ Microbiol. 1986 Jun;51(6):1293–1299. doi: 10.1128/aem.51.6.1293-1299.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz W. H., Schimming S., Rücknagel K. P., Burgschwaiger S., Kreil G., Staudenbauer W. L. Nucleotide sequence of the celC gene encoding endoglucanase C of Clostridium thermocellum. Gene. 1988;63(1):23–30. doi: 10.1016/0378-1119(88)90542-2. [DOI] [PubMed] [Google Scholar]
- Tailliez P., Girard H., Millet J., Beguin P. Enhanced Cellulose Fermentation by an Asporogenous and Ethanol-Tolerant Mutant of Clostridium thermocellum. Appl Environ Microbiol. 1989 Jan;55(1):207–211. doi: 10.1128/aem.55.1.207-211.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Wood W. E., Neubauer D. G., Stutzenberger F. J. Cyclic AMP levels during induction and repression of cellulase biosynthesis in Thermomonospora curvata. J Bacteriol. 1984 Dec;160(3):1047–1054. doi: 10.1128/jb.160.3.1047-1054.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagüe E., Béguin P., Aubert J. P. Nucleotide sequence and deletion analysis of the cellulase-encoding gene celH of Clostridium thermocellum. Gene. 1990 Apr 30;89(1):61–67. doi: 10.1016/0378-1119(90)90206-7. [DOI] [PubMed] [Google Scholar]